CME
Advances in the Treatment and Recognition of Sepsis and Septic Shock
- Authors: Adil Haider, MD, MPH ; Thomas S Mitchell, Jr, MD
- THIS ACTIVITY HAS EXPIRED FOR CREDIT
Target Audience and Goal Statement
This activity is intended for general surgeons and health care providers interested in the latest prevention and diagnostic and management options for the problems frequently encountered in the surgical setting.
The goal of this activity is to assist surgeons and healthcare providers in the diagnosis and management of common surgical problems, notably sepsis and trauma. It will also update surgeons on the current state of robotics in surgery.
Upon completion of this activity, participants will be able to:
- Identify the characteristics that distinguish sepsis as a specific disease.
- Describe some of the recent advances in treating trauma patients.
- Explain the key issues currently being discussed in the area of robotics.
Disclosures
As an organization accredited by the ACCME, Medscape requires everyone who is in a position to control the content of an education activity to disclose all relevant financial relationships with any commercial interest. The ACCME defines "relevant financial relationships" as "financial relationships in any amount, occurring within the past 12 months, that create a conflict of interest."
Medscape encourages Authors to identify investigational products or off-label uses of products regulated by the U.S. Food and Drug Administration, at first mention and where appropriate in the content.
Accreditation Statements

-
Medscape, LLC is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
Medscape designates this educational activity for a maximum of 1.25 category 1 credit(s) toward the AMA Physician's Recognition Award. Each physician should claim only those credits that reflect the time he/she actually spent in the activity.
For Physicians
For questions regarding the content of this activity, contact the accredited provider for this CME/CE activity noted above. For technical assistance, contact [email protected]
Instructions for Participation and Credit
There are no fees for participating in or receiving credit for this
online educational activity. For information on applicability and
acceptance of continuing education credit for this activity, please
consult your professional licensing board.
This activity is designed to be completed within the time
designated on the title page; physicians should claim only those
credits that reflect the time actually spent in the activity. To
successfully earn credit, participants must complete the activity
online during the valid credit period that is noted on the title page.
Follow these steps to earn CME/CE credit:
- Read the target audience, learning objectives, and author disclosures.
- Study the educational content online or printed out.
- Online, choose the best answer to each test question. To receive a certificate, you must receive a passing score as designated at the top of the test. Medscape encourages you to complete the Activity Evaluation to provide feedback for future programming.
The credit that you receive is based on your user profile.
Advances in the Treatment and Recognition of Sepsis and Septic Shock
processing....
Introduction
The recognition of sepsis as a specific disease entity has led to improvements in our understanding of its pathophysiology and to the development of innovative preventive, diagnostic, and treatment strategies against the various inciting pathological processes. Nomenclature has been developed to distinguish between physiological responses to noninfectious stimuli (ie, systemic inflammatory response syndrome [SIRS]) and conditions arising from infection. These are further categorized as sepsis, severe sepsis, septic shock, and even multiple organ dysfunction syndrome (MODS).
SIRS is present with any 2 of the following conditions[1]:
- Temperature > 38.0°C or < 36.0°C;
- Heart rate > 90 beats per minute;
- Respiratory rate > 20 breaths per minute;
- Partial pressure of carbon dioxide (PCO2) < 32 mm Hg;
- Leukocytosis (white blood cell [WBC] count > 12,000 mcL-1);
- Leukopenia (WBC count < 4000 mcL-1); and
- Normal WBC count with > 10% immature forms.
Remember, you don't have to be infected to have SIRS (eg, acute pancreatitis).
Sepsis has been defined as infection-induced organ dysfunction or hypoperfusion with 2 or more SIRS criteria.[1]
Infection may be documented or suspected -- blood cultures need not be positive. Other sources (eg, urine and sputum) may be positive, but just a suspicion is good enough. In 2002, the definition was broadened to define sepsis as documented or suspected infection with any of the SIRS criteria or 1 or more of the following[2]:
- Significant edema or positive fluid balance (> 20 mL/kg over 24 hours);
- Hyperglycemia (plasma glucose > 120 mg/dL) in the absence of diabetes;
- Inflammatory variables: plasma C-reactive protein > 2 SD above the normal value or plasma procalcitonin > 2 SD above the normal value;
- Mixed venous oxygen saturation (SVO2) > 70%; and
- Cardiac index > 3.5 L·• min-1 • M-23.
