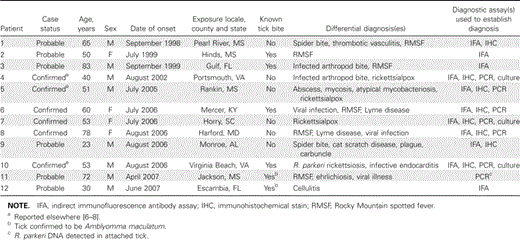
-
PDF
- Split View
-
Views
-
Cite
Cite
Christopher D. Paddock, Richard W. Finley, Cynthia S. Wright, Howard N. Robinson, Barbara J. Schrodt, Carole C. Lane, Okechukwu Ekenna, Mitchell A. Blass, Cynthia L. Tamminga, Christopher A. Ohl, Susan L. F. McLellan, Jerome Goddard, Robert C. Holman, John J. Openshaw, John W. Sumner, Sherif R. Zaki, Marina E. Eremeeva, Rickettsia parkeri Rickettsiosis and Its Clinical Distinction from Rocky Mountain Spotted Fever, Clinical Infectious Diseases, Volume 47, Issue 9, 1 November 2008, Pages 1188–1196, https://doi.org/10.1086/592254
Close - Share Icon Share
Abstract
Background. Rickettsia parkeri rickettsiosis, a recently identified spotted fever transmitted by the Gulf Coast tick (Amblyomma maculatum), was first described in 2004. We summarize the clinical and epidemiological features of 12 patients in the United States with confirmed or probable disease attributable to R. parkeri and comment on distinctions between R. parkeri rickettsiosis and other United States rickettsioses.
Methods. Clinical specimens from patients in the United States who reside within the range of A. maculatum for whom an eschar or vesicular rash was described were evaluated by ⩾1 laboratory assays at the Centers for Disease Control and Prevention (Atlanta, GA) to identify probable or confirmed infection with R. parkeri.
Results. During 1998–2007, clinical samples from 12 patients with illnesses epidemiologically and clinically compatible with R. parkeri rickettsiosis were submitted for diagnostic evaluation. Using indirect immunofluores-cence antibody assays, immunohistochemistry, polymerase chain reaction assays, and cell culture isolation, we identified 6 confirmed and 6 probable cases of infection with R. parkeri. The aggregate clinical characteristics of these patients revealed a disease similar to but less severe than classically described Rocky Mountain spotted fever.
Conclusions. Closer attention to the distinct clinical features of the various spotted fever syndromes that exist in the United States and other countries of the Western hemisphere, coupled with more frequent use of specific confirmatory assays, may unveil several unique diseases that have been identified collectively as Rocky Mountain spotted fever during the past century. Accurate assessments of these distinct infections will ultimately provide a more valid description of the currently recognized distribution, incidence, and case-fatality rate of Rocky Mountain spotted fever.
An unnamed, apparently new rickettsia has been repeatedly isolated at the Rocky Mountain Laboratory during the past nine years from specimens of Amblyomma maculatum, a tick of wide distribution in the Southern States…the presumptive evidence from animal experimentation suggests that human infection might be confusingly similar to spotted fever. Parker Ralph R. 1948 [1, p. 146]
In 1937, Parker et al. [2] identified a unique spotted fever rickettsia borne by the Gulf Coast tick (A. maculatum) (figure 1A); this rickettsia was eventually named Rickettsia parkeri in Parker's honor. When these investigators infected guinea pigs with R. parkeri, they observed a febrile illness in the animals that was similar to but milder than Rocky Mountain spotted fever (RMSF) [5], which led Parker and others to speculate that R. parkeri might also cause an RMSF-like disease in humans. In 2004, the first confirmed human infection with R. parkeri was described [6], and infections with this rickettsia were subsequently identified in other patients from Mississippi [7], Virginia [8], and, possibly, other states [9].
A, Life stages of the Gulf Coast tick (Amblyomma maculatum). From left: larva, nymph, adult male, and adult female. Each stage can feed on human hosts and can be infected with Rickettsia parkeri. The head of a pin is included for scale. B, Classic range (dark blue) of A. maculatum in the United States, based on historical and contemporary records [3, 4]; a hypothetical range (pale blue), based on published incidental tick collection data (Pete Teel, Texas A&M University, personal communication); and the locations of confirmed (shaded circles) and probable (unshaded circles) cases of R. parkeri rickettsiosis discussed in this report.
