-
PDF
- Split View
-
Views
-
Cite
Cite
Syed Ali Zaidi, Carol Singer, Gastrointestinal and Hepatic Manifestations of Tickborne Diseases in the United States, Clinical Infectious Diseases, Volume 34, Issue 9, 1 May 2002, Pages 1206–1212, https://doi.org/10.1086/339871
Close - Share Icon Share
Abstract
Signs and symptoms related to the gastrointestinal tract and liver may provide important clues for the diagnosis of various tickborne diseases prevalent in different geographic areas of the United States. We review clinical and laboratory features that may be helpful in detecting a tickborne infection. Physicians evaluating patients who live in or travel to areas where tickborne diseases are endemic and who present with an acute febrile illness and gastrointestinal manifestations should maintain a high index of suspicion for one of these disease entities, particularly if the patient has received a tick bite. If detected early, many of these potentially serious illnesses can be easily and effectively treated, thereby avoiding serious morbidity and even death.
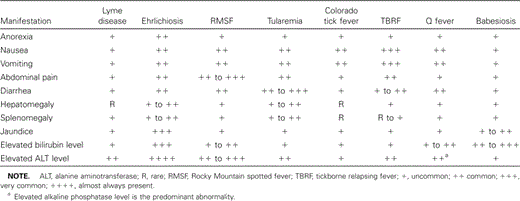
Ticks are the most common agents for vectorborne diseases in the United States. The prevalence of tickborne diseases has been increasing in certain regions as a result of greater outdoor activity and migration of the population into rural areas. Ticks normally feed on rodents; however, continued ecologic disturbance of fields and forests is increasingly exposing humans to infected ticks. A variety of organisms, including viral, protozoal, and bacterial microbes, are transmitted by ticks. We discuss the 8 most common tickborne diseases: Lyme disease, ehrlichiosis, Rocky Mountain spotted fever (RMSF), tularemia, Colorado tick fever, tickborne relapsing fever (TBRF), Q fever, and babesiosis. Each of these diseases presents with a spectrum of constitutional symptoms. Gastrointestinal and hepatic involvement, however, do not uniformly occur. We also present a review of the gastrointestinal and hepatic manifestations of tickborne diseases that are endemic in different regions of the United States (table 1), including a description of the pathogenetic mechanisms and histopathologic lesions involved.
Gastrointestinal and hepatic manifestations of tickborne diseases.
Lyme Disease
Lyme disease is the most common vectorborne infection in the United States. It is caused by the spirochete Borrelia burgdorferi and is transmitted by Ixodid ticks (by Ixodes scapularis in northeastern and midwestern states and by Ixodes pacificus in western states), generally during the months of May through October.
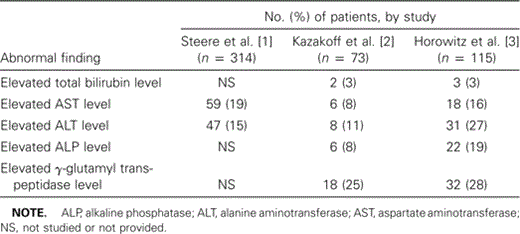
Gastrointestinal manifestations. Gastrointestinal signs and symptoms are common in the early stages of Lyme disease. In a study of 314 patients with early Lyme disease, the predominant clinical findings included anorexia (in 23% of patients), nausea (in 17%), vomiting (in 10%), abdominal pain (in 8%), right upper-quadrant tenderness (in 8%), hepatomegaly (in 5%), splenomegaly (in 6%), and diarrhea (in 2%) [1]. Approximately 10% of the patients had symptoms that were suggestive of hepatitis. Subclinical hepatitis occurred in 27% of patients during the early stages of disease, according to one study [2]. Abnormal liver function test (LFT) findings generally indicate mild hepatocellular injury. Patients with early disseminated Lyme disease are more likely to have abnormal LFT findings than are patients with localized disease [3]. The results of 3 different studies that evaluated abnormal LFT findings in patients with Lyme disease are compared in table 2. However, elevations in aspartate aminotransferase and alanine aminotransferase levels may indicate Lyme disease—associated myositis in some patients and may not be related to underlying hepatic injury.
Comparison of abnormal findings of liver function tests in patients with Lyme disease, as reported in 3 case series.
The prognosis for patients with Lyme disease—associated hepatic dysfunction is excellent with appropriate antimicrobial treatment [3]. LFT elevations usually decrease or resolve within 3 weeks after starting treatment. Hepatitis has not been reported as a late complication of the disease. In 1 case report, liver function abnormalities occurred late in the course of Lyme disease; however, recurrence of Lyme disease could not be distinguished from relapse in that case [4].
