-
PDF
- Split View
-
Views
-
Cite
Cite
Jean-Philippe Collet, Holger Thiele, Emanuele Barbato, Olivier Barthélémy, Johann Bauersachs, Deepak L Bhatt, Paul Dendale, Maria Dorobantu, Thor Edvardsen, Thierry Folliguet, Chris P Gale, Martine Gilard, Alexander Jobs, Peter Jüni, Ekaterini Lambrinou, Basil S Lewis, Julinda Mehilli, Emanuele Meliga, Béla Merkely, Christian Mueller, Marco Roffi, Frans H Rutten, Dirk Sibbing, George C M Siontis, Mohammed Chettibi, Hamlet G Hayrapetyan, Bernhard Metzler, Ruslan Najafov, Valeriy I Stelmashok, Marc Claeys, Zumreta Kušljugić, Plamen Marinov Gatzov, Bosko Skoric, Georgios Panayi, Martin Mates, Rikke Sorensen, Khaled Shokry, Toomas Marandi, Olli A Kajander, Philippe Commeau, Alexander Aladashvili, Steffen Massberg, Dimitrios Nikas, Dávid Becker, Ingibjörg J Guðmundsdóttir, Aaron J Peace, Roy Beigel, Ciro Indolfi, Nazipa Aidargaliyeva, Shpend Elezi, Medet Beishenkulov, Aija Maca, Olivija Gustiene, Philippe Degrell, Andrew Cassar Maempel, Victoria Ivanov, Peter Damman, Sasko Kedev, Terje K Steigen, Jacek Legutko, João Morais, Dragos Vinereanu, Dmitry Duplyakov, Marco Zavatta, Milan Pavlović, Marek Orban, Matjaž Bunc, Borja Ibañez, Robin Hofmann, Oliver Gaemperli, Yassin Bani Marjeh, Faouzi Addad, Eralp Tutar, Alexander Parkhomenko, Nina Karia, ESC Scientific Document Group , 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC), European Heart Journal, Volume 42, Issue 14, 7 April 2021, Pages 1289–1367, https://doi.org/10.1093/eurheartj/ehaa575
Close - Share Icon Share
 For the Supplementary Data which include background information and detailed discussion of the data that have provided the basis for the Guidelines see European Heart Journal online.
For the Supplementary Data which include background information and detailed discussion of the data that have provided the basis for the Guidelines see European Heart Journal online.
Table of contents
Abbreviations and acronyms 1293
1 Preamble 1295
2 Introduction 1296
2.1 Definitions 1296
2.1.1 Universal definition of myocardial infarction 1296
2.1.1.1 Type 1 myocardial infarction 1296
2.1.1.2 Type 2 myocardial infarction 1297
2.1.1.3 Types 3–5 myocardial infarction 1297
2.1.2 Unstable angina in the era of high-sensitivity cardiac troponin assays 1297
2.2 Epidemiology 1297
2.3 What is new? 1297
2.4 Number and breakdown of classes of recommendations (Supplementary Data) 1298
3 Diagnosis 1298
3.1 Clinical presentation (Supplementary Data) 1298
3.2 Physical examination (Supplementary Data) 1298
3.3 Diagnostic tools 1298
3.3.1 Electrocardiogram 1298
3.3.2 Biomarkers: high-sensitivity cardiac troponin 1299
3.3.2.1 Central laboratory vs. point-of-care 1300
3.3.2.2 Other biomarkers 1301
3.3.3 Rapid ‘rule-in’ and ‘rule-out’ algorithms 1301
3.3.4 Observe 1303
3.3.4.1 Caveats of using rapid algorithms 1303
3.3.4.2 Confounders of cardiac troponin concentration 1303
3.3.4.3 Practical guidance on how to implement the European Society of Cardiology 0 h/1 h algorithm 1304
3.3.4.4 Avoiding misunderstandings: time to decision = time of blood drawrn-around time 1304
3.3.5 Non-invasive imaging 1305
3.3.5.1 Functional evaluation 1305
3.3.5.2 Anatomical evaluation 1305
3.4 Differential diagnosis 1305
4 Risk assessment and outcomes 1307
4.1 Electrocardiogram indicators (Supplementary Data) 1307
4.2 Biomarkers 1307
4.3 Clinical scores for risk assessment (Supplementary Data) 1307
4.4 Bleeding risk assessment 1308
4.5 Integrating ischaemic and bleeding risks 1309
5 Pharmacological treatments 1309
5.1 Antithrombotic treatment 1309
5.1.1 Antiplatelet drugs and pre-treatment 1311
5.1.1.1 Antiplatelet drugs and dual antiplatelet therapy 1311
5.1.1.2 Pre-treatment 1312
5.1.2 Peri-interventional anticoagulant treatment 1314
5.1.3 Peri-interventional antiplatelet treatment 1315
5.1.4 Post-interventional and maintenance treatment 1315
5.2 Pharmacological treatment of ischaemia (Supplementary Data) 1318
5.2.1 Supportive pharmacological treatment (Supplementary Data) 1318
5.2.2 Nitrates and beta-blockers (Supplementary Data) 1318
5.3 Managing oral antiplatelet agents in patients requiring long-termoral anticoagulants 1318
5.3.1 Patients with atrial fibrillation without mechanical prosthetic heart valves or moderate-to-severe mitral stenosis undergoing percutaneous coronary intervention or managed medically (Supplementary Data) 1318
5.3.2 Patients requiring vitamin K antagonists or undergoing coronary artery bypass surgery 1320
5.4 Management of acute bleeding events (Supplementary Data) 1322
5.4.1 General supportivemeasures (Supplementary Data) 1322
5.4.2 Bleeding events on antiplatelet agents (Supplementary Data) 1322
5.4.3 Bleeding events on vitamin K antagonists (Supplementary Data) 1322
5.4.4 Bleeding events on non-vitamin K antagonist oral anticoagulants (Supplementary Data) 1322
5.4.5 Non-access-related bleeding events (Supplementary Data) 1322
5.4.6 Bleeding events related to percutaneous coronary intervention (Supplementary Data) 1322
5.4.7 Bleeding events related to coronary artery bypass surgery (Supplementary Data) 1322
5.4.8 Transfusion therapy (Supplementary Data) 1322
5.4.9 Recommendations for bleeding management and blood transfusion in non-ST-segment elevation acute coronary syndromes for anticoagulated patients 1322
6 Invasive treatments 1322
6.1 Invasive coronary angiography and revascularization 1322
6.1.1 Routine invasive vs. selective invasive approach (Supplementary Data) 1322
6.1.2 Timing of invasive strategy 1323
6.1.2.1 Immediate invasive strategy (<2 h) 1323
6.1.2.2 Early invasive strategy (<24 h) 1323
6.1.2.3 Selective invasive strategy 1324
6.1.3 Pattern of coronary artery disease in non-ST-segment elevation acute coronary syndrome (Supplementary Data) 1325
6.1.4 How to identify the culprit lesion? (Supplementary Data) 1325
6.1.5 Spontaneous coronary artery dissection 1325
6.1.6 Fractional flow reserve, instantaneous wave-free ratio, and other resting indices (Supplementary Data) 1326
6.1.6.1 Fractional flow reserve 1326
6.1.6.2 Instantaneous wave-free ratio and other resting indices 1326
6.1.7 Intracoronary imaging 1326
6.2 Conservative treatment 1326
6.2.1 Patients who are not candidates for invasive coronary angiography 1326
6.2.2 Patients with coronary artery disease not amenable to revascularization 1326
6.3 Technical aspects 1327
6.3.1 Technical aspects and challenges 1327
6.3.2 Vascular access 1327
6.3.3 Revascularization strategies 1327
6.4 Coronary artery bypass grafting 1327
6.5 Percutaneous coronary intervention vs. coronary artery bypass surgery 1327
6.6 Specific situations 1328
6.6.1 Management of patients with ongoing myocardial ischaemia 1328
6.6.2 Management of patients with cardiac arrest 1328
6.7 Recommendations for coronary revascularization 1328
7 Myocardial infarction with non-obstructive coronary arteries and alternative diagnoses 1329
8 Special populations 1331
8.1 Heart failure and cardiogenic shock 1331
8.2 Diabetes mellitus 1332
8.3 Chronic kidney disease 1333
8.4 Anaemia 1334
8.5 Thrombocytopenia (Supplementary Data) 1334
8.5.1 Thrombocytopenia related to glycoprotein IIb/IIIa inhibitors (Supplementary Data) 1334
8.5.2 Heparin-induced thrombocytopenia (Supplementary Data) 1334
8.6 The older person 1334
8.7 Frailty 1334
8.8 Sex disparities 1334
9 Long-term management of non-ST-segment elevation acute coronary syndrome (Supplementary Data) 1335
9.1 Lifestyle management (Supplementary Data) 1335
9.1.1 Smoking (Supplementary Data) 1335
9.1.2 Diet and alcohol (Supplementary Data) 1335
9.1.3 Weight management (Supplementary Data) 1335
9.1.3 Physical activity (Supplementary Data) 1335
9.1.4 Cardiac rehabilitation (Supplementary Data) 1335
9.1.5 Psychosocial factors (Supplementary Data) 1335
9.1.6 Environmental factors (Supplementary Data) 1335
9.1.7 Sexual activity (Supplementary Data) 1335
9.1.8 Adherence and sustainability (Supplementary Data) 1335
9.1.9 Influenza vaccination (Supplementary Data) 1335
9.2 Pharmacological management (Supplementary Data) 1335
9.2.1 Anti-ischaemic drugs 1335
9.2.1.1 Beta-blockers (Supplementary Data) 1335
9.2.2 Antithrombotic treatments 1335
9.2.3 Proton pump inhibitors (Supplementary Data) 1335
9.2.4 Statins and other lipid-lowering agents 1335
9.2.5 Glucose-lowering therapy in patients with diabetes 1336
9.2.6 Renin-angiotensin-aldosterone system blockers (Supplementary Data) 1336