The US distribution of A. maculatum extends across all states that border the Gulf of Mexico, as well as several other southern, mid-Atlantic, and central states, including Georgia, Kansas, Kentucky, North Carolina, Oklahoma, South Carolina, Tennessee, and Virginia (figure 1B) [3]. R. parkeri has been detected in or isolated from A. maculatum collected in many of these states, indicating that this pathogenic rickettsia is endemic throughout a relatively large expanse of the United States [2, 4, 5]. Here, we summarize the clinical and epidemiological characteristics of patients with confirmed or probable R. parkeri rickettsiosis, comment on the clinical differences between this disease and RMSF, and discuss the epidemiological importance of these distinctions.
Patients and Methods
Patients. Serum, whole blood, and skin biopsy specimens are routinely submitted to the Centers for Disease Control and Prevention (CDC) by clinicians and state health departments for specialized diagnostic tests when a spotted fever rickettsiosis is suspected in patients with compatible clinical signs and epidemiologic features. For patients residing within the US range of A. maculatum endemicity for whom an eschar or vesicular rash was reported to the CDC during 1998–2007, specimens were evaluated by ⩾1 laboratory methods. Accompanying epidemiological and clinical data, of varying completeness, were provided by the submitting physician or public health worker. A confirmed case of R. parkeri rickettsiosis was defined by isolation of R. parkeri in cell culture or PCR amplification of R. parkeri DNA from a clinical specimen. A probable case was defined as a clinically and epidemiologically compatible illness, with ⩾1 supportive serological or immunohistochemical test result, through use of group-specific assays for spotted fever group rickettsiae (SFGR).
Indirect immunofluorescence assays. Antigens of 4 species of SFGR (R. parkeri Hmac, Rickettsia rickettsii Sheila Smith, Rickettsia akari Hartford, and Rickettsia amblyommii GAT16) were selected on the basis of known or suggested associations with human disease in the United States. Rickettsiae were cultivated in Vero E6 cells and were purified using centrifugation through 25%–45% Renografin (Nycomed) gradients. The nuclear fraction was suspended in K-36 buffer, was irradiated, was spotted on glass slides, and was fixed in acetone. Fluorescein isothiocyanate–labeled, goat anti-human IgG (heavy chain– and light chain–specific) or anti-human IgM antibodies (Kirkegaard & Perry) were used at dilutions of 1:200 and 1:100, respectively. IgG was absorbed from serum samples before evaluation for IgM with use of a Mini Rapi-Sep-M kit (Panbio Diagnostics). Samples were screened at consecutive 2-fold serum dilutions, starting at 1:32, and titers were represented as the reciprocal of the last dilution exhibiting reactivity with the specific antigen. IgG and IgM titers ⩾64 were considered to be positive for data analysis.
Histology and immunohistochemistry. Three-micrometer sections were cut from formalin-fixed, paraffin-embedded biopsy specimens of skin and were stained with hematoxylin-eosin and with use of an immunoalkaline phosphatase technique with a polyclonal anti–R. rickettsii antiserum diluted at 1:500. In formalin-fixed tissues, this antiserum reacts with antigens of all known pathogenic SFGR that are indigenous to the United States, including R. rickettsii, R. parkeri, and R. akari [6, 10, 11].
Cell culture. Vero E6 cells were inoculated with triturated skin biopsy specimens and were incubated at 34.5°C in a 5% CO2-enriched atmosphere [6, 10]. Cultures were screened for infection with rickettsiae with use of acridine orange stain, and results were confirmed using molecular assays.
Molecular analyses. DNA was extracted, using a QIAamp DNA Mini Kit (Qiagen), from fresh biopsy samples of skin tissue; formalin-fixed, paraffin-embedded tissue sections; cell culture suspensions; and an A. maculatum specimen removed from a patient [4, 10]. Segments of the rickettsial 17-kilodalton antigen gene or the rickettsial outer membrane protein A gene (ompA) were amplified from these extracts with use of nested or seminested PCR assays [4, 12]. Purified amplicons were sequenced using an ABI PRISM 3.0 BigDye Terminator Cycle Sequencing kit (Applied Biosystems) and a 3100 Nucleic Acid Sequence Analyzer (Applied Biosystems) or using a GenomeLab Dye Terminator Cycle Sequencing Quick Start Kit (Beckman Coulter) and a Beckman CEQ 8000 automated sequencer (Beckman Coulter). Nucleotide homologies were constructed using the National Center for Biotechnology Information BLAST 2.0 program.