Pathogenesis and histopathologic findings. The pathogenesis of liver injury in patients with Lyme disease includes an interplay of direct hepatic invasion by the spirochete and immunologic responses. B. burgdorferi has been shown to penetrate through vascular endothelial cells in vitro [5], and it is this invasive capacity of the organism that is responsible for its tissue colonization in vivo [6]. Cellular and humoral immunologic mechanisms also contribute to the abnormal LFT findings commonly observed in the early stages of Lyme disease. Lyme disease can result in a variety of histologic abnormalities in the liver—in particular, sinusoidal infiltration by a mixed inflammatory infiltrate [4]. Kupffer cell hyperplasia, microvesicular fat, and hepatocyte ballooning are less commonly observed [7]. Spirochetes have been identified within hepatic sinusoids and parenchyma [4, 7]. This is most common in early, disseminated infection. Rarely, B. burgdorferi infection can result in a granulomatous hepatitis, which suggests that Lyme disease should be included in the differential diagnosis of febrile granulomatous hepatitis [8].
Conclusions. The diagnosis of Lyme disease should be considered for any patient from an area of endemicity who presents with a nonspecific illness and abnormal LFT findings, regardless of the presence of a rash. The abnormal LFT findings are usually mild and generally improve or resolve within 3 weeks after the initiation of appropriate therapy [3].
Ehrlichiosis
Ehrlichiae are obligate intracellular rickettsiae that parasitize WBCs. In the United States, human monocytic ehrlichiosis is caused primarily by Ehrlichia chaffeensis and, less often, by Ehrlichia canis. Human granulocytic ehrlichiosis is caused by Ehrlichia phagocytophila and Ehrlichia equi, and Ehrlichia ewingii has been recognized as an agent of human ehrlichiosis [9].
Gastrointestinal manifestations. Gastrointestinal manifestations of ehrlichiosis are frequently nonspecific and include nausea, vomiting, diarrhea, abdominal pain, and, occasionally, jaundice; these manifestations usually occur early in the course of illness, sometimes at the time of presentation. Although gastrointestinal hemorrhage is uncommon, Wallace et al. [10] have described 3 patients with gastrointestinal hemorrhage. Death following gastrointestinal and pulmonary hemorrhage, partly in relation to thrombocytopenia, has been reported among elderly patients. In contrast, younger patients may have mild or subclinical illness [11], although severe abdominal pain was the presenting symptom of a 5-year-old girl with human granulocytic ehrlichiosis [12]. Diarrhea occurs in up to 10% of patients and may be the primary manifestation [13]. Therefore, ehrlichiosis should be included in the differential diagnosis of febrile diarrhea in patients in areas of endemicity, especially in the presence of leukopenia, thrombocytopenia, or elevated aminotransferase levels. Hepatic involvement occurs in µ80% of patients, usually resulting in mild, transient elevations of aminotransferase levels [14]. Occasionally, elevations in transaminase levels may be more marked and cholestasis and liver failure may occur. However, these conditions generally resolve with appropriate therapy [11]. Progressive hepatosplenomegaly has been described in association with ehrlichiosis, with resolution preceding and paralleling recovery with antibiotic therapy [15].
Pathogenesis and histopathologic findings. Liver injury occurs by proliferation of organisms within hepatocytes and by stimulation of immunologic and nonspecific inflammatory mechanisms. The types of histologic lesions in the liver vary from focal hepatic necrosis to ring granulomas and cholestatic hepatitis [14]. Usually, there is evidence of a mixed portal infiltrate and sinusoidal lymphoid cellular infiltrate; foamy Kupffer cells and apoptotic hepatocytes are less frequently seen [14, 16].
Conclusions. Ehrlichiosis should be considered in the differential diagnosis of an acute febrile illness with gastrointestinal symptoms, especially in the presence of cytopenia, hepatosplenomegaly, elevated transaminase levels, and appropriate history of travel or exposure. PCR may be a useful tool for early diagnosis of Ehrlichia species. Progression to multiorgan failure and death can be avoided with early clinical suspicion and timely treatment with doxycycline.
RMSF
RMSF is the most common rickettsial disease in the United States. The causative organism, Rickettsia rickettsii, is transmitted by the dog tick Dermacentor variabilis in the eastern United States, and by the wood tick Dermacentor andersoni in the Rocky Mountain region. The highest incidences of the disease occur in Oklahoma, North Carolina, Virginia, Maryland, Georgia, Missouri, Arkansas, Montana, and South Dakota; however, cases have been reported from every state except Hawaii and Vermont.