9.2.7 Mineralocorticoid receptor antagonist therapy (Supplementary Data) 1336
9.2.8 Antihypertensive therapy (Supplementary Data) 1336
9.2.9 Hormone replacement therapy (Supplementary Data) 1336
10 Quality indicators 1337
11 Management strategy 1340
12 Key messages 1341
13 Gaps in evidence for non-ST-segment elevation acute coronary syndrome care and future research 1342
14 ‘What to do’ and ‘what not to do’messages 1343
15 Supplementary data 1347
16 Appendix 1347
17 References 1348
Tables of Recommendations
Recommendations for diagnosis, risk stratification, imaging, and rhythm monitoring in patients with suspected non-ST-segment elevation acute coronary syndrome 1306
Recommendations on biomarker measurements for prognostic stratification 1308
Recommendations for antithrombotic treatment in non-ST- segment elevation acute coronary syndrome patients undergoing percutaneous coronary intervention 1314
Recommendations for post-interventional and maintenance treatment in patients with non-ST-segment elevation acute coronary syndrome 1317
Recommendations for anti-ischaemic drugs in the acute phase of non-ST-segment elevation acute coronary syndrome 1318
Recommendations for combining antiplatelet agents and anticoagulants in non-ST-segment elevation acute coronary syndrome patients requiring chronic oral anticoagulation 1321
Recommendations for bleeding management and blood transfusion in non-ST-segment elevation acute coronary syndromes for anticoagulated patients 1322
Recommendations for coronary revascularization 1328
Recommendations for myocardial infarction with non-obstructive coronary arteries 1331
Recommendations for non-ST-segment elevation acute coronary syndrome patients with heart failure or cardiogenic shock 1332
Recommendations for diabetes mellitus in non-ST-segment elevation acute coronary syndrome patients 1333
Recommendations for patients with chronic kidney disease and non-ST-segment elevation acute coronary syndrome 1333
Recommendations for older persons with non-ST-segment elevation acute coronary syndrome 1334
Recommendations for lifestyle managements after non-STsegment elevation acute coronary syndrome 1335
Recommendations for pharmacological long-term management after non-ST-segment elevation acute coronary syndrome (excluding antithrombotic treatments) 1336
List of tables
Table 1 Classes of recommendations 1295
Table 2 Levels of evidence 1296
Table 3 Clinical implications of high-sensitivity cardiac troponin assays 1301
Table 4 Conditions other than acute type 1 myocardial infarction associated with cardiomyocyte injury (= cardiac troponin elevation) 1301
Table 5 Assay specific cut-off levels in ng/l within the 0 h/1 h and 0 h/2 h algorithms 1303
Table 6 Differential diagnoses of acute coronary syndromes in the setting of acute chest pain 1306
Table 7 Major and minor criteria for high bleeding risk according to the Academic Research Consortium for High Bleeding Risk at the time of percutaneous coronary intervention (bleeding risk is high if at least one major or two minor criteria aremet) 1309
Table 8 Dose regimen of antiplatelet and anticoagulant drugs in non-ST-segment elevation acute coronary syndrome patients 1311
Table 9 P2Y12 receptor inhibitors for use in non-ST-segment elevation acute coronary syndrome patients 1312
Table 10 Treatment options for extended dual antithrombotic or antiplatelet therapies 1316
Table 11 Risk criteria for extended treatment with a second antithrombotic agent 1316
Table 12 Suggested strategies to reduce bleeding risk related to percutaneous coronary intervention 1319
Table 13 Randomized controlled trials including patients with non-ST-segment elevation acute coronary syndrome requiring anticoagulation and antiplatelet therapy 1319
Table 14 Diagnostic criteria of myocardial infarction with non-obstructive coronary arteries 1330
Table 15 Quality indicators in non-ST-segment elevation acute coronary syndrome care 1337
List of figures
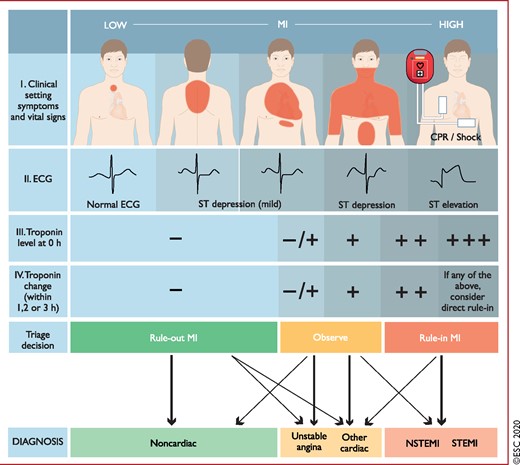
Figure 1 Diagnostic algorithm and triage in acute coronary syndrome. 1299
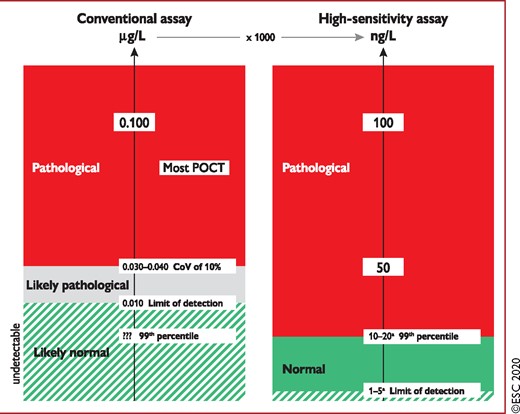
Figure 2 Value of high-sensitivity cardiac troponin. 1300
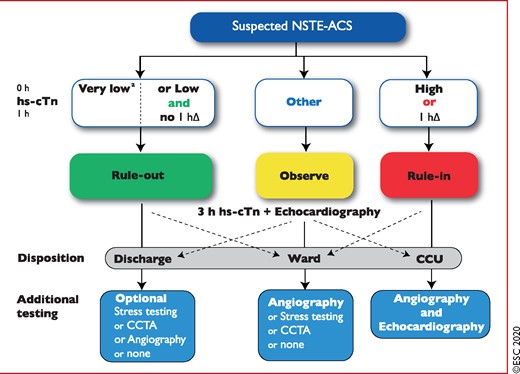
Figure 3 0 h/1 h rule-out and rule-in algorithm using high-sensitivity cardiac troponin assays in haemodynamically stable patients presenting with suspected non-ST-segment elevation acute coronary syndrome to the emergency department. 1302
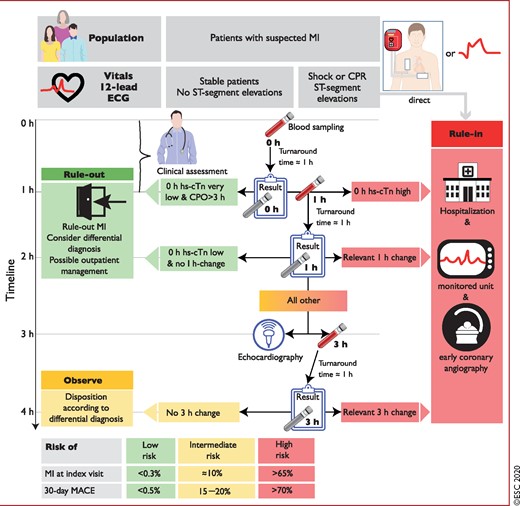
Figure 4 Timing of the blood draws and clinical decisions when using the European Society of Cardiology 0 h/1 h algorithm. 1304
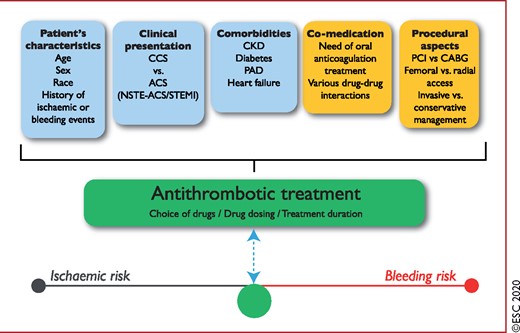
Figure 5 Determinants of antithrombotic treatment in coronary artery disease. 1310
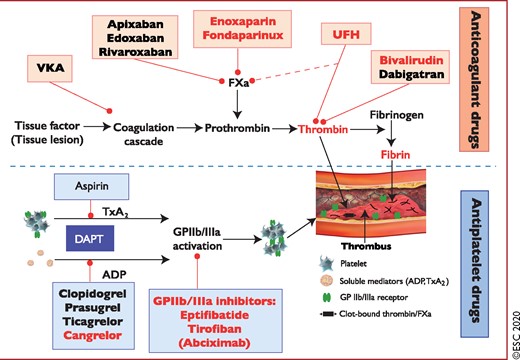
Figure 6 Antithrombotic treatments in non-ST-segment elevation acute coronary syndrome patients: pharmacological targets. 1310
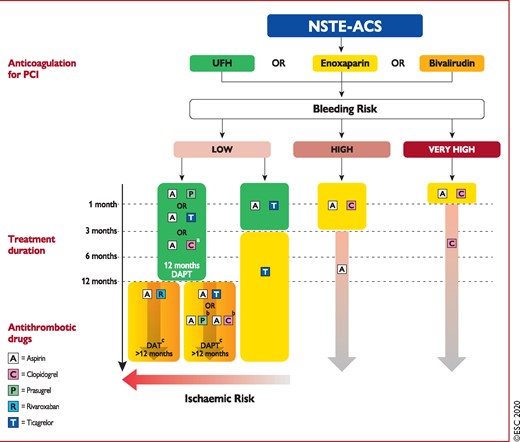
Figure 7 Algorithm for antithrombotic therapy in non-ST-segment elevation acute coronary syndrome patients without atrial fibrillation undergoing percutaneous coronary intervention 1313
Figure 8 Algorithm for antithrombotic therapy in non-ST-segment elevation acute coronary syndrome patients with atrial fibrillation undergoing percutaneous coronary intervention or medical management 1320
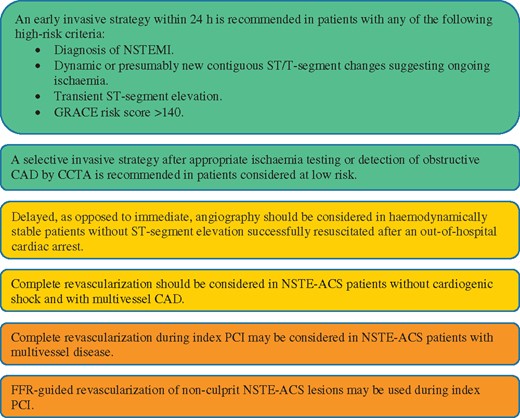
Figure 9 Selection of non-ST-segment elevation acute coronary syndrome treatment strategy and timing according to initial risk stratification. 1323
Figure 10 Time to coronary angiography in the early/immediate invasive and delayed invasive groups of included trials. 1324
Figure 11 Diagnosis and treatment of patients with non-ST-segment elevation acute coronary syndrome related to spontaneous coronary artery dissection. 1325
Figure 12 Diagnostic algorithm for myocardial infarction with non-obstructive coronary arteries using a traffic light scheme. 1331
Figure 13 Central illustration. Management strategy for non-ST-segment elevation acute coronary syndrome patients. 1340
Abbreviations and acronyms
- ACCOAST
Comparison of Prasugrel at the Time of Percutaneous Coronary Intervention or as Pretreatment at the Time of Diagnosis in Patients with Non-ST Elevation Myocardial Infarction
- ACE
Angiotensin-converting enzyme
- ACS
Acute coronary syndromes
- ACUITY
Acute Catheterization and Urgent Intervention Triage strategY
- ACVC
Association for Acute Cardiovascular Care
- ADP
Adenosine diphosphate
- AF
Atrial fibrillation
- AGRIS
Australian GRACE Risk score Intervention Study
- AHA
American Heart Association
- AMI
Acute myocardial infarction
- ARB
Angiotensin receptor blocker
- ARC-HBR
Academic Research Consortium for High Bleeding Risk
- ATLAS ACS 2– TIMI 51
Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Standard Therapy in Subjects with Acute Coronary Syndrome–Thrombolysis In Myocardial Infarction 51
- AUGUSTUS
Antithrombotic Therapy after Acute Coronary Syndrome or PCI in Atrial Fibrillation
- BARC
Bleeding Academic Research Consortium
- BEST
Randomized Comparison of Coronary Artery Bypass Surgery and Everolimus-Eluting Stent Implantation in the Treatment of Patients with Multivessel Coronary Artery Disease
- b.i.d
Bis in die (twice a day)
- BNP
B-type natriuretic peptide
- CABG
Coronary artery bypass graft(ing)
- CAD
Coronary artery disease
- CCS
Chronic coronary syndromes
- CCTA
Coronary computed tomography angiography
- CCU
Coronary care unit
- CFR
Coronary flow reserve
- CHA2DS2-VASc
Congestive heart failure, Hypertension, Age ≥75 years (2 points), Diabetes, Stroke (2 points)–Vascular disease, Age 65–74, Sex category (female)
- CHAMPION
Cangrelor versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition
- CI
Confidence interval
- CK
Creatine kinase
- CKD
Chronic kidney disease
- CK-MB
Creatine kinase myocardial band
- CMR
Cardiac magnetic resonance
- COACT
Coronary Angiography after Cardiac Arrest
- COMPASS
Cardiovascular OutcoMes for People using Anticoagulation StrategieS
- CPG
Clinical practice guidelines
- CPR
Cardiopulmonary resuscitation
- CrCl
Creatinine clearance
- CRUSADE
Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines
- CS
Cardiogenic shock
- CT
Computed tomography
- CULPRIT- SHOCK
Culprit Lesion Only PCI versus Multivessel PCI in Cardiogenic Shock
- CVD
Cardiovascular disease
- CYP
Cytochrome P450
- DAPT
Dual antiplatelet therapy
- DAT
Dual antithrombotic therapy
- DES