Case report form evaluations. RMSF case report forms (CRFs) submitted to the CDC were evaluated for the laboratory methods used to determine confirmed cases of RMSF for the period 1981–2005, as defined by the Council of State and Territorial Epidemiologists' case definition for this disease [13]. These laboratory criteria included: (1) a ⩾4-fold change in antibody titer reactive with R. rickettsii, (2) isolation of R. rickettsii in cell culture, (3) PCR amplification of DNA of R. rickettsii, or (4) immunostaining of R. rickettsii in a clinical specimen.
Data analysis. The qualitative reactivity (i.e., a positive or negative titer result) of each patient's serum sample with R. parkeri antigen was compared with his or her serum reactivity to R. rickettsii, R. akari, or R. amblyommii antigens by using McNemar's test and the κ coefficient (SAS, documentation 9.13, 2004; SAS Institute). For patients with >1 serum specimen, the first sample that showed a titer ⩾64 to any SFGR antigen was used for these comparisons. A P value ×.05 was considered to be statistically significant.
Results
Patient characteristics. Six confirmed and 6 probable cases of R. parkeri rickettsiosis were identified in patients from Alabama, Florida, Kentucky, Maryland, Mississippi, South Carolina, and Virginia during 1998–2007 (figure 1 and table 1). Patients (8 men and 4 women) had an age range of 23–83 years (median age, 53 years). Ten patients (83%) became ill between late July and early September. Seven patients reported a tick bite, and eschars were identified definitively at bite sites for 6 patients. For 2 patients, ticks bites were identified definitively as caused by A. maculatum. The first recognized symptoms occurred 2–10 days (median duration, 5 days) after the tick bite and included a “sore”; or “pimple”; at the tick bite site (6 patients), fever (3), fatigue with myalgia or headache (2), and generalized rash (1). RMSF or rickettsialpox were included in the working diagnoses for 9 patients (75%).
Selected characteristics of Rickettsia parkeri rickettsiosis among 12 patients in the United States, 1998–2007.
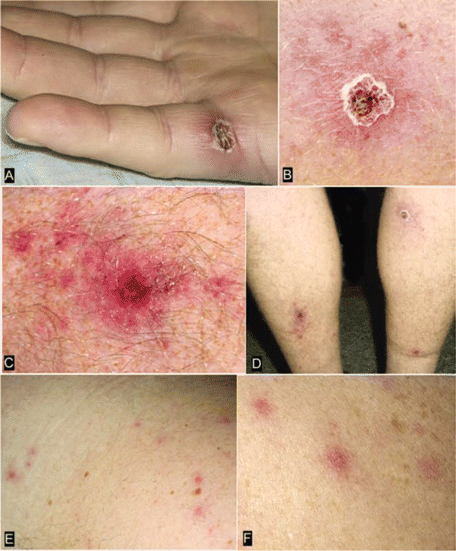
All patients described low to moderate fever (median temperature, 39.2°C; range, 37.7°C–40.3°C) that persisted for 2–11 days (median duration, 7 days), and only 2 (17%) reported temperatures ⩾40°C (table 2). Eschars were identified in 11 patients, which appeared as crusted, nonpruritic, and nontender or mildly tender ulcers, 0.5–2 cm in greatest dimension, surrounded by an indurated, erythematous halo and, occasionally, by a few scattered petechiae (figure 2A–2D). These lesions were identified on the lower leg, shoulder, upper arm, groin, flank, toe, neck, or hand; 2 patients had multiple eschars. The recognition of the eschar preceded onset of fever by 0–4 days. A maculopapular or vesiculopapular exanthem, involving primarily the trunk and extremities, was identified on 10 patients within 0.5–4 days after the onset of fever (figure 2E and 2F). These lesions were pink, nonpruritic macules and papules, 0.2–0.5 cm in greatest dimension, and some contained a small central vesicle or pustule. The estimated number of these lesions had a range between ∼15 and several hundred. The exanthem involved the palms or soles of 5 patients and the face of 2 patients. Almost all patients reported myalgia and headache, most often described as mild. Nine patients (75%) described arthralgia. Tender lymphadenopathy in proximity to the eschar was documented for 3 patients. Only 1 patient reported nausea and vomiting; none reported diarrhea. Photophobia and neck stiffness were described by 2 patients, but no severe neurological manifestations were identified on physical examination.
