Wikipedia talk:WikiProject Elements
| Main talk | Templates RELC | Articles RELC Stats | Periodic Table by Quality other PTQs | Pictures | Isotopes | Periodic Table Graphics (PTG) | Participants WikiChem IRC | Links |
| Elements Project‑class | |||||||
| |||||||
|
Archives: Index 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62 |
|
|
Sections older than 60 days may be automatically archived by lowercase sigmabot III. |
Did you know
- 04 Jul 2023 – Europium compounds (talk · edit · hist) was nominated for DYK by Praseodymium-141 (t · c); see discussion
Featured article candidates
- 02 May 2023 – Nonmetal (chemistry) (talk · edit · hist) FA nominated by Sandbh (t · c) was not promoted; see discussion
Good article nominees
- 31 May 2023 – Cerium (talk · edit · hist) was GA nominated by Praseodymium-141 (t · c); start discussion
- 09 Mar 2023 – Holmium (talk · edit · hist) was GA nominated by Praseodymium-141 (t · c); start discussion
Good topic candidates
- 16 May 2023 – Group 5 element (talk · edit · hist) GT nominated by Praseodymium-141 (t · c) was closed, see discussion
The topic includes: Dubnium, Vanadium, Niobium, Tantalum
Featured article reviews
- 30 Jan 2023 – Uranium (talk · edit · hist) was put up for FA review by Hog Farm (t · c); see discussion
Requests for comments
- 22 May 2023 – Wikipedia:WikiProject Elements (talk · edit · hist) RfC by 123957a (t · c) was closed; see discussion
Peer reviews
- 14 Apr 2023 – Protactinium (talk · edit · hist) has been put up for PR by Praseodymium-141 (t · c); see discussion
Requested moves
- 07 May 2023 – Metals close to the border between metals and nonmetals (talk · edit · hist) move request to Post-transition metal by Interstellarity (t · c) was moved to Post-transition metal (talk · edit · hist) by MaterialWorks (t · c) on 15 May 2023; see discussion
Articles to be split
- 05 Apr 2023 – Isotopes of thorium (talk · edit · hist) is proposed for splitting by Nucleus hydro elemon (t · c); see discussion
Click to watch (Subscribe via
| B | C | Start | Stub | List | Category | Disambig | Draft | File | Portal | Project | Redirect | Template | NA | ??? | Total | ||||
| 30 | 0 | 96 | 89 | 128 | 99 | 38 | 0 | 177 | 292 | 4 | 0 | 116 | 1 | 24 | 3,870 | 257 | 5 | 0 | 5,226 |
Group 5 elements GT nomination[edit]
here. 141Pr {contribs} 19:00, 10 May 2023 (UTC)
RfC on the classification of chemical elements on the periodic table[edit]
Should the classification scheme for the chemical elements on the periodic table be changed from the current 4-color block-based scheme (s-block, p-block d-block, f-block) to a scheme where all elements have the same color? (This RfC is a follow-up to this RfC.) 123957a (talk) 15:53, 22 May 2023 (UTC)
- NB. Per WP:RFC, I have closed this part of discussion since the consensus to show all the elements the same colour is uniformly opposed. Even an involved editor can so close the discussion.
- Discussion remains open on:
- Side discussion re venue
- Scary ambiguity?
- Black and white labelled PT (noting not all elements are shown as the same colour).
- --- Sandbh (talk) 07:43, 31 May 2023 (UTC)
The following discussion is closed. Please do not modify it. Subsequent comments should be made on the appropriate discussion page. No further edits should be made to this discussion.
RfC responses/discussion[edit]
- Oppose. Firstly, this amounts to removing information when there's no need to do so. Certainly, the block classification does not occupy too much space.
- Secondly, it's hard to overstate the importance of blocks. They are directly related to the azimuthal quantum number and hence to the quantum bases of the entire periodic table. A block-based division scheme is mentioned in the IUPAC Red Book as an option on par with labelling groups, which we always do:
The groups of elements in the periodic table (see inside front cover) are numbered from 1 to 18. The elements (except hydrogen) of groups 1, 2 and 13–18 are designated as main group elements and, except in group 18, the first two elements of each main group are termed typical elements. Optionally, the letters s, p, d and f may be used to distinguish different blocks of elements.