Drug-eluting stent
- EACTS
European Association for Cardio-Thoracic Surgery
- ECG
Electrocardiogram/electrocardiography
- Echo
Echocardiogram
- eGFR
Estimated glomerular filtration rate
- ELISA
Early or Late Intervention in unStable Angina
- ENTRUST- AF PCI
EdoxabaN TRreatment versUS VKA in paTients with AF undergoing PCI
- ESC
European Society of Cardiology
- FAMOUS- NSTEMI
Fractional flow reserve versus angiography in guiding management to optimize outcomes in non-ST-elevation myocardial infarction
- FFR
Fractional flow reserve
- FFR-CT
Fractional flow reserve-computed tomography
- GDF-15
Growth differentiation factor 15
- GP
Glycoprotein
- GRACE
Global Registry of Acute Coronary Events
- HAS-BLED
Hypertension, abnormal renal and liver function (1 point each), stroke, bleeding history or predisposition, labile INR, elderly (>65 years), drugs and alcohol (1 point each)
- HBR
High bleeding risk
- h-FABP
Heart-type fatty acid-binding protein
- HIT
Heparin-induced thrombocytopenia
- HR
Hazard ratio
- hs-cTn
High-sensitivity cardiac troponin
- IABP
Intra-aortic balloon pump
- IABP-SHOCK II
Intraaortic Balloon Pump in cardiogenic shock II
- ICA
Invasive coronary angiography
- iFR
Instantaneous wave-free ratio
- IMR
Index of microcirculatory resistance
- INR
International normalized ratio
- ISAR-REACT
Intracoronary stenting and Antithrombotic regimen–Rapid Early Action for Coronary Treatment
- ISAR-TRIPLE
Triple Therapy in Patients on Oral Anticoagulation After Drug Eluting Stent Implantation
- i.v.
Intravenous
- IVUS
Intravascular ultrasound
- LBBB
Left bundle branch block
- LD
Loading dose
- LDL-C
Low-density lipoprotein cholesterol
- LIPSIA-NSTEMI
Leipzig Immediate versus early and late PercutaneouS coronary Intervention triAl in NSTEMI
- LMWH
Low-molecular-weight heparin
- LV
Left ventricular
- LVEF
Left ventricular ejection fraction
- MACE
Major adverse cardiovascular events
- MATRIX
Minimizing Adverse Haemorrhagic Events by TRansradial Access Site and Systemic Implementation of angioX
- MD
Maintenance dose
- MDCT
Multidetector computed tomography
- MI
Myocardial infarction
- MINOCA
Myocardial infarction with non-obstructive coronary arteries
- MRA
Mineralocorticoid receptor antagonist
- NOAC
Non-vitamin K antagonist oral anticoagulant
- NPV
Negative predictive value
- NSTE-ACS
Non-ST-segment elevation acute coronary syndrome
- NSTEMI
Non-ST-segment elevation myocardial infarction
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
- OAC
Oral anticoagulation/anticoagulant
- OASIS-5
Fifth Organization to Assess Strategies in Acute Ischemic Syndromes
- OCT
Optical coherence tomography
- o.d.
Once daily
- OR
Odds ratio
- P
Prasugrel
- PAD
Peripheral artery disease
- PCI
Percutaneous coronary intervention
- PCSK9
Proprotein convertase subtilisin kexin 9
- Pd/Pa
Distal coronary to aortic pressure ratio
- PEGASUS-TIMI 54
Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin-Thrombolysis in Myocardial Infarction 54
- PLATO
PLATelet inhibition and patient Outcomes
- POCT
Point-of-care test
- PPV
Positive predictive value
- PRECISE-DAPT
PREdicting bleeding Complications In patients undergoing Stent implantation and subsEquent Dual Anti Platelet Therapy
- PRECOMBAT
Premier of Randomized Comparison of Bypass Surgery versus Angioplasty Using Sirolimus-Eluting Stent in Patients with Left Main Coronary Artery Disease
- PROMs
Patient-reported outcome measures
- QI
Quality indicator
- RBBB
Right bundle branch block
- RCT
Randomized controlled trial
- RE-DUAL PCI
Randomized Evaluation of Dual Antithrombotic Therapy with Dabigatran versus Triple Therapy with Warfarin in Patients with Nonvalvular Atrial Fibrillation Undergoing Percutaneous Coronary Intervention
- REDUCE-IT
Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial
- RFR
Resting full-cycle ratio
- RIDDLE-NSTEMI
Randomized Study of Immediate Versus Delayed Invasive Intervention in Patients With Non-ST-Segment Elevation Myocardial Infarction
- RIVAL
RadIal Vs femorAL access for coronary intervention
- RR
Relative risk
- SAPT
Single antiplatelet therapy
- SCAAR
Swedish Coronary Angiography and Angioplasty Registry
- SCAD
Spontaneous coronary artery dissection
- SISCA
Comparison of Two Treatment Strategies in Patients With an Acute Coronary Syndrome Without ST Elevation
- SMILE
Impact of Different Treatment in Multivessel Non ST Elevation Myocardial Infarction Patients: One Stage Versus Multistaged Percutaneous Coronary Intervention
- SPECT
Single-photon-emission tomography
- STEMI
ST-segment elevation myocardial infarction
- STS
Society of Thoracic Surgeons
- SYNTAX
Synergy between PCI with Taxus and cardiac surgery
- TAT
Triple antithrombotic therapy
- TIMACS
Timing of Intervention in Patients with Acute Coronary Syndromes
- TIMI
Thrombolysis In Myocardial Infarction
- TRITON-TIMI 38
TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel–Thrombolysis In Myocardial Infarction 38
- TROPICAL-ACS
Testing Responsiveness to Platelet Inhibition on Chronic Antiplatelet Treatment for Acute Coronary Syndromes
- TWILIGHT
Ticagrelor With Aspirin or Alone in High-Risk Patients After Coronary Intervention
- UFH
Unfractionated heparin
- UKGRIS
UK GRACE Risk Score Intervention Study
- ULTIMATE
Intravascular Ultrasound Guided Drug Eluting Stents Implantation in “All-Comers” Coronary Lesions
- VALIDATE- SWEDEHEART
Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies
- VERDICT
Very EaRly vs Deferred Invasive evaluation using Computerized Tomography
- VKA
Vitamin K antagonist
- WOEST
What is the Optimal antiplatElet and anticoagulant therapy in patients with oral anticoagulation and coronary StenTing
1 Preamble
Guidelines summarize and evaluate available evidence with the aim of assisting health professionals in proposing the best management strategies for an individual patient with a given condition. Guidelines and their recommendations should facilitate decision making of health professionals in their daily practice. However, the final decisions concerning an individual patient must be made by the responsible health professional(s) in consultation with the patient and caregiver as appropriate.
A great number of guidelines have been issued in recent years by the European Society of Cardiology (ESC), as well as by other societies and organizations. Because of their impact on clinical practice, quality criteria for the development of guidelines have been established in order to make all decisions transparent to the user. The recommendations for formulating and issuing ESC Guidelines can be found on the ESC website (https://www.escardio.org/Guidelines/Clinical-Practice-Guidelines/Guidelines-development/Writing-ESC-Guidelines). The ESC Guidelines represent the official position of the ESC on a given topic and are regularly updated.
In addition to the publication of Clinical Practice Guidelines, the ESC carries out the EurObservational Research Programme of international registries of cardiovascular diseases and interventions which are essential to assess, diagnostic/therapeutic processes, use of resources and adherence to Guidelines. These registries aim at providing a better understanding of medical practice in Europe and around the world, based on high-quality data collected during routine clinical practice.
Furthermore, the ESC has developed and embedded in this document a set of quality indicators (QIs), which are tools to evaluate the level of implementation of the Guidelines and may be used by the ESC, hospitals, healthcare providers and professionals to measure clinical practice as well as used in educational programmes, alongside the key messages from the guidelines, to improve quality of care and clinical outcomes.
The Members of this Task Force were selected by the ESC, including representation from its relevant ESC sub-specialty groups, in order to represent professionals involved with the medical care of patients with this pathology. Selected experts in the field undertook a comprehensive review of the published evidence for management of a given condition according to ESC Committee for Practice Guidelines (CPG) policy. A critical evaluation of diagnostic and therapeutic procedures was performed, including assessment of the risk–benefit ratio. The level of evidence and the strength of the recommendation of particular management options were weighed and graded according to predefined scales, as outlined below.
