
Early Neonatal Sepsis Screening: Procalcitonin and C-Reactive Protein Diagnostic Value
*Corresponding Author(s):
Joana BorgesDepartment Of Paediatrics, Hospital De Santa Maria, Centro Hospitalar Universitário De Lisboa Norte, Lisbon, Portugal
Tel:+351 217805000,
Email:borges.joana@gmail.com
Abstract
Objective
To evaluate the utility of procalcitonin and C-reactive protein for screening asymptomatic neonates with risk factors for early-onset sepsis.
Study Design
A consecutive cohort of term/late preterm newborns with at least one infectious risk factor was recruited. The primary outcome was to compare the accuracy of procalcitonin (cut-off 5ng/ml) for deciding antibiotic therapy and to compare its predictive value that of C-reactive protein (cut-off 2mg/dl). Authors were blinded to procalcitonin results and clinical decisions were made using C-reactive protein only, per protocol.
Results
Ninety-three neonates were enrolled. Seven patients had a positive septic screen and started antibiotics. All blood cultures were negative. If procalcitonin was used to estimate a positive C-reactive protein value, it would show 57% of sensitivity and 82% of specificity (21% positive predictive value; 96% negative predictive value).
Conclusion
Our results discourage the use of procalcitonin for the diagnosis of early-onset sepsis in newborns with risk factors when using conventional cut-off values.
Keywords
Biomarkers; Neonatal sepsis; Neonatal screening; Newborn
INTRODUCTION
Diagnosis of Early-Onset neonatal sepsis (EOS) remains challenging and sepsis substantially contributes to neonatal morbidity and mortality [1,2]. EOS is usually associated with mother to foetus transmission of microbiological agents during the perinatal period, shortly before or during birth, and occurs, by definition, within the first 72 hours of life [1,3].
The incidence of EOS in term and late preterm infants is low, generally 1-2/1000 live births, but the potential for serious adverse outcomes should lead to a low threshold for evaluation and treatment of possible sepsis in neonates [4,5]. EOS mortality is about 10%, with most deaths occurring within 48 hours from the onset of the infection [6]. Neonates with EOS can present initially with nonspecific and subtle clinical symptoms. Low sensitivity and specificity of current laboratory findings further complicate EOS diagnosis [1,5]. In the absence of clinical signs, most neonatal units do not treat based only on risk factors for EOS, requiring a positive biomarker to start antibiotics. Therefore, screening for EOS should be performed in infants with identifiable risk factors and/or signs and symptoms suggestive of sepsis, but the ideal combination of EOS biomarkers is still to be found.
A wide variety of acute-phase reactants have been evaluated in neonates as potential biomarkers for ruling out neonatal sepsis and to determine antibiotics suspension [4,7]. Although C-Reactive Protein (CRP) and Procalcitonin (PCT) concentrations are the most frequently used and both were investigated in sufficiently large studies, their diagnostic advantage remains controversial [4,7,8].
CRP is considered to be a good biomarker for screening of neonatal sepsis since its concentration increases within 6 to 8 hours of an infectious episode in neonates and peaks at 24 hours [9,10]. The sensitivity of a CRP determination is low at birth but improves dramatically if the first determination is made 6 to 12 hours after birth [11]. Nevertheless, CRP increases are also detected in autoimmunity, post-surgery, perinatal asphyxia or other inflammatory processes and this marker also increases physiologically in newborns within the first days after birth [9,12,13].
PCT is a biomarker with a potential earlier rise in response to infection, often used to differentiate sepsis from systemic inflammation, that can be useful to reduce antibiotic exposure in paediatric patients [14]. In response to infection, PCT concentrations increase within 2 hours and peak values are reached after 12 hours, even though a physiologic increase in PCT concentration has been shown to occur within the first 24 hours after birth [12,15].
Normal values for PCT and CRP concentrations according to hours of life and gestational age, as well as the influence of various prenatal and perinatal variables, were already published [12]. Some perinatal factors associated with changes in acute phase reactants were also described (prolonged rupture of membranes, antenatal steroid administration and intrapartum antimicrobial prophylaxis increase CRP, while only prolonged rupture of membranes increases PCT) [12,16].
CRP is most commonly used in screening for neonatal sepsis, but some studies had shown that PCT can have better sensitivity and specificity [4,17].