(p. 51). Unlike the other names for sets of elements given by the Red Book, blocks cover every element once clearly and there is not much debate on which elements belong to which block, so they can well serve as a classification. A colouring is necessary to make it clear that helium is an s-block element despite its position. Double sharp (talk) 20:58, 22 May 2023 (UTC)
- Secondly, it's hard to overstate the importance of blocks. They are directly related to the azimuthal quantum number and hence to the quantum bases of the entire periodic table. A block-based division scheme is mentioned in the IUPAC Red Book as an option on par with labelling groups, which we always do:
- Oppose' not enough justification, with 4 colors there is at least some good simple important information that can be extracted visually. Removing colors will just make it more plain.--ReyHahn (talk) 21:18, 22 May 2023 (UTC)
- Oppose Perhaps I'm missing something, but I can't see any good reason for removing this information. With the 10-colour option that was previously proposed (at the link above), there was some ambiguity as to what counted as what, but there's a pretty strong consensus as to where the blocks are, so unless there's some strong reason why we don't want that information, I can't see what harm it does to retain it, and it's potentially useful. Anaxial (talk) 21:35, 22 May 2023 (UTC)
- Oppose. The 4-color scheme has all (or nearly all) of the positives of the schemes with more categories (display useful information, categorize elements, more eye-catching) but none (or nearly none) of the negatives (border ambiguities, elements in multiple classes, lack of consensus in the literature for category selection/names/membership, no single classification principle, nearly-endless talk-page arguments). I say this even though the discussion and perfection of the larger category scheme is what really got me involved in WP. But in the end, I have to say that all the time spent by me and several other collaborators over the years would have been much better spent making edits that significantly improved the encyclopedia rather back-and-forth tweaks that verge on the meaningless. YBG (talk) 01:14, 23 May 2023 (UTC)
- Oppose. While the 4-color block scheme is not representative of the literature, showing all elements with the same color would be even worse. Sandbh (talk) 02:10, 23 May 2023 (UTC)
- Oppose. The block names (s, p, d, and f) are in common use using colour to highlight them is useful. Other colour schemes are seen elsewhere but they can become too fussy. Monochrome has no advantages, in my opinion. Mike Turnbull (talk) 14:55, 24 May 2023 (UTC)
- Oppose there is already another plain table around. The coloured one look better, and is useful for its purpose of highlighting blocks. Perhaps it is not accessible, but those that cannot see colour can find the facts in text elsewhere. Graeme Bartlett (talk) 23:55, 24 May 2023 (UTC)
- Oppose I saw the RfC at WT:PHYS and so I'm responding as a casual bystander. Frankly, I'm amazed that the 10-color table was eliminated. The four-color table is eye-bleedingly ugly, and almost devoid of information. The whole point of colors is to say "some of these are not like others", and white-washing the thing, by removing information, just makes the affair boring and stultifying. WP should make readers think "Wow! This is interesting! Something is going on here! Let me investigate!". Coloring it in only a handful of colors just promotes a know-it-all syndrome: "its just four colors, what more is there to know?" and the know-it-all syndrome is a mind-killer. (And yes, I understand the 10-color coloring was ambiguous; but there is a way of coloring it indicate that ambiguity! Ambiguity itself is a terrible excuse!) 67.198.37.16 (talk) 17:01, 26 May 2023 (UTC)
Side discussion re venue[edit]
- Note. This not the right forum. RfC's such as these should be posted at talk periodic table. Sandbh (talk) 02:15, 23 May 2023 (UTC)
- Presumably you mean Talk:Periodic table. Discussions there should concern that one specific article, but this RfC is somewhat broader in its scope, having potentially far-reaching effects. We don't have a WikiProject dedicated to the periodic table, so it's either Chemistry, Physics or here. --Redrose64 🌹 (talk) 09:57, 23 May 2023 (UTC)
- I did indeed mean Talk:Periodic table for the reason that the PT appearing in the lede of that article is generally regarded as the Wikipedia PT. The chemistry project is too disinterested, the ELEM project is too small and non-representative (I speak here as a member of that project) and since the PT is an organising icon of chemistry and the relationships among the elements, Physics is not appropriate. YBG has the right idea; post any further RfC at Talk:Periodic table, then at least ping ELEM, CHEM and PHYSICS. Sandbh (talk) 04:27, 24 May 2023 (UTC)
Scary ambiguity?[edit]
I was impressed by the comment from the IP editor in support of their oppose, namely:
- "And yes, I understand the 10-color coloring was ambiguous; but there is a way of coloring it indicate that ambiguity! Ambiguity itself is a terrible excuse!)"
It is indeed true that chemistry has all sorts of fuzzy definitions.
And yet the 4-colour table was adopted to eliminate this very fuzziness. How unrepresentative, especially for an encyclopedia. I wonder what gives? What is so concerning about ambiguity? --- Sandbh (talk) 08:22, 29 May 2023 (UTC)
- WP:NPOV, as copiously explained at the previous RFC. And also off-topic for this one. Double sharp (talk) 08:44, 29 May 2023 (UTC)
Well, a table showing just the four blocks is not representative of the way “feature” periodic tables are shown in textbooks and is therefore in breach of WP:NPOV. Equally, a table showing ten categories is not representative. A representative table would show just the Ln and An, the TM, metalloids, the noble gases, and the unknowns, and it would need to mark out the four blocks. (Yes I know, one would think that the halogens too would be commonly shown in such tables, but they aren’t.)