2 Introduction
2.1 Definitions
The clinical presentation of acute coronary syndromes (ACS) is broad. It ranges from cardiac arrest, electrical or haemodynamic instability with cardiogenic shock (CS) due to ongoing ischaemia or mechanical complications such as severe mitral regurgitation, to patients who are already pain free again at the time of presentation.1 The leading symptom initiating the diagnostic and therapeutic cascade in patients with suspected ACS is acute chest discomfort described as pain, pressure, tightness, and burning. Chest pain-equivalent symptoms may include dyspnoea, epigastric pain, and pain in the left arm. Based on the electrocardiogram (ECG), two groups of patients should be differentiated:
Patients with acute chest pain and persistent (>20 min) ST-segment elevation. This condition is termed ST-segment elevation ACS and generally reflects an acute total or subtotal coronary occlusion. Most patients will ultimately develop ST-segment elevation myocardial infarction (STEMI). The mainstay of treatment in these patients is immediate reperfusion by primary percutaneous coronary intervention (PCI) or, if not available in a timely manner, by fibrinolytic therapy.2
Patients with acute chest discomfort but no persistent ST-segment elevation [non-ST-segment elevation ACS (NSTE-ACS)] exhibit ECG changes that may include transient ST-segment elevation, persistent or transient ST-segment depression, T-wave inversion, flat T waves, or pseudo-normalization of T waves; or the ECG may be normal.
The pathological correlate at the myocardial level is cardiomyocyte necrosis [non-ST-segment elevation myocardial infarction (NSTEMI)] or, less frequently, myocardial ischaemia without cell damage (unstable angina). A small proportion of patients may present with ongoing myocardial ischaemia, characterized by one or more of the following: recurrent or ongoing chest pain, marked ST-segment depression on 12-lead ECG, heart failure, and haemodynamic or electrical instability.1 Due to the amount of myocardium in jeopardy and the risk of developing CS and/or malignant ventricular arrhythmias, immediate coronary angiography and, if appropriate, revascularization are indicated (see section 6).
2.1.1 Universal definition of myocardial infarction
Acute myocardial infarction (AMI) defines cardiomyocyte necrosis in a clinical setting consistent with acute myocardial ischaemia.1,3 A combination of criteria is required to meet the diagnosis of AMI, namely the detection of an increase and/or decrease of a cardiac biomarker, preferably high-sensitivity cardiac troponin (hs-cTn) T or I, with at least one value above the 99th percentile of the upper reference limit and at least one of the following:
Symptoms of myocardial ischaemia.
New ischaemic ECG changes.
Development of pathological Q waves on ECG.
Imaging evidence of loss of viable myocardium or new regional wall motion abnormality in a pattern consistent with an ischaemic aetiology.
Intracoronary thrombus detected on angiography or autopsy.
2.1.1.1 Type 1 myocardial infarction
Type 1 myocardial infarction (MI) is characterized by atherosclerotic plaque rupture, ulceration, fissure, or erosion with resulting intraluminal thrombus in one or more coronary arteries leading to decreased myocardial blood flow and/or distal embolization and subsequent myocardial necrosis. The patient may have underlying severe coronary artery disease (CAD) but, on occasion (5–10% of cases), there may be non-obstructive coronary atherosclerosis or no angiographic evidence of CAD, particularly in women.1,3–5
2.1.1.2 Type 2 myocardial infarction
Type 2 MI is myocardial necrosis in which a condition other than coronary plaque instability causes an imbalance between myocardial oxygen supply and demand.3 Mechanisms include hypotension, hypertension, tachyarrhythmias, bradyarrhythmias, anaemia, hypoxaemia, but also by definition, coronary artery spasm, spontaneous coronary artery dissection (SCAD), coronary embolism, and coronary microvascular dysfunction.6–8
2.1.1.3 Types 3–5 myocardial infarction
The universal definition of MI also includes type 3 MI (MI resulting in death when biomarkers are not available) and types 4 and 5 MI [related to PCI and coronary artery bypass grafting (CABG), respectively].3
2.1.2 Unstable angina in the era of high-sensitivity cardiac troponin assays
Unstable angina is defined as myocardial ischaemia at rest or on minimal exertion in the absence of acute cardiomyocyte injury/necrosis. Among unselected patients presenting to the emergency department with suspected NSTE-ACS, the introduction of hs-cTn measurements in place of standard troponin assays resulted in an increase in the detection of MI (∼4% absolute and 20% relative increases) and a reciprocal decrease in the diagnosis of unstable angina.9–13 Compared with NSTEMI patients, individuals with unstable angina do not experience acute cardiomyocyte injury/necrosis, have a substantially lower risk of death, and appear to derive less benefit from intensified antiplatelet therapy, as well as an invasive strategy within 72 h.1,3–5,9–19 Pathophysiology and epidemiology are discussed in detail elsewhere.1
2.2 Epidemiology
The proportion of patients with NSTEMI in MI surveys increased from one third in 1995 to more than half in 2015, mainly accounted for by a refinement in the operational diagnosis of NSTEMI20. As opposed to STEMI, no significant changes are observed in the baseline characteristics of the NSTEMI population with respect to age and smoking, while diabetes, hypertension, and obesity increased substantially. The use of early angiography (≤72 h from admission) increased from 9% in 1995 to 60% in 2015 [adjusted odds ratio (OR) 16.4, 95% confidence interval (CI) 12.0–22.4, P<0.001] and PCI during the initial hospital stay increased from 12.5% to 67%. The main consequences of these changes are a reduction in 6-month mortality from 17.2% to 6.3% and the adjusted hazard ratio (HR) decreased to 0.40 (95% CI 0.30–0.54) in 2010, remaining stable at 0.40 (0.30–0.52) in 2015.20
2.3 What is new?

Diagnosis

Risk stratification

Antithrombotic treatment

Invasive treatment

Diagnosis
Risk assessment

Pharmacological treatments
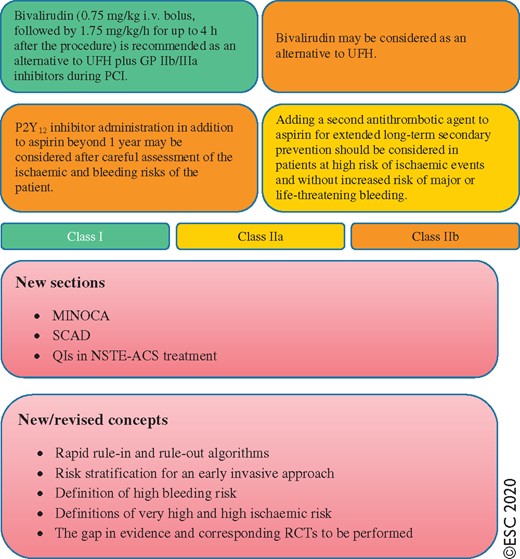
ACS = acute coronary syndromes; AF = atrial fibrillation; BNP = B-type natriuretic peptide; CAD = coronary artery disease; CCTA = coronary computed tomography angiography; CHA2DS2-VASc = Congestive heart failure, Hypertension, Age ≥75 years (2 points), Diabetes, Stroke (2 points)–Vascular disease, Age 65–74, Sex category (female); CK = creatine kinase; CK-MB = creatine kinase myocardial band; DAPT = dual antiplatelet therapy; DAT = dual antithrombotic therapy; ECG = electrocardiogram/electrocardiography; ESC = European Society of Cardiology; FFR = fractional flow reserve; GP = glycoprotein; GRACE = Global Registry of Acute Coronary Events; h-FABP = heart-type fatty acid-binding protein; hs-cTn = high-sensitivity cardiac troponin; MDCT = multidetector computed tomography; MINOCA = myocardial infarction with non-obstructive coronary arteries; NOAC = non-vitamin K antagonist oral anticoagulant; NSTE-ACS = non-ST-segment elevation acute coronary syndrome; NSTEMI = non-ST-segment elevation myocardial infarction; NT-proBNP = N-terminal pro-B-type natriuretic peptide; OAC = oral anticoagulation/anticoagulant; PCI = percutaneous coronary intervention; QI = quality indicator; RCT = randomized controlled trial; SCAD = spontaneous coronary artery dissection; TAT = triple antithrombotic therapy; UFH = unfractionated heparin.
2.4 Number and breakdown of classes of recommendations (Supplementary Data)
The total number of recommendations is 131. The breakdown of the recommendations according to ESC classes of recommendations and levels of evidence are summarized in Supplementary Figure 1.
3 Diagnosis
3.1 Clinical presentation (Supplementary Data)
3.2 Physical examination (Supplementary Data)
3.3 Diagnostic tools
3.3.1 Electrocardiogram
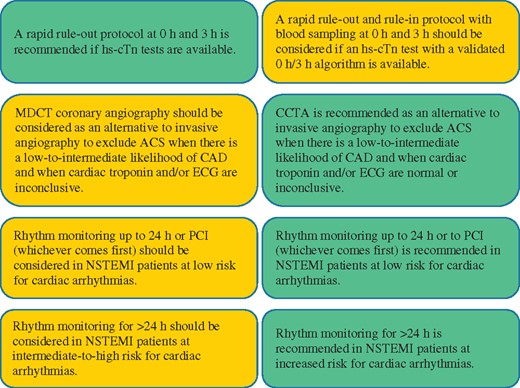
The resting 12-lead ECG is the first-line diagnostic tool in the assessment of patients with suspected ACS (Figure 1). It is recommended to perform it within 10 min of the patient’s arrival in the emergency room or, ideally, at first contact with the emergency medical services in the pre-hospital setting and to have it immediately interpreted by a qualified physician.21 While the ECG in the setting of NSTE-ACS may be normal in more than 30% of patients, characteristic abnormalities include ST-segment depression, transient ST-segment elevation, and T-wave changes.6–8,10–13,22
If the standard leads are inconclusive and the patient has signs or symptoms suggestive of ongoing myocardial ischaemia, additional leads should be recorded; left circumflex artery occlusion may be detected only in V7–V9 or right ventricular MI only in V3R and V4R.3 In patients with suggestive signs and symptoms, the finding of persistent ST-segment elevation indicates STEMI, which mandates immediate reperfusion.2 Comparison with previous tracings is valuable, particularly in patients with pre-existing ECG abnormalities. It is recommended to obtain additional 12-lead ECGs in case of persistent or recurrent symptoms or diagnostic uncertainty. In patients with left bundle branch block (LBBB), specific ECG criteria (Sgarbossa’s criteria) may help in the detection of candidates for immediate coronary angiography.23,24 Patients with a high clinical suspicion of ongoing myocardial ischaemia and LBBB should be managed in a way similar to STEMI patients, regardless of whether the LBBB is previously known.2 In contrast, haemodynamically stable patients presenting with chest pain and LBBB only have a slightly higher risk of having MI compared to patients without LBBB. Therefore, the result of the hs-cTn T/I measurement at presentation should be integrated into the decision regarding immediate coronary angiography.24
In patients with right bundle brunch block (RBBB), ST-elevation is indicative of STEMI while ST-segment depression in lead I, aVL, and V5–6 is indicative of NSTE-ACS.25 In patients with paced ventricular beats, the ECG is often of no help for the diagnosis of NSTE-ACS. Novel ECG algorithms using digital ECG data are in development.26–28 In general, it is advisable to perform ECG interpretation using remote technologies at the pre-hospital stage.