Severe sepsis is defined as sepsis associated with organ dysfunction, hypoperfusion, or hypotension.[1] The organ-dysfunction variables include:
- Arterial hypoxemia (PaO2/fraction of inspired oxygen [FiO2] ratio of < 300 torr);
- Acute oliguria (urine output < .5 mL·kg-1·hour-1 or 45 mmol/L for at least 2 hours);
- Creatinine > 2.0 mg/dL;
- Coagulation abnormalities (international normalized ratio > 1.5 or activated partial thromboplastin time > 60 seconds);
- Thrombocytopenia (platelet count < 100,000 mcL-1);
- Hyperbilirubinemia (plasma total bilirubin > 2.0 mg/dL or 35 mmol/L);
- Tissue-perfusion variable: hyperlactatemia (> 2 mmol/L); and
- Hemodynamic variables: arterial hypotension (systolic blood pressure [SBP] < 90 mm Hg, mean arterial pressure [MAP] < 70 mm Hg, or SBP decrease > 40 mm Hg).
Septic shock is defined as acute circulatory failure after crystalloid fluid challenge unexplained by other causes.[1] Acute circulatory failure is defined as persistent arterial hypotension (SBP < 90 mm Hg, MAP < 60, or a reduction in SBP > 40 mm Hg from baseline despite adequate volume resuscitation).
MODS is the presence of the altered function of 2 or more organs in an acutely ill patient, such that homeostasis cannot be maintained without intervention.[1]
To update its fellows on the diagnosis and management of these conditions, the American College of Surgeons' Committee on Peri-operative Care sponsored a session on "Advances in the Treatment and Recognition of Sepsis and Septic Shock" at its 90th Clinical Congress. David H. Reines, MD, FACS, from Falls Church, Virginia, Chair of the panel, reported that sepsis is the leading cause of death in noncoronary intensive care units (ICUs), particularly surgical ICUs, and the 11th most common cause of death in the United States.[3,4] With 750,000 new cases annually and a daily mortality of over 500, sepsis has the highest mortality rate of any major disease, up to 50% (Figure).[3,5]
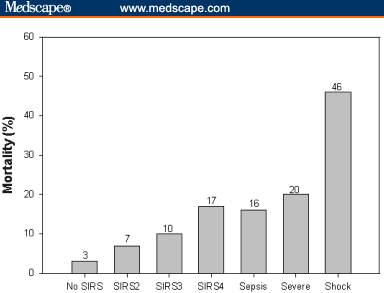
Figure. Mortality rises with increases in the number of systemic inflammatory response syndrome symptoms and in the severity of the disease process. (Adapted from: Rangel-Frausto M, Pttet D, Costigan M, et al. The natural history of the systemic inflammatory response syndrome (SIRS). JAMA. 1995;273:117-123.)
Dr. Reines reminded the audience of the various interventions that have been employed against sepsis, with little success. Use of extremely high-dose corticosteroids (30 mg/kg), for example, was eventually found to be detrimental in the long term.[6] Administration of antiendotoxin against the lipid A moiety of endotoxin[7] in patients with gram-negative infection also did not improve outcomes, possibly because the infection could not be diagnosed in time for the therapy to be effective. Antithromboxane and cyclooxygenase inhibitors, such as ibuprofen, have not been shown to be beneficial in septic shock.[8] Similarly, nitric oxide was also not found to be helpful despite early enthusiasm.
In many instances, in a septic patient, the cause of the sepsis may have already been stabilized. For example, a successful Hartmann's procedure for perforated sigmoid diverticulitis stabilizes the patient's surgical problem, but sepsis persists during and after the operation. Sepsis itself -- and not just its inciting cause -- must be directly treated.
Evidence-Based Practice Guidelines From the Surviving Sepsis Campaign
In 2003, representatives from 11 international medical societies collaborated to develop evidence-based practice guidelines for severe sepsis and septic shock, under the auspices of the Surviving Sepsis Campaign.[2] The goal of the campaign is to achieve a 25% reduction in sepsis mortality by 2009 (campaign details at: http://www.ihi.org/ihi/topics/criticalcare/sepsis). Lena Napolitano, MD, FACS, from Baltimore Maryland, reviewed the Surviving Sepsis guidelines for the audience. These guidelines are continually being reviewed and updated. The next update will be published in 2006.
According to these guidelines, the mainstays of the treatment of sepsis include:
- Controlling the source of the infection;
- Antimicrobial therapy;
- Resuscitation and hemodynamic support;
- Organ support, including mechanical ventilation and renal replacement therapy;
- Sedation/analgesia as needed; and
- Adequate nutrition.
One of the most important factors in the outcome of severe sepsis is the initial choice of antibiotics. Over 30% to 70% of antibiotics prescribed empirically do not cover the bacterial flora that are eventually grown in cultures.[9] The mortality rate in patients who initially receive the wrong antibiotic is 10% to 40% higher than in patients who were adequately covered to start with.[9,10] It is therefore important to understand the bacteriology of the organ system involved and the resistance and susceptibility patterns specific to the particular hospital environment (hence the importance of the hospital antibiotogram). Antibiotics must be started broadly and then narrowed down according to microbiology.