There would need to be some kind of fuzziness at least for group 12, to show that they are sometimes counted as TM and sometimes not.
As well, while the elements commonly recognised as metalloids are B, Si, Ge, As, Sb, and Te it is undeniably true that Po and At are sometimes counted as metalloids, and to a lesser extent Se too.
Past problems with the PT in the lede have originated in the fact that we have tried to categorise every element into precisely one category which works fine for most elements but is not representative of some of the elements at the boundaries, such as Po, At and Se.
Pinging other editors to ask if you could chime in with your views. --- Sandbh (talk)
- You are still re-arguing the previous RFC in this one. It's not a particular NPOV concern to say that selenium is in the p-block, and similarly for the other elements. On the other hand, has anyone ever seen a periodic table that uses precisely the categories you advocate for a "representative table" and no others? At least such tables exist for the 4-colour scheme. Double sharp (talk) 12:00, 29 May 2023 (UTC)
- Responding to the ping. As I understand it, you are requesting that the previous RfC be re-opened? If so, while I have no strong opinion on the outcome, surely 23 days is too soon to re-open a formally closed RfC, and it would be off-topic to discuss as part of this RfC anyway. But I perhaps I misunderstand what you're asking? Anaxial (talk) 12:04, 29 May 2023 (UTC)
- Also responding to ping. Let's just close this RfC first, which will lead to us retaining the status quo. If the IP editor or anyone else wants to make a new multi-coloured proposal, let them do so. I would oppose the current 10-coloured version replacing the 4-coloured one. Mike Turnbull (talk) 15:21, 29 May 2023 (UTC)
- Thank you for separating this aside in a separate section. Nevertheless I strongly encourage you to refrain from further discussion off the current topic. Having one IP agree with your POV surely should not be reason for an off-topic discussion. YBG (talk) 23:08, 29 May 2023 (UTC)
- I feel the 10-color scheme is a compromise between the many different schemes found in external sources; I think it's okay to settle for a compromise if it shows more useful information. To me, the switch from the 10-color scheme to the 4-color block-based scheme seems like it may be a case of "perfect is the enemy of good"; by trying to change from a scheme with fuzzy areas to a scheme with no fuzzy areas, we may have made the periodic table less useful. I also feel the current scheme puts too much emphasis on the blocks and fails to show the huge differences among elements in the p-block. 123957a (talk) 23:20, 30 May 2023 (UTC)
- 123957a: I recall the 10-color scheme was deprecated due to it implying sharp boundaries between all categories; and a lack of consistency in the literature on what to label the leftover metals and nonmetals immediately to either side of the metalloids.
- A 4-color-block-based scheme is sharp, and useful from a physics perspective, and not representative of the fuzziness associated with chemistry.
- Looking at the featured periodic tables appearing in 101 chemistry textbooks (steering clear of the leftover metals and nonmetals) resulted in eleven labels with the indicated appearance %'s:
- Actinides (66)
- Lanthanides (66)
- Nonmetals (30)
- Metals (26)
- Transition metals (24)
- Metalloids (23)
- s-block (13)
- f-block (13)
- d-block (13)
- p-block (13)
- Noble gases (11)
- Actinides (66)
- All other labels have appearance frequencies of < 10% e.g. halogens (6).
Double sharp: Thanks. 1. The closure note to the prior RFC noted, in part, "Further, at least a few editors contemplated continued discussion of the various proposals in subsequent conversations and RFCs." That is what I'm doing, since the current RfC is effectively a non-starter. 2. Of course, there is no NPOV concern about placing Se in the p-block. Rather, the NPOV concern is that a 4-colour block-based colour scheme is unrepresentative of feature tables appearing in the textbooks. 3. I don't know if anyone has ever seen a PT that uses just the categories I suggested, nor is that the point. Instead, the point is that if an NPOV is taken then the categories I suggested are most representative of feature tables appearing in textbooks rather than four coloured blocks. 4. A 4-colour block PT has a place in the periodic table article but not in the lede.
Anaxial: Thanks. No, I'm not requesting the prior RfC be re-opened. Since this current RfC is effectively a non-starter, I'm starting—as flagged in the closing comments of the previous RfC—a discussion of another proposal.
Michael D. Turnbull: Thank you.