It is important to highlight that more than 50% of patients presenting with acute chest pain and LBBB to the emergency department or chest pain unit will ultimately be found to have a diagnosis other than MI.24 Similarly, more than 50% of patients presenting with acute chest pain and RBBB to the emergency department will ultimately be found to have a diagnosis other than MI and should, therefore, also await the result of the hs-cTn T/I measurement at presentation.25
Diagnostic algorithm and triage in acute coronary syndrome. The initial assessment is based on the integration of low likelihood and/or high likelihood features derived from the clinical setting (i.e. symptoms, vital signs), the 12-lead ECG, and the cardiac troponin concentration determined at presentation to the emergency department and serially thereafter. ‘Other cardiac’ includes – among others – myocarditis, Takotsubo syndrome, or congestive heart failure. ‘Non-cardiac’ refers to thoracic diseases such as pneumonia or pneumothorax. Cardiac troponin and its change during serial sampling should be interpreted as a quantitative marker: the higher the 0 h level or the absolute change during serial sampling, the higher the likelihood for the presence of MI. In patients presenting with cardiac arrest or haemodynamic instability of presumed cardiovascular origin, echocardiography should be performed/interpreted by trained physicians immediately following a 12-lead ECG. If the initial evaluation suggests aortic dissection or pulmonary embolism, D-dimers and CCTA are recommended according to dedicated algorithms.1,29–33 CPR = cardiopulmonary resuscitation; ECG = electrocardiogram/electrocardiography; MI = myocardial infarction; NSTEMI = non-ST-segment elevation myocardial infarction; STEMI = ST-segment elevation myocardial infarction. Listen to the audio guide of this figure online.
3.3.2 Biomarkers: high-sensitivity cardiac troponin
Biomarkers complement clinical assessment and 12-lead ECG in the diagnosis, risk stratification, and treatment of patients with suspected NSTE-ACS. Measurement of a biomarker of cardiomyocyte injury, preferably hs-cTn, is mandatory in all patients with suspected NSTE-ACS.1,3,10–13 Cardiac troponins are more sensitive and specific markers of cardiomyocyte injury than creatine kinase (CK), its myocardial band isoenzyme (CK-MB), and myoglobin.1,3,4,10–13,29,30 If the clinical presentation is compatible with myocardial ischaemia, then a dynamic elevation of cardiac troponin above the 99th percentile of healthy individuals indicates MI. In patients with MI, levels of cardiac troponin rise rapidly (i.e. usually within 1 h from symptom onset if using high-sensitivity assays) after symptom onset and remain elevated for a variable period of time (usually several days).1,3,4,10–13,29,30 Advances in technology have led to a refinement in cardiac troponin assays and have improved the ability to detect and quantify cardiomyocyte injury.1,3,4,6–8,10–13,29,30,34–36 Data from large multicentre studies have consistently shown that hs-cTn assays increase diagnostic accuracy for MI at the time of presentation as compared with conventional assays (Figure 2), especially in patients presenting early after chest pain onset, and allow for a more rapid ‘rule-in’ and ‘rule-out’ of MI (see section 3.3.3 and Table 3).1,3,4,6–8,10–13,29,30,35,36 Overall, hs-cTn T and hs-cTn I assays seem to provide comparable diagnostic accuracy in the early diagnosis of MI.37–40
Clinical implications of high-sensitivity cardiac troponin assays
| Compared with standard cardiac troponin assays, hs-cTn assays: |
|
|
|
|
| Levels of hs-cTn should be interpreted as quantitative markers of cardiomyocyte damage (i.e. the higher the level, the greater the likelihood of MI): |
|
|
|
| Rising and/or falling cardiac troponin levels differentiate acute (as in MI) from chronic cardiomyocyte damage (the more pronounced the change, the higher the likelihood of AMI). |
| Compared with standard cardiac troponin assays, hs-cTn assays: |
|
|
|
|
| Levels of hs-cTn should be interpreted as quantitative markers of cardiomyocyte damage (i.e. the higher the level, the greater the likelihood of MI): |
|
|
|
| Rising and/or falling cardiac troponin levels differentiate acute (as in MI) from chronic cardiomyocyte damage (the more pronounced the change, the higher the likelihood of AMI). |
AMI = acute myocardial infarction; hs-cTn = high-sensitivity cardiac troponin; MI = myocardial infarction; NPV = negative predictive value; PPV = positive predictive value.
Clinical implications of high-sensitivity cardiac troponin assays
| Compared with standard cardiac troponin assays, hs-cTn assays: |
|
|
|
|
| Levels of hs-cTn should be interpreted as quantitative markers of cardiomyocyte damage (i.e. the higher the level, the greater the likelihood of MI): |
|
|
|
| Rising and/or falling cardiac troponin levels differentiate acute (as in MI) from chronic cardiomyocyte damage (the more pronounced the change, the higher the likelihood of AMI). |
| Compared with standard cardiac troponin assays, hs-cTn assays: |
|
|
|
|
| Levels of hs-cTn should be interpreted as quantitative markers of cardiomyocyte damage (i.e. the higher the level, the greater the likelihood of MI): |
|
|
|
| Rising and/or falling cardiac troponin levels differentiate acute (as in MI) from chronic cardiomyocyte damage (the more pronounced the change, the higher the likelihood of AMI). |
AMI = acute myocardial infarction; hs-cTn = high-sensitivity cardiac troponin; MI = myocardial infarction; NPV = negative predictive value; PPV = positive predictive value.
3.3.2.1 Central laboratory vs. point-of-care
The vast majority of cardiac troponin assays that are run on automated platforms in the central laboratory are sensitive (i.e. allow for detection of cardiac troponin in ∼20–50% of healthy individuals) or high-sensitivity (detection in ∼50–95% of healthy individuals) assays. High-sensitivity assays are recommended over less sensitive ones, as they provide higher diagnostic accuracy at identical low cost.1,3,4,6–8,10–13,29,30,33,35,36
The majority of currently used point-of-care tests (POCTs) cannot be considered sensitive or high-sensitivity assays41. Therefore, the obvious advantage of POCTs, namely the shorter turn-around time, is counterbalanced by lower sensitivity, lower diagnostic accuracy, and lower negative predictive value (NPV). Overall, automated assays have been more thoroughly evaluated than POCTs and seem to be preferable at this point in time.1,3,4,6–8,10–13,29,30,33,35,36
As these techniques continue to improve, and performance characteristics are both assay and hospital dependent, it is important to re-evaluate this preference once extensively validated high-sensitivity POCTs become clinically available.42 The first hs-cTn I POCTs have recently been shown to provide comparable performance characteristics to that of central laboratory hs-cTn I/T assays.43,44
Value of high-sensitivity cardiac troponin. hs-cTn assays (right) are reported in ng/L and provide identical information as conventional assays (left, reported in μg/L) if the concentration is substantially elevated, e.g. above 100 ng/L. In contrast, only hs-cTn allows a precise differentiation between ‘normal’ and mildly elevated. Therefore, hs-cTn detects a relevant proportion of patients with previously undetectable cardiac troponin concentrations with the conventional assay who have hs-cTn concentrations above the 99th percentile possibly related to AMI. ??? = unknown due to the inability of the assay to measure in the normal range;6–8,10–13,29–31 AMI = acute myocardial infarction; CoV = coefficient of variation; hs-cTn = high-sensitivity cardiac troponin; POCT = point-of-care test. aThe limit of detection varies among the different hs-cTn assays between 1 ng/L and 5 ng/L. Similarly, the 99th percentile varies among the different hs-cTn assays, mainly being between 10 ng/L and 20 ng/L. Listen to the audio guide of this figure online.
Many cardiac pathologies other than MI also result in cardiomyocyte injury and, therefore, cardiac troponin elevations (Table 4). Tachyarrhythmias, heart failure, hypertensive emergencies, critical illness, myocarditis, Takotsubo syndrome, and valvular heart disease are the most frequent ones. Most often in elderly patients with renal dysfunction, elevations in cardiac troponin should not be primarily attributed to impaired clearance and considered harmless, as cardiac conditions such as chronic coronary syndromes (CCS) or hypertensive heart disease seem to be the most important contributor to cardiac troponin elevation in this setting.35,45 Other life-threatening conditions presenting with chest pain, such as aortic dissection and pulmonary embolism, may also result in elevated cardiac troponin concentrations and should be considered as differential diagnoses (Table 4).
Conditions other than acute type 1 myocardial infarction associated with cardiomyocyte injury (= cardiac troponin elevation)
| Tachyarrhythmias |
| Heart failure |
| Hypertensive emergencies |
| Critical illness (e.g. shock/sepsis/burns) |
| Myocarditisa |
| Takotsubo syndrome |
| Valvular heart disease (e.g. aortic stenosis) |
| Aortic dissection |
| Pulmonary embolism, pulmonary hypertension |
| Renal dysfunction and associated cardiac disease |
| Acute neurological event (e.g. stroke or subarachnoid haemorrhage) |
| Cardiac contusion or cardiac procedures (CABG, PCI, ablation, pacing, cardioversion, or endomyocardial biopsy) |
| Hypo- and hyperthyroidism |
| Infiltrative diseases (e.g. amyloidosis, haemochromatosis, sarcoidosis, scleroderma) |
| Myocardial drug toxicity or poisoning (e.g. doxorubicin, 5-fluorouracil, herceptin, snake venoms) |
| Extreme endurance efforts |
| Rhabdomyolysis |
| Tachyarrhythmias |
| Heart failure |
| Hypertensive emergencies |
| Critical illness (e.g. shock/sepsis/burns) |
| Myocarditisa |
| Takotsubo syndrome |
| Valvular heart disease (e.g. aortic stenosis) |
| Aortic dissection |
| Pulmonary embolism, pulmonary hypertension |
| Renal dysfunction and associated cardiac disease |
| Acute neurological event (e.g. stroke or subarachnoid haemorrhage) |
| Cardiac contusion or cardiac procedures (CABG, PCI, ablation, pacing, cardioversion, or endomyocardial biopsy) |
| Hypo- and hyperthyroidism |
| Infiltrative diseases (e.g. amyloidosis, haemochromatosis, sarcoidosis, scleroderma) |
| Myocardial drug toxicity or poisoning (e.g. doxorubicin, 5-fluorouracil, herceptin, snake venoms) |
| Extreme endurance efforts |
| Rhabdomyolysis |
Bold = most frequent conditions.
CABG = coronary artery bypass graft(ing); PCI = percutaneous coronary intervention.
Includes myocardial extension of endocarditis or pericarditis.