The objectives of this study were to evaluate the diagnostic accuracy of PCT in asymptomatic neonates with risk factors for EOS, when compared to CRP.
METHODS
We designed a prospective single-center study conducted at a university hospital (HSM-CHULN) between May and October 2016, aimed to compare the diagnostic usefulness of PCT to CRP in EOS screening. A consecutive cohort of newborns was recruited according to protocol inclusion criteria: term or late preterm newborns with at least one risk factor for EOS. The study protocol was based on local guidelines for EOS screening.
Approval was obtained from the local ethics committee. Written Informed consent was obtained from legal representatives before patient enrollment. The presence of signs or symptoms compatible with EOS, age above 30 hours of life at the time of recruitment or parental refusal of informed consent were exclusion criteria.
Peripheral blood samples for routine laboratory investigations were collected from a peripheral vein, between 18 and 30 hours of life. CRP measurements were obtained by immunoturbidimetric technique (CRPL3, Cobas® reagent) and PCT by an electrochemiluminescence immunoassay - ECLIA (Elecsys BRAHMS PCT Cobas® reagent) using, for both assays, the Cobas 6000 analyzer (Roche®).
The primary outcome of the study was to assess the sensitivity and specificity of PCT as a biomarker to support the decision to initiate antibiotic therapy in infants with risk factors for EOS and to compare its predictive value that of CRP. The cut-off value used was 5 ng/mL for PCT, corresponding to the median value for PCT in our sample plus 1 SD, a value consistent with the physiological elevation that occurs in the first 72 hours of life, and 2mg/dL for CRP, which corresponds to local protocol’s cut-off to start antibiotics in the absence of clinical signs of infection [16,18-20,21]. Secondary outcomes were to evaluate the usefulness of the combined use of CRP and PCT in the decision to initiate antibiotic therapy, to increase the sensitivity and specificity of the diagnosis of EOS in this risk group.
The algorithm used for inclusion, clinical and laboratory evaluations and decision on therapeutic initiation is summarized in figure 1.
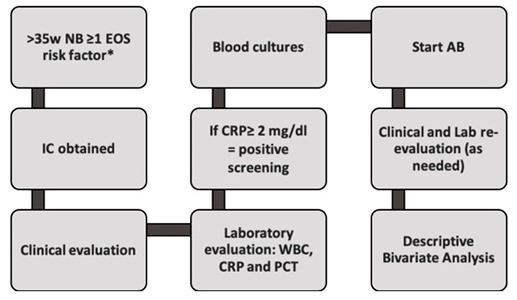 Figure 1: Flowchart of study protocol (AB: Antibiotics; CRP: C-Reactive Protein; EOS: Early Onset Sepsis; IC: Informed Consent; NB: Newborn; PCT: Procalcitonin; WBC: White Blood cell Count).
Figure 1: Flowchart of study protocol (AB: Antibiotics; CRP: C-Reactive Protein; EOS: Early Onset Sepsis; IC: Informed Consent; NB: Newborn; PCT: Procalcitonin; WBC: White Blood cell Count).
*EOS risk factors: Prolonged membrane rupture (≥ 18 hours); Intrapartum fever (≥ 38ºC); Maternal positive septic screening (CRP >2mg/dL or leukocytosis > 20000 cells/mL with neutrophilia); Aminionitis; Positive Group B Streptococcus (GBS) vaginal/rectal smear without adequate prophylaxis (≥ 2 Ampicillin administrations) or without elective C-section; GBS bacteriuria without adequate prophylaxis; Peripartum urinary tract infection without adequate treatment; Previous newborn with GBS infection, without adequate prophylaxis.
Researchers were blind to PCT values during the study to avoid changes in practice related to these results. Nevertheless, PCT values could be unblinded if requested by the clinician in particular circumstances, which was not necessary.
Statistical analysis was performed using SPSS® v23.0. Categorical variables were analyzed using Fisher's exact test. The diagnostic usefulness of PCT compared to CPR was assessed by calculating its sensitivity, specificity, Positive Predictive Value (PPV) and Negative Predictive Value (NPV).
RESULTS
During the study period, 113 patients were screened and 93 neonates (82%) were enrolled. Mean birth weight was 3266g (± 479g) and gestational age was 39,0 weeks (± 1 week).