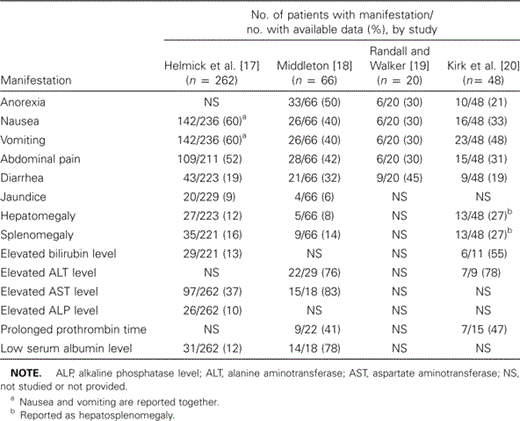
Gastrointestinal manifestations. Gastrointestinal manifestations, as reported from 4 different case series, are listed in table 3. These manifestations, which include nausea, vomiting, diarrhea, and abdominal pain, are present more frequently than rash during the first 2–3 days of illness. The classically described tetrad of fever, headache, rash, and a history of tick bite is rarely encountered at this stage of the illness. Thus, to reduce the mortality rate associated with RMSF, these clinical manifestations must achieve wider recognition [19]. The gastrointestinal abnormalities associated with RMSF may lead to an erroneous diagnosis of an acute abdomen, and they have resulted in surgical intervention in cases in which appendicitis and acute cholangitis were clinically suspected [21–23]. Diarrhea occurs in up to one-third of patients and may be the primary presenting symptom. Guaiac-positive stool samples and vomitus suggest severe underlying intestinal vasculitis [18]. Massive hemorrhage of the upper gastrointestinal tract has occurred in some patients [18]. Hepatic involvement usually results in mild to moderate elevation of aminotransferase levels and occasional jaundice. The latter is a predictor of mortality and is likely the result of a combination of inflammatory bile ductular obstruction and hemolysis [24]. Pancreatic rickettsial infection and vasculitis occur frequently, although the parenchymal injury may not be severe enough to qualify as pancreatitis [19].
Gastrointestinal and hepatic manifestations of Rocky Mountain spotted fever, as reported in 4 case series.
Pathogenesis and histopathologic findings. Although vasculitis with ischemic necrosis is a possible mechanism for gastrointestinal injury, this does not occur frequently in other organs. The most likely mechanism appears to be an effect on the gastrointestinal tract nerves of humoral inflammatory reaction, edema, and ischemia without infarction [25]. The main pathologic lesion of RMSF is vasculitis in the stomach, small and large intestines, and pancreas [19, 26]. Rickettsiae may infect the endothelial lining, the liver sinusoids, and the portal vasculature, but not hepatocytes, leading to a mild focal hepatitis and periportal inflammation. In cases of fulminant RMSF, actively growing rickettsiae can result in vast destruction of hepatic vasculature, with or without significant vasculitis [27].
Conclusions. The diagnosis of RMSF is often delayed, usually because of failure to consider diagnosis at the initial presentation. Mortality rates may be decreased by the administration of empiric treatment within the first 5 days of the illness [28].
RMSF must be included in the differential diagnosis for febrile patients who live in or have traveled to an area of endemicity and who present with headache, nausea, vomiting, diarrhea, or abdominal pain, particularly in spring or summer. Receipt of a tick bite may not be recalled by the patient, and a rash may not yet have appeared. Awareness of the possibility of hepatic involvement in RMSF is essential. In febrile patients for whom an exploratory laparotomy does not demonstrate the cause of an acute abdomen, it may be worthwhile to search for a rickettsial vasculitis. The finding of mononuclear-predominant vasculitis, with or without thrombosis, should lead to consideration of a diagnosis of RMSF [24].
Tularemia
Tularemia is caused by a gram-negative coccobacillus, Francisella tularensis, which is transmitted by the tick Amblyomma americanum in the southeastern and south-central United States and by D. andersoni and D. variabilis in the western United States. The disease has a bimodal distribution: a peak incidence in summer reflects tick-associated disease, and a peak incidence in winter is associated with animal contact, especially rabbits [29]. F. tularensis infection has been reported in all 50 states, but the majority of cases occur in Missouri, Arkansas, Tennessee, Oklahoma, Kansas, and Utah.