Conditions other than acute type 1 myocardial infarction associated with cardiomyocyte injury (= cardiac troponin elevation)
| Tachyarrhythmias |
| Heart failure |
| Hypertensive emergencies |
| Critical illness (e.g. shock/sepsis/burns) |
| Myocarditisa |
| Takotsubo syndrome |
| Valvular heart disease (e.g. aortic stenosis) |
| Aortic dissection |
| Pulmonary embolism, pulmonary hypertension |
| Renal dysfunction and associated cardiac disease |
| Acute neurological event (e.g. stroke or subarachnoid haemorrhage) |
| Cardiac contusion or cardiac procedures (CABG, PCI, ablation, pacing, cardioversion, or endomyocardial biopsy) |
| Hypo- and hyperthyroidism |
| Infiltrative diseases (e.g. amyloidosis, haemochromatosis, sarcoidosis, scleroderma) |
| Myocardial drug toxicity or poisoning (e.g. doxorubicin, 5-fluorouracil, herceptin, snake venoms) |
| Extreme endurance efforts |
| Rhabdomyolysis |
| Tachyarrhythmias |
| Heart failure |
| Hypertensive emergencies |
| Critical illness (e.g. shock/sepsis/burns) |
| Myocarditisa |
| Takotsubo syndrome |
| Valvular heart disease (e.g. aortic stenosis) |
| Aortic dissection |
| Pulmonary embolism, pulmonary hypertension |
| Renal dysfunction and associated cardiac disease |
| Acute neurological event (e.g. stroke or subarachnoid haemorrhage) |
| Cardiac contusion or cardiac procedures (CABG, PCI, ablation, pacing, cardioversion, or endomyocardial biopsy) |
| Hypo- and hyperthyroidism |
| Infiltrative diseases (e.g. amyloidosis, haemochromatosis, sarcoidosis, scleroderma) |
| Myocardial drug toxicity or poisoning (e.g. doxorubicin, 5-fluorouracil, herceptin, snake venoms) |
| Extreme endurance efforts |
| Rhabdomyolysis |
Bold = most frequent conditions.
CABG = coronary artery bypass graft(ing); PCI = percutaneous coronary intervention.
Includes myocardial extension of endocarditis or pericarditis.
3.3.2.2 Other biomarkers
Among the multitude of additional biomarkers evaluated for the diagnosis of NSTE-ACS, only CK-MB, myosin-binding protein C,46 and copeptin47–58 may have clinical relevance in specific clinical settings when used in combination with cardiac troponin T/I. Compared with cardiac troponin, CK-MB shows a more rapid decline after MI and may provide added value for the timing of myocardial injury and the detection of early reinfarction.1 However, it is important to highlight that little is known on how to best diagnose early reinfarction. Detailed clinical assessment including chest pain characteristics (same characteristics as index event), 12-lead ECG for the detection of new ST-segment changes or T-wave inversion, as well as serial measurement of cardiac troponin T/I and CK/CK-MB is recommended. Myosin-binding protein C is more abundant than cardiac troponin and may therefore provide value as an alternative to, or in combination with, cardiac troponin.46 Assessment of copeptin, the C-terminal part of the vasopressin prohormone, may quantify the endogenous stress level in multiple medical conditions including MI. As the level of endogenous stress appears to be high at the onset of MI in most patients, the added value of copeptin to conventional (less sensitive) cardiac troponin assays is substantial.49,50,53 Therefore, the routine use of copeptin as an additional biomarker for the early rule-out of MI should be considered in the increasingly uncommon setting where hs-cTn assays are not available. However, copeptin does not have relevant added value for institutions using one of the well-validated hs-cTn-based rapid protocols in the early diagnosis of MI.47,48,51,52,54–58 Other widely available laboratory variables, such as estimated glomerular filtration rate (eGFR), glucose, and B-type natriuretic peptide (BNP) provide incremental prognostic information and may therefore help in risk stratification.59 The determination of D-dimer is recommended in outpatients/emergency department patients with low or intermediate clinical probability, or those that are unlikely to have pulmonary embolism, to reduce the need for unnecessary imaging and irradiation. D-dimers are key diagnostic elements whenever pulmonary embolism is suspected.32,60
3.3.3 Rapid ‘rule-in’ and ‘rule-out’ algorithms
Due to the higher sensitivity and diagnostic accuracy for the detection of MI at presentation, the time interval to the second cardiac troponin assessment can be shortened with the use of hs-cTn assays. This seems to substantially reduce the delay to diagnosis, translating into shorter stays in the emergency department and lower costs.11,56,61–66 It is recommended to use the 0 h/1 h algorithm (best option, blood draw at 0 h and 1 h) or the 0 h/2 h algorithm (second-best option, blood draw at 0 h and 2 h Figure 3). These have been derived and well-validated in large multicentre diagnostic studies using central adjudication of the final diagnosis for all currently available hs-cTn assays.33,35,36,39,67–69 Optimal thresholds for rule-out were selected to allow for a minimal sensitivity and NPV of 99%. Optimal thresholds for rule-in were selected to allow for a minimal positive predictive value (PPV) of 70%. The algorithms were developed in large derivation cohorts and then validated in large independent validation cohorts. As an alternative, the previous European Society of Cardiology (ESC) 0 h/3 h algorithm70 should be considered.1 However, three recent large diagnostic studies have suggested that the ESC 0 h/3 h algorithm seems to balance efficacy and safety less well in comparison to more rapid protocols using lower rule-out concentrations including the ESC 0 h/1 h algorithm.71–73 Moreover, the very high safety and high efficacy of applying the ESC 0 h/1 h algorithm has recently been confirmed in three real-life implementation studies, including one randomized controlled trial (RCT) .66,73,74
The 0 h/1 h and 0 h/2 h algorithms rely on two concepts: first, hs-cTn is a continuous variable and the probability of MI increases with increasing hs-cTn values,35,36,39,68,69,75,76 second, early absolute changes of the levels within 1 h or 2 h can be used as surrogates for absolute changes over 3 h or 6 h and provide incremental diagnostic value to the cardiac troponin assessment at presentation.33,35,36,39,68,69,75,76 The cut-off concentrations within the 0 h/1 h and 0 h/2 h algorithms are assay specific (Table 5).33,35,36,39,68,69,75,76 The NPV for MI in patients assigned ‘rule-out’ exceeded 99% in several large validation cohorts.35,36,39,68,69,77 Used in conjunction with clinical and ECG findings, the 0 h/1 h and 0 h/2 h algorithm will allow the identification of appropriate candidates for early discharge and outpatient management. Even after the rule-out of MI, elective non-invasive or invasive imaging may be indicated according to clinical assessment. Invasive coronary angiography (ICA) will still be the best option in patients with very high clinical likelihood of unstable angina, even after NSTEMI has been ruled out. In contrast, stress testing with imaging or coronary computed tomography angiography (CCTA) will be the best option in patients with low-to-modest clinical likelihood of unstable angina. No testing is necessary in patients with a clear alternative diagnosis.
The PPV for MI in patients meeting the ‘rule-in’ criteria is about 70–75%.35,36,39,69 Most of the ‘rule-in’ patients with diagnoses other than MI did have conditions that usually still require ICA or cardiac magnetic resonance (CMR) imaging for accurate diagnosis, including Takotsubo syndrome and myocarditis.35,36,39,68,69,75,76 Therefore, the vast majority of patients triaged towards the rule-in group are candidates for early ICA and admission to a coronary care unit (CCU).
These algorithms should always be integrated with a detailed clinical assessment and 12-lead ECG, and repeat blood sampling is mandatory in case of ongoing or recurrent chest pain.
0 h/1 h rule-out and rule-in algorithm using high-sensitivity cardiac troponin assays in haemodynamically stable patients presenting with suspected non-ST-segment elevation acute coronary syndrome to the emergency department. 0 h and 1 h refer to the time from first blood test. NSTEMI can be ruled out at presentation if the hs-cTn concentration is very low. NSTEMI can also be ruled out by the combination of low baseline levels and the lack of a relevant increase within 1 h (no 1hΔ). Patients have a high likelihood of NSTEMI if the hs-cTn concentration at presentation is at least moderately elevated or hs-cTn concentrations show a clear rise within the first hour (1hΔ).1,6–8,10–13,29–31,33 Cut-offs are assay specific (see Table 3) and derived to meet predefined criteria for sensitivity and specificity for NSTEMI. CCU = coronary care unit; CCTA = coronary computed tomography angiography; CPO = chest pain onset; hs-cTn = high-sensitivity cardiac troponin; NSTE-ACS = non-ST-segment elevation acute coronary syndrome; NSTEMI = non-ST-segment elevation myocardial infarction. aOnly applicable if CPO >3 h. Listen to the audio guide of this figure online.
The same concept applies to the 0 h/2 h algorithm. Cut-off levels are assay-specific and shown in Table 5. Cut-off levels for other hs-cTn assays are in development.
Assay specific cut-off levels in ng/l within the 0 h/1 h and 0 h/2 h algorithms
| 0 h/1 h algorithm | Very low | Low | No 1hΔ | High | 1hΔ |
| hs-cTn T (Elecsys; Roche) | <5 | <12 | <3 | ≥52 | ≥5 |
| hs-cTn I (Architect; Abbott) | <4 | <5 | <2 | ≥64 | ≥6 |
| hs-cTn I (Centaur; Siemens) | <3 | <6 | <3 | ≥120 | ≥12 |
| hs-cTn I (Access; Beckman Coulter) | <4 | <5 | <4 | ≥50 | ≥15 |
| hs-cTn I (Clarity; Singulex) | <1 | <2 | <1 | ≥30 | ≥6 |
| hs-cTn I (Vitros; Clinical Diagnostics) | <1 | <2 | <1 | ≥40 | ≥4 |
| hs-cTn I (Pathfast; LSI Medience) | <3 | <4 | <3 | ≥90 | ≥20 |
| hs-cTn I (TriageTrue; Quidel) | <4 | <5 | <3 | ≥60 | ≥8 |
| 0 h/2 h algorithm | Very low | Low | No 2hΔ | High | 2hΔ |
| hs-cTn T (Elecsys; Roche) | <5 | <14 | <4 | ≥52 | ≥10 |
| hs-cTn I (Architect; Abbott) | <4 | <6 | <2 | ≥64 | ≥15 |
| hs-cTn I (Centaur; Siemens) | <3 | <8 | <7 | ≥120 | ≥20 |
| hs-cTn I (Access; Beckman Coulter) | <4 | <5 | <5 | ≥50 | ≥20 |
| hs-cTn I (Clarity; Singulex) | <1 | TBD | TBD | ≥30 | TBD |
| hs-cTn I (Vitros; Clinical Diagnostics) | <1 | TBD | TBD | ≥40 | TBD |
| hs-cTn I (Pathfast; LSI Medience) | <3 | TBD | TBD | ≥90 | TBD |
| hs-cTn I (TriageTrue; Quidel) | <4 | TBD | TBD | ≥60 | TBD |
| 0 h/1 h algorithm | Very low | Low | No 1hΔ | High | 1hΔ |
| hs-cTn T (Elecsys; Roche) | <5 | <12 | <3 | ≥52 | ≥5 |
| hs-cTn I (Architect; Abbott) | <4 | <5 | <2 | ≥64 | ≥6 |
| hs-cTn I (Centaur; Siemens) | <3 | <6 | <3 | ≥120 | ≥12 |
| hs-cTn I (Access; Beckman Coulter) | <4 | <5 | <4 | ≥50 | ≥15 |
| hs-cTn I (Clarity; Singulex) | <1 | <2 | <1 | ≥30 | ≥6 |
| hs-cTn I (Vitros; Clinical Diagnostics) | <1 | <2 | <1 | ≥40 | ≥4 |
| hs-cTn I (Pathfast; LSI Medience) | <3 | <4 | <3 | ≥90 | ≥20 |
| hs-cTn I (TriageTrue; Quidel) | <4 | <5 | <3 | ≥60 | ≥8 |
| 0 h/2 h algorithm | Very low | Low | No 2hΔ | High | 2hΔ |
| hs-cTn T (Elecsys; Roche) | <5 | <14 | <4 | ≥52 | ≥10 |
| hs-cTn I (Architect; Abbott) | <4 | <6 | <2 | ≥64 | ≥15 |
| hs-cTn I (Centaur; Siemens) | <3 | <8 | <7 | ≥120 | ≥20 |
| hs-cTn I (Access; Beckman Coulter) | <4 | <5 | <5 | ≥50 | ≥20 |
| hs-cTn I (Clarity; Singulex) | <1 | TBD | TBD | ≥30 | TBD |
| hs-cTn I (Vitros; Clinical Diagnostics) | <1 | TBD | TBD | ≥40 | TBD |
| hs-cTn I (Pathfast; LSI Medience) | <3 | TBD | TBD | ≥90 | TBD |
| hs-cTn I (TriageTrue; Quidel) | <4 | TBD | TBD | ≥60 | TBD |
These cut-offs apply irrespective of age and renal function. Optimized cut-offs for patients above 75 years of age and patients with renal dysfunction have been evaluated, but not consistently shown to provide better balance between safety and efficacy as compared to these universal cut-offs.35,36,69 The algorithms for additional assays are in development.