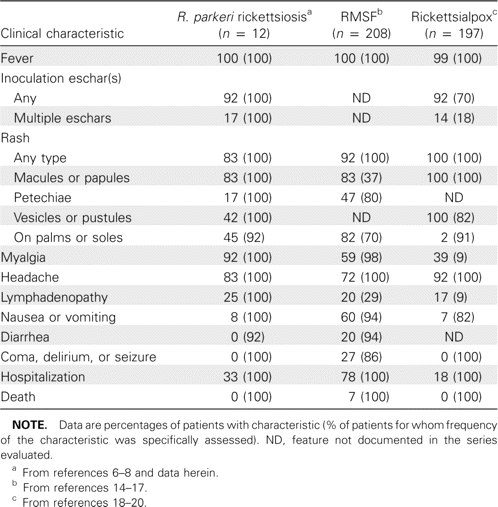
Comparison of selected clinical features of Rickettsia parkeri rickettsiosis with those of Rocky Mountain spotted fever (RMSF) and rickettsialpox, as reported in well-characterized case series.
Cutaneous lesions from patients with confirmed Rickettsia parkeri rickettsiosis. Inoculation eschars, representing the site of primary infection following a bite from an R. parkeri–infected tick, are present on the lateral aspect of the palm of patient 5 (A) and on the lower extremities of patient 4 (B–D). These lesions are 0.5–1.5 cm wide, with a central area of ulcerated or scabbed skin surrounded by a halo of erythema (A and B) or petechiae (C). Panel D shows multiple eschars on patient 4. The rash of R. parkeri rickettsiosis, as seen on patients 4 (E) and 5 (F), is a maculopapular or papulovesicular eruption on the trunk and extremities, occasionally involving the palms and soles.
Modest elevations of aspartate aminotransferase (median level, 56 U/L; range, 38–92 U/L) and alanine aminotransferase (median level, 78 U/L; range, 41–195 U/L) were observed in 7 of 9 and 4 of 8 patients, respectively. Mild thrombocytopenia (median platelet count, 145×106 platelets/L; range, 122–149×106 platelets/L) and leukopenia (median WBC count, 3.4×106 cells/L; range, 3.2–4.4×106 cells/L) were identified in 4 and 5, respectively, of 10 patients. Two patients with confirmed infection and 2 with probable infection were hospitalized for a median of 4 days (range, 2–9 days). Eleven patients received doxycycline and defervesced within 12–48 h after receipt of this antibiotic; 8 patients received 1–3 other antibiotics (i.e., penicillins, cephalosporins, or sulfa-containing antimicrobials) before receipt of doxycycline, and 1 patient recovered without receipt of a tetracycline-class drug.
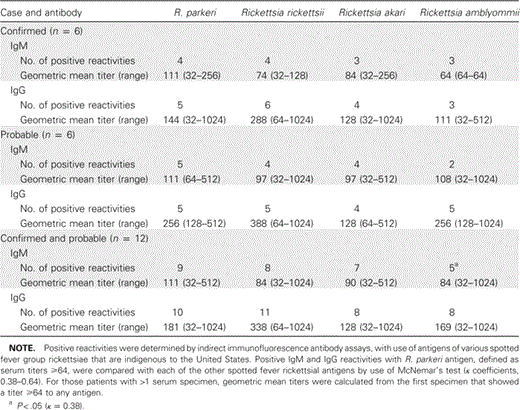
Indirect immunofluorescence assays. Serum specimens collected from patients with confirmed and probable infection demonstrated broad IgM and IgG reactivity with all antigens, although the highest geometric titers were obtained using R. rickettsii as the antigen (table 3). Antibody titers ⩾64 to each of the antigens were identified in serum samples from 4 patients with confirmed infection, including 3 samples with cross-reacting IgM and 4 with cross-reacting IgG; all confirmed cases had IgG reactive with R. parkeri and R. rickettsii. Titers ⩾64 to each of the antigens, including 3 with cross-reacting IgM and 4 with cross-reacting IgG, were identified in ⩾1 serum samples from the patients with probable infection; 5 patients with probable infection had IgG that was reactive with R. parkeri and R. rickettsii. Four patients with confirmed R. parkeri infection had higher IgG titers with heterologous R. rickettsii antigens in ⩾1 serum sample; however, qualitative analysis of each patient's IgG reactivities with R. parkeri showed no statistically significant differences, when compared with the reactivities with any of the other 3 antigens (table 3). R. amblyommii was the only antigen that generated a significantly different IgM result from reaction with R. parkeri (P=.046).