Most frequently found EOS risk factors were prolonged rupture of membranes (66%), positive Group B Streptococcus (GBS) vaginal or rectal smear culture without adequate prophylaxis (12%), positive maternal septic screen (11%), maternal fever (10%) and preterm membrane rupture (7,5%) (Figure 2). None of the newborns presented with signs or symptoms of clinical sepsis during the study.
Blood samples were obtained at a median age of 24 hours of life. Mean values observed for CRP were 0,64 mg/dL±0,7 (95% CI 0,50-0,79) and 2,85 ng/mL±3,1 (95% CI 2,22-3,49) for PCT. Among the screened newborns, 94% had CRP values below the 2mg/dL cut-off, whereas 80% had PCT values below the 5ng/mL cut-off (Figure 3).
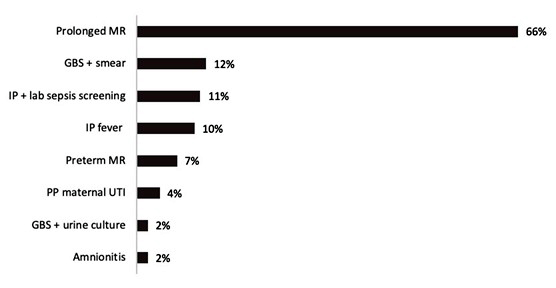
Figure 2: Incidence of early neonatal sepsis risk factors on study population. MR: Membrane rupture; GBS: Group B Streptococcus; IP: Intrapartum; lab: Laboratory; PP: Peripartum; UTI: Urinary tract infection; +: Positive.
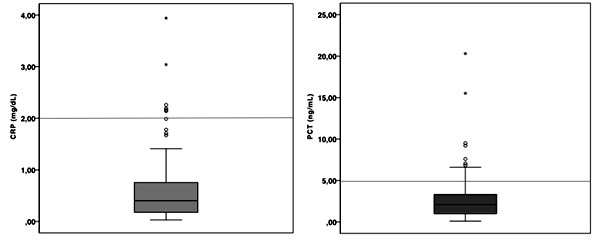
Figure 3: Laboratorial screening values obtained for CRP (left) and PCT (right). Horizontal lines indicate study cut-offs of 2mg/dL for CRP and 5ng/mL for PCT. Legend: CRP: C-reactive protein; PCT: Procalcitonin.
Seven patients (7.5%) had a positive septic screen based on CRP values above 2mg/dL. Those patients were started on antibiotics based in the local protocol, independently of other laboratory tests findings. Blood cultures were negative in all 7 cases and all studied infants were asymptomatic throughout their hospital stay. If a PCT value above 5ng/mL had been used alone as a sepsis risk discriminator instead of CRP, 19 patients (20%) would have been considered with positive screening. Out of these 19 patients, 4 had also a positive CRP and therefore were initiated on antibiotics. As so, the use of PCT alone would have configured a 55% increase in antibiotic exposure. None of those untreated patients according to PCT screening developed symptoms nor was readmitted during the first month of life.
If PCT was used to estimate a CRP value >2 mg/dL, it would show low sensitivity (57%) and moderate specificity (82%), which translates to a very low PPV (21%) and quite high NPV (96%). Thus, PCT and CRP exhibited a different profile when used to screen EOS in those newborns with risk factors, with a statistically significant difference on Fisher´s exact test (p<0.05).
DISCUSSION
Multiple biomarkers have been investigated for early diagnosis of neonatal sepsis, including CRP, PCT, serum amyloid A or hepcidin [5]. CRP is the most commonly use biomarker for bacterial sepsis in neonates and children, but PCT has demonstrated diagnostic and therapeutic utility in a variety of clinical situations. It is a well-recognized and reliable serum marker for the presence or absence of invasive bacterial infection and response to antibiotic therapy in the paediatric population [13,22]. Recent studies also suggest that the use of PCT combined with other inflammatory markers such as IL-6 or IL-8 may represent a reasonable diagnostic approach to EOS [23]. However, the newborns have particularities that are reflected in a wide heterogeneity of reference ranges reported in the literature. There is no evidence to support the isolated use of PCT as a biomarker on those newborns at risk for sepsis.