Practice parameters for hemodynamic support give volume resuscitation a top priority. The use of a pulmonary artery catheter may help optimize resuscitation. Alternatively, some centers monitor central venous pressure, maintaining it at 8-12 mm Hg. Such "filling pressures" must be adjusted for ventilator settings, especially if positive end-expiratory pressure is being used. Pressor therapy with catecholamines should not be initiated unless hypotension and hypoperfusion persist despite adequate fluid resuscitation (eg, pulmonary capillary wedge pressure > 20 mm Hg). The goal is not only to restore blood pressure but to restore microcirculation and perfusion. The Society of Critical Care Medicine now recommends the use of norepinephrine (Levophed) and dopamine for pressor therapy.[2,9] "Renal-dose" dopamine to support renal function and urine output is no longer considered to be of any value.
In a randomized trial by Rivers and colleagues,[11] the investigators evaluated "early goal directed therapy." The main goal of this approach was to achieve within 6 hours an SVO2 > 70%, as measured by an oximetric central venous catheter (not a pulmonary artery catheter). This was done with aggressive fluid resuscitation, transfusions, and pressor therapy. In patients managed with this approach, the mortality rate was 30.5%, as compared with 46.5% in patients managed with standard therapy.[11] The improved survival with very early, large-volume fluid-replacement therapy supports the aggressive treatment of septic patients.
Vasopressor Therapy for Septic Shock
Vasopressin physiology in septic shock is another area of investigation. In one study, patients in septic shock were found to have relatively low plasma vasopressin levels compared with patients in cardiogenic shock (3.1 pg/mL vs 22.7 pg/mL). Initial clinical trials have shown that there may be some improvement in survival with its use, and larger, controlled studies need to be performed before its routine use can be recommended.[12] Rozenfeld and Cheng[13] reviewed 11 retrospective, 6 prospective cohort, and 4 prospective randomized studies on the role of exogenous vasopressin in patients with septic shock. Vasopressin produced rapid and persistent increases in systemic vascular resistance and arterial blood pressure, reducing the dosage requirements of adrenergic agents. They concluded that vasopressin should be considered if response to 1 or 2 adrenergic agents is inadequate or as a method to reduce the dosage of adrenergic agents, but it should not be started as first-line therapy. Dr. Napolitano reported that at her center, patients who require high-dose vasopressor therapy with norepinephrine and dopamine or who have refractory hypotension are treated with vasopressin, .01-.04 mcg/min, in addition to these other agents. They are then slowly weaned off the other pressors, increasing vasopressin to keep MAP > 60 mm Hg.[2,14]
For patients with low cardiac output despite fluid resuscitation, dobutamine may be considered. In such cases, vasopressors should be titrated to achieve an MAP of > 64 mm Hg.
Adrenal Insufficiency in Sepsis
Adrenal insufficiency is present in approximately 30% of patients with sepsis or septic shock. Adrenal insufficiency is diagnosed with a cosyntropin stimulation test, which is performed by measuring the response to a dose of adrenocorticotropic hormone ( cosyntropin 250 mcg intravenously [IV]) after 30 and 60 minutes. An increase in plasma cortisol of less than 9 mcg/dL is considered to indicate adrenal insufficiency.[15]
A trial by Annane and associates[16] demonstrated the early benefits of corticosteroid therapy in sepsis. Patients, who received hydrocortisone 50 mg IV every 6 hours and fludrocortisone 50 mcg via a nasogastric tube daily for 7 days came off the pressors sooner (7 vs 10 days) and had a statistically significantly lower mortality rate than the patients not treated with steroids (53% vs 63% at 28 days). However, at 1-year follow-up no difference was seen in survival between the 2 groups despite the initial improvement in the group receiving steroid treatment.[16]
The role of the peripheral glucocorticoid resistance syndrome in sepsis is also under investigation. It is thought that elevated cytokine levels in the blood induce glucocorticoid resistance in target tissues by altering steroid receptor function and affinity. Superdoses of steroids in late septic shock are currently being tested in septic patients.[17]
Activated Protein C for Septic Shock
Activated protein C (APC) was approved by the US Food and Drug Administration (FDA) in 2001 for the treatment of septic shock. In severe sepsis, protein C levels are decreased and D dimers are increased, resulting in a procoagulatory state at the microvascular level. APC has been shown to downregulate inflammation and promote fibrinolysis. Shifting the coagulation cascade toward fibrinolysis helps prevent the formation of microthrombi and emboli, improving circulation at the microvascular level. APC also inhibits inflammation by downregulating interleukin (IL)-1 and tumor necrosis factor (TNF).[18] The Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study showed that APC induced a 13% reduction in mortality at 28 days and 11% reduction at 90 days, in patients with Acute Physiologic and Chronic Health Evaluation (APACHE) scores in the third and fourth quartiles (APACHE score, > 24). Long-term reduction in mortality over 2 years remains at 12% for these patients as well.[19] The most significant side effect of this drug is serious bleeding, and it must be used with caution, especially in postsurgical patients. APC is most beneficial in the sickest patients, and hence it is reserved for those with an APACHE score > 24. Of all the cohorts of patients in which it has been investigated, APC has been shown to be most effective in patients with intra-abdominal sepsis.