Assay specific cut-off levels in ng/l within the 0 h/1 h and 0 h/2 h algorithms
| 0 h/1 h algorithm | Very low | Low | No 1hΔ | High | 1hΔ |
| hs-cTn T (Elecsys; Roche) | <5 | <12 | <3 | ≥52 | ≥5 |
| hs-cTn I (Architect; Abbott) | <4 | <5 | <2 | ≥64 | ≥6 |
| hs-cTn I (Centaur; Siemens) | <3 | <6 | <3 | ≥120 | ≥12 |
| hs-cTn I (Access; Beckman Coulter) | <4 | <5 | <4 | ≥50 | ≥15 |
| hs-cTn I (Clarity; Singulex) | <1 | <2 | <1 | ≥30 | ≥6 |
| hs-cTn I (Vitros; Clinical Diagnostics) | <1 | <2 | <1 | ≥40 | ≥4 |
| hs-cTn I (Pathfast; LSI Medience) | <3 | <4 | <3 | ≥90 | ≥20 |
| hs-cTn I (TriageTrue; Quidel) | <4 | <5 | <3 | ≥60 | ≥8 |
| 0 h/2 h algorithm | Very low | Low | No 2hΔ | High | 2hΔ |
| hs-cTn T (Elecsys; Roche) | <5 | <14 | <4 | ≥52 | ≥10 |
| hs-cTn I (Architect; Abbott) | <4 | <6 | <2 | ≥64 | ≥15 |
| hs-cTn I (Centaur; Siemens) | <3 | <8 | <7 | ≥120 | ≥20 |
| hs-cTn I (Access; Beckman Coulter) | <4 | <5 | <5 | ≥50 | ≥20 |
| hs-cTn I (Clarity; Singulex) | <1 | TBD | TBD | ≥30 | TBD |
| hs-cTn I (Vitros; Clinical Diagnostics) | <1 | TBD | TBD | ≥40 | TBD |
| hs-cTn I (Pathfast; LSI Medience) | <3 | TBD | TBD | ≥90 | TBD |
| hs-cTn I (TriageTrue; Quidel) | <4 | TBD | TBD | ≥60 | TBD |
| 0 h/1 h algorithm | Very low | Low | No 1hΔ | High | 1hΔ |
| hs-cTn T (Elecsys; Roche) | <5 | <12 | <3 | ≥52 | ≥5 |
| hs-cTn I (Architect; Abbott) | <4 | <5 | <2 | ≥64 | ≥6 |
| hs-cTn I (Centaur; Siemens) | <3 | <6 | <3 | ≥120 | ≥12 |
| hs-cTn I (Access; Beckman Coulter) | <4 | <5 | <4 | ≥50 | ≥15 |
| hs-cTn I (Clarity; Singulex) | <1 | <2 | <1 | ≥30 | ≥6 |
| hs-cTn I (Vitros; Clinical Diagnostics) | <1 | <2 | <1 | ≥40 | ≥4 |
| hs-cTn I (Pathfast; LSI Medience) | <3 | <4 | <3 | ≥90 | ≥20 |
| hs-cTn I (TriageTrue; Quidel) | <4 | <5 | <3 | ≥60 | ≥8 |
| 0 h/2 h algorithm | Very low | Low | No 2hΔ | High | 2hΔ |
| hs-cTn T (Elecsys; Roche) | <5 | <14 | <4 | ≥52 | ≥10 |
| hs-cTn I (Architect; Abbott) | <4 | <6 | <2 | ≥64 | ≥15 |
| hs-cTn I (Centaur; Siemens) | <3 | <8 | <7 | ≥120 | ≥20 |
| hs-cTn I (Access; Beckman Coulter) | <4 | <5 | <5 | ≥50 | ≥20 |
| hs-cTn I (Clarity; Singulex) | <1 | TBD | TBD | ≥30 | TBD |
| hs-cTn I (Vitros; Clinical Diagnostics) | <1 | TBD | TBD | ≥40 | TBD |
| hs-cTn I (Pathfast; LSI Medience) | <3 | TBD | TBD | ≥90 | TBD |
| hs-cTn I (TriageTrue; Quidel) | <4 | TBD | TBD | ≥60 | TBD |
These cut-offs apply irrespective of age and renal function. Optimized cut-offs for patients above 75 years of age and patients with renal dysfunction have been evaluated, but not consistently shown to provide better balance between safety and efficacy as compared to these universal cut-offs.35,36,69 The algorithms for additional assays are in development.
3.3.4 Observe
Patients who do not qualify for ‘rule-out’ or ‘rule-in’, are assigned to observe. They represent a heterogeneous group that usually requires a third measurement of cardiac troponin at 3 h and echocardiography as the next steps.85 ICA should be considered in patients for whom there is a high degree of clinical suspicion of NSTE-ACS (e.g. relevant increase in cardiac troponin from presentation to 3 h), while in patients with low-to-intermediate likelihood for this condition according to clinical judgment, non-invasive imaging using CCTA or stress testing [stress echocardiography, positron emission tomography, single-photon-emission tomography (SPECT), or CMR for the detection of ACS features (oedema, late gadolinium enhancement, perfusion defect, etc.)] should be considered after discharge from the emergency department to the ward. No further diagnostic testing is indicated when alternative conditions, such as rapid ventricular rate response to atrial fibrillation (AF) or hypertensive emergency, have been identified.
3.3.4.1 Caveats of using rapid algorithms. When using any algorithm, three main caveats apply
Algorithms should only be used in conjunction with all available clinical information, including detailed assessment of chest pain characteristics and ECG.
The ESC 0 h/1h and 0 h/2 h algorithms apply to all patients irrespective of chest pain onset. The safety (as quantified by the NPV) and sensitivity are very high (>99%), including in the subgroup of patients presenting very early (e.g. <2 h).69 However, due to the time dependency of troponin release and the only moderate number of patients presenting <1 h after chest pain onset in previous studies, obtaining an additional cardiac troponin concentration at 3 h in patients presenting <1 h and triaged towards rule-out should be considered.
As late increases in cardiac troponin have been described in ∼1% of patients, serial cardiac troponin testing should be pursued if the clinical suspicion remains high or whenever the patient develops recurrent chest pain.35,36,39,68,69,75,76,86
3.3.4.2 Confounders of cardiac troponin concentration. In patients presenting with suspected NSTE-ACS, beyond the presence or absence of MI, four clinical variables affect hs-cTn concentrations:35,36,39,69,79,87–93
Age (to a large extent as a surrogate for pre-existing cardiac disease).
Renal dysfunction (to a large extent as a surrogate for pre-existing cardiac disease).
Time from chest pain onset.
Sex.
The effect of age (differences in concentration between healthy very young vs. healthy very old individuals up to 300%), renal dysfunction (differences in concentration between otherwise healthy patients with very high vs. very low eGFR up to 300%), and chest pain onset (>300%) is substantial, and modest for sex (≈40%).11,35,36,39,69,79,88–93 Until information technology tools that allow the incorporation of the effect of all four variables are available, the use of uniform cut-off concentrations should remain the standard of care in the early diagnosis of MI.35,36,39,68,69,75,76
3.3.4.3 Practical guidance on how to implement the European Society of Cardiology 0 h/1 h algorithm
In order to maximize the safety and feasibility of the process, the nursing team should, in general, obtain blood samples for hs-cTn at 0 h and 1 h irrespective of other clinical details and pending results. This introduces unnecessary cardiac troponin measurements in perhaps 10–15% of patients with very low 0 h concentrations and chest pain onset >3 h, but substantially facilitates the process and thereby further increases patient safety. Documentation of the time of the 0 h blood draw allows exact determination of the time window (± 10 min) of the 1 h blood draw. If the 1 h (± 10 min) blood draw was not feasible, then blood should be drawn at 2 h and the ESC 0 h/2 h algorithm applied.
3.3.4.4 Avoiding misunderstandings: time to decision = time of blood draw + turn-around time
The use of the ESC 0 h/1 h algorithm is irrespective of the local turn-around time. 0 h and 1 h refer to the time point at which blood is taken (Figure 4).
The clinical and economic benefit of the ESC 0 h/1 h algorithm vs. the ESC 0 h/3 h algorithm or other algorithms with the second blood draw later than 1 h is therefore independent of the local turn-around time.61
Timing of the blood draws and clinical decisions when using the European Society of Cardiology 0 h/1 h algorithm. 0 h and 1 h refer to the time points at which blood is taken. The turn-around time is the time period from blood draw to reporting back the results to the clinician. It is usually about 1 h using an automated platform in the central laboratory. It includes transport of the blood tube to the lab, scanning of the probe, centrifugation, putting plasma on the automated platform, the analysis itself, and the reporting of the test result to the hospital information technology/electronic patient record. The turn-around time is identical whether using a hs-cTn assay vs. a conventional assay, as long as both are run on an automated platform. Adding the local turn-around time to the time of blood draw determines the earliest time point for clinical decision making based on hs-cTn concentrations. e.g. for the 0 h time point, time to decision is at 1 h if the local turn-around time is 1 h. For the blood drawn at 1 h, the results are reported back at 2 h (1 h + 1 h) if the local turn-around time is 1 h. Relevant 1 h changes are assay dependent and listed in Table 3. CPO = chest pain onset; CPR = cardiopulmonary resuscitation; ECG = electrocardiogram/electrocardiography; hs-cTn = high-sensitivity cardiac troponin; MACE = major adverse cardiovascular events; MI = myocardial infarction. Listen to the audio guide of this figure online.