Gastrointestinal manifestations. Tularemia acquired by a tick bite presents in either the ulceroglandular or typhoidal form. Gastrointestinal manifestations are most prominent in the latter presentation and include anorexia, nausea, vomiting, diarrhea, and abdominal pain with tenderness. Hepatosplenomegaly is uncommon in the acute stage but becomes more common as the disease progresses. Diarrhea can be severe, although it is only rarely bloody, and it is occasionally accompanied by intestinal necrosis in severe cases [30]. Hepatic involvement occurs in up to 75% of patients [31]. The most frequently observed abnormal LFT findings are mild to moderate elevations in aminotransferase levels. Jaundice, marked cholestasis, and, occasionally, granulomatous hepatitis are seen in more-severe cases [32–34]. Ascites [32], cholangitis [35], and liver abscess [36] have been reported infrequently.
Histopathologic findings. The most frequently observed hepatic pathologic lesion is coagulative necrosis with sinusoidal dilatation and a mixed inflammatory infiltrate [30, 37, 38]. The lesions are considered in the spectrum of granulomatous hepatitis, although epithelioid and giant cell granulomas are rare [34]. Organisms are uncommonly noted by use of Gram staining [33].
Conclusions. Tularemia is a rare cause of cholestatic hepatitis. It should be considered in patients from an area of endemicity who present with cholestasis of an unclear etiology and who have an appropriate exposure history. Dissemination to the liver may occur more frequently than has been recognized.
Colorado Tick Fever
Colorado tick fever is an acute febrile illness caused by a virus of the Coltivirus genus and transmitted by the tick D. andersoni. The geographic distribution of the virus is similar to that of its tick vector, including the United States and Canadian Rocky Mountain, Wasatach, and Sierra Nevada ranges. Most infections result from occupational or recreational exposure to infected ticks in these areas.
Gastrointestinal manifestations. Gastrointestinal manifestations of Colorado tick fever are not prominent and mainly comprise nonspecific complaints of nausea, vomiting, and abdominal pain. In a review of 228 patients who had Colorado tick fever diagnosed, abdominal pain and vomiting were the most common gastrointestinal symptoms, occurring in 20% of the cases [39]. Hepatitis may occur as an unusual complication of late-stage disease [40]. The occurrence of hepatitis may be a primarily immunologic process secondary to immune complex deposition. However, an infectious mechanism cannot be ruled out entirely.
Conclusions. Colorado tick fever is not commonly associated with overt gastrointestinal manifestations. However, hepatitis may occur as an unusual manifestation of the disease. The illness has a benign course, and, with supportive treatment, prognosis is good for a complete recovery.
TBRF
TBRF is caused by spirochetes of the genus Borrelia (Borrelia turicatae, Borrelia hermsii, and Borrelia parkeri in North America) transmitted by Ornithodoros ticks. It occurs throughout the world, except for a few areas in the southwest Pacific islands, with endemic foci found in the western United States, southern British Columbia, and Central and South America.
Gastrointestinal manifestations. In a review of 133 confirmed cases of TBRF, nausea and vomiting were the most prominent gastrointestinal complaints, occurring in 77.6% and 73% of patients, respectively. Other features were less common, including abdominal pain (in 46.3% of patients), diarrhea (in 19.6%), jaundice (in 10.3%), hepatomegaly (in 7.5%), and splenomegaly (in 4.7%) [41]. Thrombocytopenia and hemorrhagic complications, including hematemesis and bloody diarrhea, are common, although they are rarely severe; they are more frequently associated with louseborne relapsing fever than with TBRF [41]. Borreliae multiply in the liver, invading hepatocytes and causing focal necrosis. In severe cases of TBRF, tender hepatosplenomegaly and jaundice may develop. Hepatic failure is a common cause of death for patients with severe cases, and autopsy findings have revealed hepatitis, hepatic necrosis, and hemorrhagic gastrointestinal lesions in many patients [42]. The disease may be more severe in pregnant woman and may be a common cause of miscarriages [42].
Conclusions. TBRF is an underrecognized and underreported disease. Because of the cross-reactivity of serologic assays, TBRF may be falsely identified as Lyme disease [43]. The current diagnostic standard for TBRF is detection of spirochetes in peripheral blood smears. Dworkin et al. [41] indicated that there are 3 factors that may be responsible for poor detection: inexperience of the microscopist in the detection of spirochetemia, increased use of automated differential counts, and examination of blood samples during the interval of asymptomatic disease between bouts of febrile disease. The authors recommended that a manual differential count be performed for patients with an appropriate exposure history who have the combination of flulike symptoms, thrombocytopenia, and gastrointestinal symptoms, including hepatitis.