Qualitative assessment of IgM and IgG antibody reactivity and geometric mean titers of confirmed and probable cases of Rickettsia parkeri rickettsiosis.
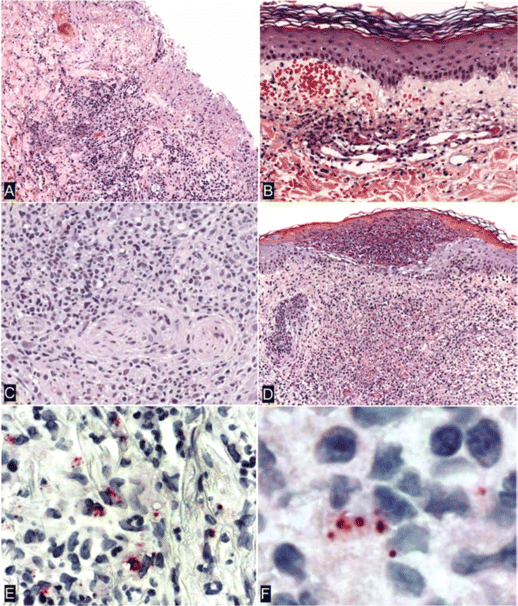
Histology and immunohistochemistry. SFGR were identified in all eschar and rash specimens obtained for biopsy that were evaluated with use of immunohistochemical stain (table 1). Eschar samples obtained for biopsy showed extensive necrosis of the epidermis and superficial dermis (figure 3A) and prominent vasculitis of small- to medium-sized dermal vessels. Partially occlusive fibrin thrombi associated with localized hemorrhage were evident in some inflamed vessels (figure 3B). The mural and perivascular infiltrates comprised predominantly lymphocytes, macrophages, and occasional neutrophils (figure 3C), and similar inflammatory infiltrates often involved sweat glands, small nerves, and subcutaneous fat. Evaluation of a papular rash lesion from 1 patient showed an intraepidermal pustule and a diffuse mixed inflammatory cell infiltrate of the superficial to middermis comprising neutrophils and mononuclear cells, associated with karyorrhectic and necrotic debris and edema (figure 3D). SFGR were found predominantly in the cytoplasm of mononuclear cells (figures 3E and 3F). In most eschars, rickettsiae were sparse; however, relatively abundant SFGR were identified by immunohistochemical stain in the single rash lesion that was available for testing.
Histopathological and immunohistochemical characteristics of eschars (A–C and F) and rash lesions (D and E) of patients with Rickettsia parkeri rickettsiosis. A, Denuded epidermis with dermal necrosis and mixed inflammatory cell infiltrates (patient 7). B, Lymphocytic vasculitis in a small vessel in the superficial dermis associated with partially occlusive fibrin thrombi and extravasated erythrocytes (patient 1). C, Dense perivascular lymphohistiocytic infiltrates in the deep dermis (patient 5). D, Intraepidermal pustule and extensive neutrophilic and mononuclear inflammatory cell infiltrates associated with necrosis in the middermis. E, Immunohistochemical localization of intact R. parkeri and rickettsial antigens in the cytoplasm of mononuclear cells (patient 6). F, Coccobacillary forms of R. parkeri in mononuclear inflammatory cells in an eschar (patient 7). Hematoxylin and eosin stain (A–D) and alkaline phosphatase with polyclonal anti–R. rickettsii antibody (1:500) and naphthol-fast red, with hematoxylin counterstain (E and F). Original magnifications, ×25 (A and D), ×50 (B), ×100 (C and E), and ×158 (F).
Cell culture and molecular analyses. Three isolates of R. parkeri (Portsmouth, Horry-SC2006, and Ft. Story strains) were obtained in Vero E6 cells and were confirmed by PCR and sequencing of 208–base pair segments of the 17-kilodalton antigen gene and 532–base pair segments of ompA. Homologous segments of ompA were also amplified from biopsy specimens of fresh skin from 4 patients, formalin-fixed skin samples from 2 patients, and from an A. maculatum removed from 1 patient (table 1). All samples had 100% sequence identity with the corresponding ompA segment of R. parkeri (GenBank accession number U17008).