Effect of Intensive Insulin Therapy on Sepsis Outcomes
Intensive insulin therapy has been shown to improve outcomes in sepsis as well. With tight glucose control between 80 and 110 mg/dL, van de Berghe and colleagues[20] showed a reduction in mortality from 20.2% to 10.6%, especially in patients with a proven septic focus. Maintaining normoglycemia may be the ICU intervention that results in the most "bang for the buck," as it is the easiest intervention to accomplish in most ICUs. At the Westchester Medical Center, Valhalla, New York, for example, after reading van de Berghe's paper in journal club, 2 surgery interns -- Travis Kemp, MD, and Roy Cheung, MD -- were able to develop and implement an intensive insulin therapy protocol before they finished their 1-month rotation through the surgical ICU. The key to their success was to involve the nursing leadership in developing the protocol as the nurses hang the drips and run the protocol once it is ordered.
Molecular Biology and the Differentiation of Sepsis From Infection
Stephen Lowry, MD, FACS, from New Brunswick, New Jersey, presented an update on the current understanding of different mediators in sepsis. According to the "2-hit theory," he said, a stimulus initially provokes an inflammatory response that is followed by an anti-inflammatory response. During this compensatory anti-inflammatory response, if patients are exposed to another stimulus or "hit" (eg, nosocomial infection), they are at greater risk of developing MODS.
Initial stimuli (infectious or otherwise) that use the Toll receptor system to induce intracellular transcription of inflammatory mediators lead to SIRS. Just the presence of SIRS increases mortality, as it activates the procoagulatory cascade, the complement system, and promotes leukocyte and cytokine release. Early mediators of SIRS are TNF and IL-1, -6, -12, and -18. Late mediators are monocyte chemoattractant protein-1, granulocyte-macrophage colony-stimulating factor, granulocyte colony-stimulating factor, and high mobility group protein. Inflammatory changes disturb homeostasis. In tightly bound systems as the body, altering just 1 organ system can make it more and more difficult to restore normalcy; as more systems fail, patients are pushed into MODS. Therefore, it is important to determine when the body starts to falter to prevent a breakdown in homeostasis and to determine when an intervention would bring the patient back to homeostasis.
Distinguishing inflammation from infections that require antibiotics remains a challenge. Two markers that are being investigated for this purpose are procalcitonin[21] and triggering receptors in myeloid cells 1,[22] with varying degrees of specificity.
J. Perren Cobb, MD, FACS, from St. Louis, Missouri, described how modern genetics may permit the accurate differentiation of infection from inflammation. This distinction is important clinically, Dr. Cobb pointed out, as surgeons tend to mistreat or overtreat patients for suspected infectious illnesses, leading to increasing antimicrobial resistance.[23] This is especially important because pharmaceutical companies are not finding it feasible to continue developing new antibiotics.
The body's reaction to sepsis can vary from a single SIRS criterion to MODS. As discussed previously, patients with sepsis usually suffer a hyperinflammatory response followed by a compensatory anti-inflammatory response. In immunocompromised or debilitated populations, however, such as the elderly, an inability to mount a compensatory response may lead to death. If we could identify patients as soon as sepsis sets in, we could help prevent them from decompensating by treating them earlier. Just as troponin I is highly specific for myocardial infarction and has enabled faster clinical intervention, a specific marker for sepsis would be useful, especially when laboratory results and physical exam findings could be interpreted differently by different specialists.
This separation of infection from inflammation is the focus of genomics research in sepsis. With DNA microarrays, it is possible to determine whether a gene is in abundance in tissue, and differential gene expression[24] may be used to see whether that gene expresses itself in the septic state. In murine models, microarray gene-expression profiles[25] can accurately distinguish between the host response to systemic inflammation and sepsis infection (at 24 hours when clinical data could not).