Q Fever
Unlike other tickborne diseases, Q fever is not transmitted to humans directly by tick bites. The causative organism, Coxiella burnetti, is maintained in ticks (D. andersoni) or other arthropods and is transmitted by them to domestic animals, most commonly cattle, sheep, and goats. Human infection occurs after inhalation of contaminated aerosols, which can lead to pneumonia, or after ingestion of raw milk or fresh goat cheese, which can lead to hepatitis [44].
Gastrointestinal manifestations. The most prominent gastrointestinal manifestation of Q fever is hepatitis, although liver damage may be subclinical in many cases. Three presentations of Q fever hepatitis have been described: an infectious hepatitis-like presentation; fever of unknown origin with characteristic granulomas, which are found by examination of liver biopsy specimens; and the incidental finding in a patient with acute Q fever pneumonia [45]. Receipt of appropriate antibiotic therapy for 2 weeks usually results in gradual resolution of symptoms and abnormal LFT findings, although chronic Q fever may necessitate a longer duration of treatment.
Histopathologic findings. The usual histologic presentation is focal hepatocellular necrosis. Widespread necrosis and granulomas may be seen in severe cases [46, 47]. The most common type of granuloma, the “doughnut granuloma,” consists of a fibrinoid ring with a clear lipid center. Although strongly suggestive of Q fever, it is not specific and may be seen in other diseases, such as Hodgkin's lymphoma and infectious mononucleosis [48].
Conclusions. Q fever must be included in the differential diagnosis of granulomatous hepatitis. The presence of fibrinoid material, either in a ringlike configuration or in broken rod-shaped strands, is highly suggestive of Q fever [47].
Babesiosis
In the United States, the most common causative organism for babesiosis is the parasite Babesia microti. Most cases occur in the northeastern United States, but cases have been reported in Virginia, Maryland, Wisconsin, Minnesota, and Missouri [49]. A newly emerging babesia-like piroplasm may be responsible for cases of this disease in the western United States [50].
Gastrointestinal manifestations. The initial gastrointestinal manifestations are nonspecific complaints of nausea, vomiting, anorexia, and abdominal pain. Hepatosplenomegaly may be present, although it is less commonly seen in patients with babesiosis than it is in patients with ehrlichiosis. Mild LFT elevations are usually observed, although they may be normal in subclinical infection [50]. The degree of indirect hyperbilirubinemia is dependent on the extent of hemolysis. Initiation of appropriate antimicrobial therapy, along with exchange transfusion for patients with severe hemolysis, usually results in resolution of the abnormal LFT findings. Bovine babesiosis, caused by Babesia divergens and Babesia bovis, has been frequently reported in Europe and occurs in asplenic patients. It is a fulminant infection associated with multiorgan and hepatic failure.
Conclusions. Mild LFT elevations are common in babesiosis. Patients without spleens tend to have a more-severe clinical course. Bovine babesiosis has a high mortality rate and may be associated with hepatic failure.
Summary
In patients who live in or travel to areas where tickborne diseases are endemic, the index of suspicion for one of these entities should be high. Early diagnosis and timely institution of treatment may avoid progression to severe disease, thereby decreasing rates of morbidity and mortality. Signs and symptoms related to the gastrointestinal tract may provide useful clinical clues for early diagnosis. Abdominal complaints are more common among patients with RMSF and may occasionally lead to an erroneous diagnosis of an acute abdomen and result in unnecessary surgery. Diarrhea is most frequently seen in patients with ehrlichiosis, RMSF, tularemia, and TBRF. Almost all of these infections involve the liver with varying degrees of severity and should be included in the differential diagnosis of acute hepatitis in areas of endemicity. The exceptions are Colorado tick fever and babesiosis, which do not commonly lead to hepatocellular injury. Q fever, in particular, and Lyme disease, ehrlichiosis, and tularemia, to a lesser extent, should be kept in mind when considering a diagnosis of granulomatous hepatitis in patients with an appropriate history of travel or exposure. Cholestasis and jaundice are most pronounced in patients with ehrlichiosis. They are uncommon in patients with Lyme disease, tularemia, Colorado tick fever, and TBRF, and they are seen variably in the rest. Finally, Lyme disease, ehrlichiosis, and babesiosis are transmitted by a common tick vector (Ixodes species ticks). Coinfection with any combination of the 3 pathogens is possible. Diagnosis of one of these diseases should prompt an evaluation for the presence of coexistent tickborne infections.





Comments