CRF evaluations. A total of 5735 laboratory-confirmed cases of RMSF were submitted to the CDC through CRFs for the 25-year interval that we evaluated; these represented ∼50% of the total number of RMSF CRFs submitted to the CDC for this period. Only 305 (5.3%) of the total “confirmed”; RMSF CRFs were actually confirmed by ⩾1 laboratory assay (i.e., culture isolation or PCR) with sufficient specificity to identify the infecting agent as R. rickettsii.
Discussion
During 1998–2007, we identified 6 confirmed and 6 probable cases of R. parkeri rickettsiosis by passive surveillance of specimen submission to the CDC for diagnostic assays. Cases occurred across a wide geographic region that approximated the US distribution of A. maculatum. The probable cases were identified by assays specific only to the level of SFGR; however, other supportive data strongly suggest that each of the patients with probable cases was infected with R. parkeri. All had a relatively mild, eschar-associated rickettsiosis that occurred within the expected range of A. maculatum (e.g., coastal Mississippi, Alabama, and Florida), 2 sustained well-documented bites by A. maculatum (including 1 from which DNA of R. parkeri was subsequently detected by PCR), and 1 had a cutaneous lesion that was histopathologically and immunohistochemically identical to those observed with the confirmed cases.
When the clinical features that we identified for R. parkeri rickettsiosis were compared with those obtained from well-supported and classic descriptions of other historically recognized US spotted fevers (i.e., RMSF and rickettsialpox), many common characteristics were identified (table 2). R. parkeri rickettsiosis and RMSF are each characterized by fever, myalgia, malaise, headache, and a maculopapular eruption that may involve the palms or soles, and both diseases occur ∼1 week after a tick bite [21]. Indeed, 50% of the patients described in this report received a diagnosis of RMSF at some point in their illnesses; however, R. parkeri rickettsiosis appears to be a milder illness than classically described RMSF. Notably, only 33% of the patients in this series were hospitalized, only 2 had a peak temperature >40°C, none had a temperature ⩾40.5°C, none reported severe neurological disease or organ system failure, and there were no deaths. By comparison, 75% of patients with classic RMSF are hospitalized; 75% have peak temperatures >40°C (almost one-half have peak temperatures ⩾40.5°C) [22]; ∼25% develop stupor, delirium, ataxia, or coma; and the aggregate case-fatality rate derived from several contemporary and well-characterized US case series is ∼7% (table 2) [14–17].
The natural history of well-characterized cases of untreated RMSF follows a progression of disease severity that most often culminates in hospitalization or death [1, 11, 14–17, 21–25]. By comparison, untreated patients with R. parkeri rickettsiosis remained only moderately ill after as many as 7–10 days of fever. Other features that distinguish R. parkeri rickettsiosis from RMSF include the occurrence of an eschar (identified in >90% of the patients in this series), a vesicular or pustular rash (observed in ∼40% of the patients in this series), and the relative absence of nausea, vomiting, and diarrhea. By comparison, patients with classic RMSF rarely have eschars; there is seldom, if ever, documented vesicular or pustular exanthems; and gastrointestinal manifestations occur in >60% of patients [1, 14–17, 21–27].
R. parkeri rickettsiosis also resembles rickettsialpox, an eschar-associated disease caused by R. akari; and a diagnosis of rickettsialpox was considered for 25% of the patients in this series. However, most US cases of rickettsialpox have been described from large metropolitan centers in the northeastern states and are associated with the bite of the house mouse mite, Liponyssoides sanguineus [28]. Indeed, a recently reported case of rickettsialpox from rural North Carolina occurred within the recognized range of A. maculatum and showed many clinical similarities with R. parkeri rickettsiosis [29]. The diagnostic dilemma posed by these clinically similar infections is compounded by the fact that antibodies generated to antigens of R. parkeri can react with R. rickettsii, R. akari, and other SFGR in conventional serological assays. The frequency with which R. parkeri rickettsiosis has been misidentified as RMSF or rickettsialpox is unknown, but the epidemiological consequences of misdiagnosis are potentially profound: if routine serological tests had been used to diagnose the 12 cases in this series, these patients could have been classified as 2 laboratory-confirmed and 9 laboratory-supported cases of RMSF by the current Council of State and Territorial Epidemiologists' case definition for RMSF. From a clinical perspective, it is important to recognize that patients infected with R. parkeri, R. rickettsii, R. akari, or any other Rickettsia species will respond to therapy with doxycycline and that this therapy should never be delayed while awaiting diagnostic certainty [21].