Eventually, genomic techniques may replace blood and tissue cultures as the standard of diagnosis in sepsis patients. Researchers hope to use gene- and protein-expression profiles from patients' blood to distinguish between SIRS and sepsis as well as between the types of infecting organisms. If current research support for molecular diagnostic techniques improves, in 5-8 years, we may see the birth of genomic "vital signs" that would tell us whether the patient is healing -- or developing sepsis.
Here are the following take-home messages:
- Clear definitions allow a better understanding of sepsis and associated problems;
- Identifying sepsis is the key to initiating early and effective therapy;
- The Surviving Sepsis Campaign provides excellent guidelines for treating septic patients;
- Intensive insulin therapy may be the easiest intervention to initiate in any ICU; and
- In the future, a genomic marker may be able to tell us when a patient is developing sepsis before clinical signs appear, allowing more prompt treatment.
Anytime you are making love, you have -- per definition -- SIRS.
Arthur Buoy
References
- Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. Chest. 1992;101:1644-1655. Abstract
- Dellinger RP, Carlet JM, Masur H, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858-873. Abstract
- National Center for Health Statistics. National Vital Statistics Reports. Table E: deaths and percentage of total deaths for the ten leading causes of death, by race: United States, 2001. National Center for Health Statistics. 2003;52:9. Available at: http://www.cdc.gov/nchs/data/dvs/nvsr52_09p9.pdf Accessed January 11, 2005.
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303-1310. Abstract
- Sands KE, Bates DW, Lanken PN, et al. Epidemiology of sepsis syndrome in 8 academic medical centers. Academic Medical Center Consortium Sepsis Project Working Group. JAMA. 1997;278:234-240. Abstract
- Lefering R, Neugebauer EA. Steroid controversy in sepsis and septic shock: a meta-analysis. Crit Care Med. 1995;23:1294-1303. Abstract
- Ziegler E, McCutchan JA, Fierer J, et al. Treatment of gram-negative bacteremia and shock with human antiserum to a mutant Escherichia coli. N Engl J Med. 1982;307:1225-1230. Abstract
- Bernard GR, Wheeler AP, Russell JA, et al. The effects of ibuprofen on the physiology and survival of patients with sepsis. The Ibuprofen in Sepsis Study Group. N Engl J Med. 1997;336:912-918. Abstract
- Wheeler AP, Bernard GR. Treating patients with severe sepsis. N Engl J Med. 1999;340:207-214. Abstract
- Luna CM, Vujacich P, Niederman MS, et al. Impact of BAL data on therapy and outcome of ventilator associated pneumonia. Chest. 1997;111:676-685. Abstract
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368-1377. Abstract
- Holmes CL. Vasopressin in septic shock: does dose matter? Crit Care Med. 2004;32:1423-1424.
- Rozenfeld V, Cheng JW. The role of vasopressin in the treatment of vasodilatation in shock states. Ann Pharmacotherapy. 2000;34:250-254.
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138-150. Abstract
- Marik P, Zaloga GP. Adrenal sufficiency during septic shock. Crit Care Med. 2003;31:141-145. Abstract
- Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288;862-871. Abstract
- Bollaert PE, Charpentier C, Levy B, Debouverie M, Audibert G, Larcan A. Reversal of late septic shock using supraphysiologic doses of hydrocortisone. Crit Care Med. 1998;26:645-650. Abstract
- Bernard GR, Margolis BD, Shanies HM et al; Extended Evaluation of Recombinant Human Activated Protein C United States Investigators. Extended evaluation of recombinant human activated protein C United States Trial (ENHANCE US): a single-arm, phase 3B, multicenter study of drotrecogin alfa (activated) in severe sepsis. Chest. 2004;125:2206-2216. Abstract
- Bernard GR, Vincent JL, Laterre PF, et al; Recombinant human protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study group. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699-709. Abstract
- van de Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359-1367. Abstract
- Christ-Crain M, Jaccard-Stolz D, Bingisser R, et al. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet. 2004;363:600-607. Abstract
- Gibot S, Cravoisy A, Levy B, Bene MC, Faure G, Bollaert PE. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N Engl J Med. 2004;350:451-458. Abstract
- Clarke T. Drug companies snub antibiotics as the pipeline threatens to run dry. Nature. 2003;425:225.
- Southern E, Mir K, Shchepinov M. Molecular interactions on microarrays. Nat Genet. 1999;21(suppl):5-9.
- Cobb JP, Laramie JM, Stormo GD, et al. Sepsis gene expression profiling: murine splenic compared with hepatic responses determined by using complementary DNA microarrays. Crit Care Med. 2002;30:2711-2721. Abstract