The rapid increase of PCT levels would configure this marker as the preferred in EOS diagnosis. Since both CRP and PCT concentrations increase physiologically during the first days of life of a healthy newborn and are also dependent on factors such as birth weight or gestational age, their use in EOS diagnostic is less accurate than desired, particularly if used immediately after birth [12]. There are no universally accepted reference values in this population for PCT, which limits its use in clinical practice. Recently, cut-off values for both CRP and PCT were investigated in a large study with healthy term and preterm newborns [12]. The predicted PCT normal ranges for term neonates were 0.01-0.55 ng/mL at birth, rising to peak levels of 0.4-18.7 ng/mL at 24 hours after birth, followed by a slow reduction to 0.04-1.8 ng/mL at about 80 hours of life [12]. Other authors report median values of 1.2 ng/mL and 2.2 ng/mL for late preterm and term infants, respectively [13].
The use of a PCT cut-off of 5ng/mL identified more potentially positive screening cases than CRP. Therefore, if PCT was used alone as sepsis risk discriminator, 19 patients would have been exposed to antibiotics, instead of the 7 patients that were treated based on CRP screening. Four patients presented both simultaneously PCT and CRP above cut-off values. Since our protocol preconizes that only newborns that cumulatively present EOS risk factors and positive CRP (above 2mg/dL) must initiate treatment, 15 patients with a “positive” PCT remained untreated. None of those patients developed sepsis and none was readmitted to the hospital during the first month of life.
According to our results, a 13.6% reduction in exposure to antibiotics would have been observed if the screening protocol based the initial antibiotic decision on a concomitant positive CPR and PCT. Although all patients have negative blood cultures, we cannot be sure that this minimal approach is safe.
A previously meta-analysis demonstrated that PCT has a higher likelihood ratio than CRP to predict neonatal sepsis, but it is far more accurate in Late-Onset neonatal sepsis (LOS) than in EOS [17]. Other recent publications reported higher sensitivity and similar specificity for PCT for EOS when compared to CRP [3,4]. In our study, PCT and CRP also exhibited a significantly different profile when used to screen EOS in those newborns with risk factors (p<0.05).
Our conclusions are limited by the absence of clinical sepsis or positive blood cultures. A much larger multicenter study would be necessary to confirm the diagnostic value of PCT and CRP in EOS, as well as to evaluate the utility (safety and costs) of their combined use. On the other hand, this study led to question the adequacy of our protocol. Based on current scientific evidence a new center’s protocol was adopted with a categorical assessment of risk factors and consequently fewer newborns with an indication for screening. To reduce prolonged and unnecessary exposure to antibiotic therapy this new protocol advised its suspension after a negative preliminary result of blood culture (after 48-72h) in the absence of clinical signs of sepsis. The development of locally adapted guidelines for the assessment of EOS risks is recommended and it is essential to maintain surveillance of its implementation [24].
CONCLUSION
The use of PCT as a determining factor for starting antibiotics would have resulted in a significant increase in the number of newborns exposed to unnecessary treatment. Given the absence of sepsis confirmed by culture or clinical signs, both during the hospitalization and after discharge, our results discourage the use of PCT alone for the screening of EOS in newborns with risk factors, when using the PCT cut-off of 5ng/mL.
CONFLICT OF INTERESTS
The authors have nothing to declare.
FINANCING SOURCES OR AWARDS
None.
ACKNOWLEDGEMENT
The authors would like to thank Dr. Teresa Amaral, from HSM-CHULN Clinical Pathology Service, for the support in the implementation and conduct of laboratory tests.
REFERENCES
- Bhandari V (2014) Effective biomarkers for diagnosis of neonatal sepsis. J Pediatric Infect Dis Soc 3: 234-245.
- Dessì A, Corsello G, Stronati M, Gazzolo D, Caboni P, et al. (2014) New diagnostic possibilities in systemic neonatal infections: Metabolomics. Early Hum Dev 90: 90-92.
- Ahmed AM, Mohammed AT, Bastawy S (2019) Original research 14: 26-32.
- Eschborn S, Weitkamp JH (2019) Procalcitonin versus C-reactive protein: Review of kinetics and performance for diagnosis of neonatal sepsis. J Perinatol 39: 893-903.
- Hedegaard SS, Wisborg K, Hvas AM (2015) Diagnostic utility of biomarkers for neonatal sepsis - a systematic review. Infect Dis (Auckl) 47:117-124.
- Shane AL, Stoll BJ (2013) Recent developments and current issues in the epidemiology, diagnosis, and management of bacterial and fungal neonatal sepsis. Am J Perinatol 30: 131-141.