Approximately 95% of the nearly 6000 “confirmed”; US cases of RMSF reported to the CDC during 1981–2005 were identified by using serological assays that do not conclusively differentiate the various SFGR indigenous to the United States. The full impact of this level of nonspecific testing on the epidemiological accuracy of RMSF is unknown; however, antibodies to SFGR are detected in the general US population at relatively high frequencies–5%–10% in several studies [30, 31]–suggesting that confirmatory methods more rigorous than existing serological techniques should be considered when diagnosing cases of presumed spotted fever rickettsiosis. In several countries considerably smaller than the United States, including Algeria, France, Morocco, Portugal, and Spain, investigators have identified as many as 5 different and sympatrically distributed SFGR in human-biting ticks [32–37] that may cause clinically similar, yet epidemiologically distinct, rickettsial diseases.
Many confirmed rickettsial pathogens (e.g., R. parkeri and Rickettsia massiliae) and rickettsiae of unknown pathogenicity in humans (e.g., Rickettsia rhipicephali, R. amblyommii, and serotypes 364D and Tillamook) reside in US ticks at frequencies far greater than those observed for R. rickettsii [4, 38–40]. It is possible that the true clinical spectrum, incidence, and distribution of RMSF in the United States have been clouded by blending epidemiological and clinical characteristics of several etiologically distinct diseases, caused by R. rickettsii, R. parkeri, and possibly other agents, under the umbrella diagnosis of RMSF. The descriptive mixing of these infections may extend throughout the Western hemisphere: R. parkeri has been detected recently in Argentina, Brazil, and Uruguay [41–43], and an eschar-associated disease that closely resembles R. parkeri rickettsiosis has also been described in several of these countries [44, 45]. In this context, rigorous efforts to obtain species-specific diagnoses through existing and developing technologies are needed to more accurately define the epidemiology and clinical presentation of spotted fever infections in the Americas.
Acknowledgments
We thank Gregory Dasch (CDC), for growing the rickettsiae and preparing the antigens used in the serological tests; James Gathany (CDC), for the tick photography; and Sheryl Hand (Mississippi State Department of Health), Stephanie Thouvenel-Romans (Escambia County Department of Health), Jennifer Del Gallo, William Taylor, Robert Cox, and Mary Ziegler, for their assistance in collecting patient information and for their interest in this work.
Potential conflicts of interest. All authors: no conflicts.
References
The findings and conclusions in this article are those of the authors and do not necessarily reflect the views of the US Department of Health and Human Services.
- polymerase chain reaction
- cell culture techniques
- immunohistochemistry
- centers for disease control and prevention (u.s.)
- rickettsia infections
- rocky mountain spotted fever
- signs and symptoms
- infections
- antibodies
- diagnosis
- rickettsia
- ticks
- spotted fevers
- case fatality rate
- eschar
- rickettsia parkeri
- vesicular rash




![A, Life stages of the Gulf Coast tick (Amblyomma maculatum). From left: larva, nymph, adult male, and adult female. Each stage can feed on human hosts and can be infected with Rickettsia parkeri. The head of a pin is included for scale. B, Classic range (dark blue) of A. maculatum in the United States, based on historical and contemporary records [3, 4]; a hypothetical range (pale blue), based on published incidental tick collection data (Pete Teel, Texas A&M University, personal communication); and the locations of confirmed (shaded circles) and probable (unshaded circles) cases of R. parkeri rickettsiosis discussed in this report.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cid/47/9/10.1086/592254/2/m_47-9-1188-fig001.gif?Expires=1715017146&Signature=p1EC1SGbuKoKS5D2-fyMJ88H2xaerpp6lGzg8eIS2JWrZ-UAvuDRnYNBnP6IqnI-SzGxW9ENo3PVRqTpviAzd5Pa0gesRtAQklzlbfC-TdXkCFwNdy-vVGjmB6SKo2CGdBjol-6vLW-GVU2KW4tMzFKtUN2wnRNdVd-WxOiKEc97LLd2sVYVpf7tbNy9B942fs987TMMQrymuHKithB1NZAfDh51qDXFYDYiXXM6A5DjkssrQrzslD4LyLy6Kk3XmFR7O65off3E3ZL46xXtAn2dVDdUAuYvdm0pFq8w4Ce0XCScMN6UfPeNBdamAfqH4s45BZx3V4Qh~-U1p26HAQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

Comments