YBG: Thank you. I feel that such strong encouragement is misplaced given (i) the current RfC is effectively dead; and (ii) the closing comments in the previous RfC. --- Sandbh (talk) 00:15, 30 May 2023 (UTC)
Black and white labelled PT[edit]

Upon further consideration this actually looks surprisingly good:
- the blocks are all there;
- the traditional dividing line between metals and nonmetals is there;
- the ambiguity of the group 12 metals is there;
- the elements commonly recognised as metalloids are there, as are the sometimes runners, Po and At, plus Se further back in the pack (is the shading for Se too light?);
- the real location of the Ln and An is nicely shown;
- the caption is spot on, in terms of its reference to the most or more commonly named sets of elements in periodic tables;
- there is no need for arguments around the borders;
- helium as an s-block element is accommodated;
- the links in the caption are nice;
- colour-challenged folks are accommodated;
- there are sufficient accoutrements to prompt further interest.
How this looks in the PT article can be seen here, noting the caption is an earlier draft.
--- Sandbh (talk) 06:25, 30 May 2023 (UTC)
Support I think this is an incredible image that adds more detail about the periodic table without putting classifications that are objective (e.g. having multiple colors for categories) I'm all for it being the lead image. OmegaMantis (call now!) 10:16, 30 May 2023 (UTC)
- I don't understand this comment. Surely we want objective classifications if feasible? Double sharp (talk) 15:55, 31 May 2023 (UTC)
![]() Self-trout Sorry, I meant subjective instead of objective. Yes, objective classifications are desirable. OmegaMantis (talk) 17:03, 31 May 2023 (UTC)
Self-trout Sorry, I meant subjective instead of objective. Yes, objective classifications are desirable. OmegaMantis (talk) 17:03, 31 May 2023 (UTC)
Colour labelled PT[edit]
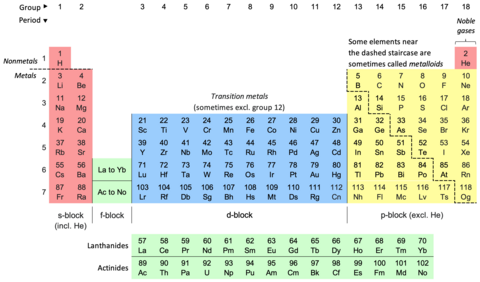
I've added some colour to the b/w image:
- the blocks are retained;
- the traditional dividing line between metals and nonmetals is there;
- the ambiguity of the group 12 metals is there;
- the real location of the Ln and An is nicely shown;
- the caption is spot on, in terms of its reference to the most or more commonly named sets of elements in periodic tables;
- there is no need for arguments around the borders;
- helium as an s-block element is accommodated;
- the links in the caption are nice;
- colour-challenged folks are accommodated;
- there are sufficient accoutrements to prompt further interest.
Rather than highlighting the metalloids I've noted that elements in the vicinity of the traditional dividing line are sometimes counted as metalloids.
I intend to upload this image into the PT article. It captures the precision of the blocks and at the same time some of the ambiguity elsewhere in the PT. --- Sandbh (talk) 07:33, 3 June 2023 (UTC)
- This is actually something I wouldn't mind so much. While the metal-nonmetal divide is subjective, this is at least a very traditional place to put it, and the caption notes that it is a matter of tradition. Of course I still dislike "germanium the metal" because by conductivity it is not one, but crucially here there is not a fully agreed definition of "metal" and it depends on context: if one's criterion for the p-block is "forms an aqua cation that doesn't immediately protonate water", then Ge and Sb as metals (but not At) actually becomes correct. (I suspect Og would form such a cation, though. But who cares about it?) So this is okay with me.
Also, typos: element 73 is tantalum (Ta), the group number 18 is duplicated, and I think that if you abbreviate "excluded" to "excl." then "included" should be abbreviated to "incl.".Double sharp (talk) 10:11, 3 June 2023 (UTC)
Thanks. Typos fixed.
I recall that early samples of Ge were found to be fairly good conductors due to the presence of impurities hence the notion of it being a metal. Later, when sufficiently pure samples became available it was realised that Ge was in fact a semiconductor. --- Sandbh (talk) 00:37, 4 June 2023 (UTC)
- The font size for the label text has been increased marginally. The ragged right margin of the metalloid note now has more of staircase look. --- Sandbh (talk) 01:47, 4 June 2023 (UTC)
- As flagged, I've added his image to the lede of periodic table. It looks quite crisp. --- Sandbh (talk) 01:55, 4 June 2023 (UTC)
Renaming of "Alternative periodic tables" to "Types of periodic tables"[edit]
I've renamed the "Alternative periodic tables" article as Types of periodic tables. At the same time I renovated, expanded, and restructured the contents. --- Sandbh (talk) 02:56, 12 June 2023 (UTC)