- Çelik HT, Portakal O, Yi?it ?, Hasçelik G, Korkmaz A, et al. (2016) Efficacy of new leukocyte parameters versus serum C-reactive protein, procalcitonin, and interleukin-6 in the diagnosis of neonatal sepsis. Pediatr Int 58: 119-125.
- Benitz WE (2010) Adjunct laboratory tests in the diagnosis of early-onset neonatal sepsis. Clin Perinatol 37: 421-438.
- Adib M, Bakhshiani Z, Navaei F, Fosoul FS, Fouladi S, et al. (2012) Procalcitonin: A reliable marker for the diagnosis of Neonatal sepsis. Iran J Basic Med Sci 15: 777-782.
- Philip A (1985) Response of C-reactive protein in neonatal Group B streptococcal infection. Pediatr Infect Dis 4: 145-148.
- Benitz WE, Han MY, Madan A, Ramachandra P (1998) Serial Serum C-Reactive Protein Levels in the Diagnosis of Neonatal Infection. Pediatrics 102.
- Chiesa C, Natale F, Pascone R, Osborn JF, Pacifico L, et al. (2011) C reactive protein and procalcitonin: Reference intervals for preterm and term newborns during the early neonatal period. Clin Chim Acta 412: 1053-1059.
- Fukuzumi N, Osawa K, Sato I, Iwatani S, Ishino R, et al. (2016) Age-specific percentile-based reference curve of serum procalcitonin concentration in Japanese preterm infants. Nat Publ Gr 6: 23871.
- Agarwal S, Akbas N, Soundar EP, Gonzalez G, Devaraj S (2015) Validation of the procalcitonin (PCT) assay: Experience in a pediatric hospital. Clin Biochem 48: 886-890.
- Das T, Johnson A (1998) Lack of specificity of procalcitonin for sepsis diagnosis in premature infants. Lancet 351: 1211-1212.
- Chiesa C, Pellegrini G, Panero A, Osborn JF, Signore F, et al. (2003) C-reactive protein, interleukin-6, and procalcitonin in the immediate postnatal period: Influence of illness severity, risk status, antenatal and perinatal complications, and infection. Clin Chem 49: 60-68.
- Vouloumanou EK, Plessa E, Karageorgopoulos DE, Mantadakis E, Falagas ME (2011) Serum procalcitonin as a diagnostic marker for neonatal sepsis: A systematic review and meta-analysis. Intensive Care Med 37: 747-762.
- Chiesa C, Pacifico L, Osborn JF, Bonci E, Hofer N, et al. (2015) Early-onset neonatal sepsis. Medicine (Baltimore) 94: 1230.
- Hofer N, Müller W (2012) An update on the use of C-reactive protein in early-onset neonatal sepsis: Current insights and new tasks. Neonatology 102: 25-36.
- Stocker M, Hop WC, van Rossum AM (2010) Neonatal procalcitonin intervention study (neopins): effect of procalcitonin-guided decision making on duration of antibiotic therapy in suspected neonatal early-onset sepsis: A multi-centre randomized superiority and non-inferiority intervention Study. BMC Pediatr 10: 89.
- Chiesa C, Panero A, Osborn JF, Simonetti AF, PAcifico L (2004) Opinion Diagnosis of Neonatal Sepsis?: A Clinical and Laboratory Challenge. Clin Chem 50: 279-287.
- Pierce R, Bigham MT, Giuliano JS (2014) Use of procalcitonin for the prediction and treatment of acute bacterial infection in children 26: 292-298.
- Pontrelli G, Crescenzo F De, Buzzetti R, Jenkner A, Balduzzi S, et al. (2017) Accuracy of serum procalcitonin for the diagnosis of sepsis in neonates and children with systemic inflammatory syndrome: A meta-analysis. BMC Infect Dis 17: 302.
- Puopolo KM, Benitz WE, Zaoutis T (2018) Management of neonates born at ≤34 6/7 weeks’ gestation with suspected or proven early-onset bacterial sepsis. Pediatrics 142: 20182896.
Citation: Borges J, Ventura A, Costa P, Abrantes M, Graça A (2020) Early Neonatal Sepsis Screening: Procalcitonin and C-Reactive Protein Diagnostic Value. J Neonatol Clin Pediatr 7: 054.
Copyright: © 2020 Joana Borges, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