3.3.5 Non-invasive imaging
3.3.5.1 Functional evaluation
Transthoracic echocardiography should be routinely available in emergency rooms and chest pain units and performed/interpreted by trained physicians in all patients during hospitalization for NSTE-ACS. This imaging modality is useful to identify abnormalities suggestive of myocardial ischaemia or necrosis (i.e. segmental hypokinesia or akinesia). In the absence of significant wall motion abnormalities, impaired myocardial perfusion detected by contrast echocardiography or reduced regional function using strain and strain rate imaging might improve the diagnostic and prognostic value of conventional echocardiography.94–96 Moreover, echocardiography can help in detecting alternative pathologies associated with chest pain, such as acute aortic dissection, pericardial effusion, aortic valve stenosis, hypertrophic cardiomyopathy, mitral valve prolapse, or right ventricular dilatation suggestive of acute pulmonary embolism. Similarly, echocardiography is the diagnostic tool of choice for patients with haemodynamic instability of suspected cardiac origin.96,97 Evaluation of left ventricular (LV) systolic function, at the latest by the time of hospital discharge, is important to estimate prognosis, and echocardiography (as well as other imaging modalities) can provide this information.
In patients without ischaemic changes on 12-lead ECGs and normal hs-cTn, who are free from chest pain for several hours, stress imaging can be performed during hospitalization or shortly after discharge. Stress imaging is preferred over exercise ECG due to its greater diagnostic accuracy.98 Various studies have shown that normal exercise or dobutamine or dipyridamole stress echocardiograms have high NPV for ischaemia and are associated with excellent patient outcomes.99,100 Moreover, stress echocardiography has demonstrated superior prognostic value over exercise ECG.101 If the acoustic window is not adequate to assess regional wall motion abnormalities, the use of echocardiographic contrast is recommended to improve the accuracy of such an assessment and facilitate the detection of ischaemia.98,101–103
CMR can assess both perfusion and wall motion abnormalities, and patients presenting with acute chest pain with a normal stress CMR have an excellent short- and mid-term prognosis.104 Additionally, CMR permits detection of scar tissue (using late gadolinium enhancement) and can differentiate this from recent infarction (using T2-weighted imaging to delineate myocardial oedema).98 Moreover, CMR can facilitate the differential diagnosis between infarction, myocarditis, or Takotsubo syndrome, among others.98 In a recent randomized trial in patients with unclear NSTEMI diagnosis, upfront imaging with CMR reduced the need for ICA and provided an alternative diagnosis in a relevant proportion of patients.105
Similarly, SPECT has been shown to be useful for the risk stratification of patients with acute chest pain suggestive of ACS. Resting myocardial scintigraphy, by detecting fixed perfusion defects suggestive of myocardial necrosis, can be helpful for the initial triage of patients presenting with chest pain without ECG changes or elevated cardiac troponins.98 Combined stress–rest imaging and/or stress-only imaging may further enhance assessment of ischaemia, while a normal study is associated with an excellent outcome.106,107 Stress–rest imaging modalities are usually not widely available on 24 h service and some (e.g. SPECT) are associated with substantial radiation exposure.
3.3.5.2 Anatomical evaluation
CCTA allows visualization of the coronary arteries and a normal scan excludes CAD. CCTA has a high NPV to exclude ACS (by excluding CAD) and an excellent outcome in patients presenting to the emergency department with low-to-intermediate pre-test probability for ACS and a normal CCTA.108 Seven RCTs have tested CCTA vs. usual care in the triage of low-to-intermediate-risk patients presenting with acute chest pain to emergency departments without signs of ischaemia on ECG and normal cardiac troponins.109 However, the majority of studies used only conventional, less sensitive assays.110–113 At a follow-up of 1–6 months, there were no deaths, and a meta-analysis demonstrated comparable outcomes with the two approaches (i.e. no difference in the incidence of MI, post-discharge emergency department visits, or re-hospitalizations) and showed that CCTA was associated with a reduction in emergency department costs and length of stay.114 However, none of these studies used hs-cTn assays, which also reduce hospital stay. In a randomized study, in which the standard of care included hs-cTn, CCTA was no longer able to improve patient flow.115 It was also noted that CCTA was associated with an increase in the use of invasive angiography.114 In contrast, in a recent randomized trial of unclear NSTEMI diagnosis, upfront imaging with CCTA reduced the need for ICA105 Similar results were observed in a sub-analysis of the Very EaRly vs Deferred Invasive evaluation using Computerized Tomography (VERDICT) trial, where upfront CCTA in NSTE-ACS patients had an NPV of 90.9%.116 However, a relatively large patient group had to be excluded for specific reasons and an NPV of 90.9% is not entirely perfect.116 Accordingly, CCTA can be used to exclude CAD and is thus less useful in patients with known CAD. Other factors limiting CCTA include severe calcifications (high calcium score) and elevated or irregular heart rate; in addition, a 24 h service is currently not widely available. Finally, the use of CCTA in the acute setting in patients with stents or previous CABG has not been validated. Importantly, computed tomography (CT) imaging can effectively exclude other causes of acute chest pain that, if untreated, are associated with high mortality, namely pulmonary embolism and aortic dissection.
3.4 Differential diagnosis
Among unselected patients presenting with acute chest pain to the emergency department, disease prevalence can be expected to be the following: 5–10% STEMI, 15–20% NSTEMI, 10% unstable angina, 15% other cardiac conditions, and 50% non-cardiac diseases.35,36,39,69,79,87–93 Several cardiac and non-cardiac conditions may mimic NSTE-ACS (Table 6).
Conditions that should always be considered in the differential diagnosis of NSTE-ACS because they are potentially life-threatening but also treatable include aortic dissection, pulmonary embolism, and tension pneumothorax. Echocardiography should be performed urgently in all patients with haemodynamic instability of suspected cardiovascular origin. Takotsubo syndrome has recently been observed more often as a differential diagnosis and usually requires coronary angiography to rule out ACS.117
Differential diagnoses of acute coronary syndromes in the setting of acute chest pain
| Cardiac . | Pulmonary . | Vascular . | Gastro-intestinal . | Orthopaedic . | Other . |
|---|---|---|---|---|---|
| Myopericarditis | Pulmonary embolism | Aortic dissection | Oesophagitis, reflux, or spasm | Musculoskeletal disorders | Anxiety disorders |
| Cardiomyopathiesa | (Tension)- pneumothorax | Symptomatic aortic aneurysm | Peptic ulcer, gastritis | Chest trauma | Herpes zoster |
| Tachyarrhythmias | Bronchitis, pneumonia | Stroke | Pancreatitis | Muscle injury/inflammation | Anaemia |
| Acute heart failure | Pleuritis | Cholecystitis | Costochondritis | ||
| Hypertensive emergencies | Cervical spine pathologies | ||||
| Aortic valve stenosis | |||||
| Takotsubo syndrome | |||||
| Coronary spasm | |||||
| Cardiac trauma |
| Cardiac . | Pulmonary . | Vascular . | Gastro-intestinal . | Orthopaedic . | Other . |
|---|---|---|---|---|---|
| Myopericarditis | Pulmonary embolism | Aortic dissection | Oesophagitis, reflux, or spasm | Musculoskeletal disorders | Anxiety disorders |
| Cardiomyopathiesa | (Tension)- pneumothorax | Symptomatic aortic aneurysm | Peptic ulcer, gastritis | Chest trauma | Herpes zoster |
| Tachyarrhythmias | Bronchitis, pneumonia | Stroke | Pancreatitis | Muscle injury/inflammation | Anaemia |
| Acute heart failure | Pleuritis | Cholecystitis | Costochondritis | ||
| Hypertensive emergencies | Cervical spine pathologies | ||||
| Aortic valve stenosis | |||||
| Takotsubo syndrome | |||||
| Coronary spasm | |||||
| Cardiac trauma |
Bold = common and/or important differential diagnoses.
Dilated, hypertrophic and restrictive cardiomyopathies may cause angina or chest discomfort.
Differential diagnoses of acute coronary syndromes in the setting of acute chest pain
| Cardiac . | Pulmonary . | Vascular . | Gastro-intestinal . | Orthopaedic . | Other . |
|---|---|---|---|---|---|
| Myopericarditis | Pulmonary embolism | Aortic dissection | Oesophagitis, reflux, or spasm | Musculoskeletal disorders | Anxiety disorders |
| Cardiomyopathiesa | (Tension)- pneumothorax | Symptomatic aortic aneurysm | Peptic ulcer, gastritis | Chest trauma | Herpes zoster |
| Tachyarrhythmias | Bronchitis, pneumonia | Stroke | Pancreatitis | Muscle injury/inflammation | Anaemia |
| Acute heart failure | Pleuritis | Cholecystitis | Costochondritis | ||
| Hypertensive emergencies | Cervical spine pathologies | ||||
| Aortic valve stenosis | |||||
| Takotsubo syndrome | |||||
| Coronary spasm | |||||
| Cardiac trauma |
| Cardiac . | Pulmonary . | Vascular . | Gastro-intestinal . | Orthopaedic . | Other . |
|---|---|---|---|---|---|
| Myopericarditis | Pulmonary embolism | Aortic dissection | Oesophagitis, reflux, or spasm | Musculoskeletal disorders | Anxiety disorders |
| Cardiomyopathiesa | (Tension)- pneumothorax | Symptomatic aortic aneurysm | Peptic ulcer, gastritis | Chest trauma | Herpes zoster |
| Tachyarrhythmias | Bronchitis, pneumonia | Stroke | Pancreatitis | Muscle injury/inflammation | Anaemia |
| Acute heart failure | Pleuritis | Cholecystitis | Costochondritis | ||
| Hypertensive emergencies | Cervical spine pathologies | ||||
| Aortic valve stenosis | |||||
| Takotsubo syndrome | |||||
| Coronary spasm | |||||
| Cardiac trauma |
Bold = common and/or important differential diagnoses.
Dilated, hypertrophic and restrictive cardiomyopathies may cause angina or chest discomfort.
Chest X-ray is recommended in all patients in whom NSTE-ACS is considered unlikely in order to detect pneumonia, pneumothorax, rib fractures, or other thoracic disorders. Stroke may be accompanied by ECG changes, myocardial wall motion abnormalities, and cardiomyocyte injury (= increase in cardiac troponin concentrations). The majority of patients presenting to the emergency department with acute chest pain have non-cardiac conditions causing the chest discomfort.35,36,39,69,79,87–93 In many instances, the pain is musculoskeletal and is therefore benign, self-limiting, and does not require hospitalization. Chest pain characteristics help – to some extent – in the early identification of these patients.
Recommendations for diagnosis, risk stratification, imaging, and rhythm monitoring in patients with suspected non-ST-segment elevation acute coronary syndrome
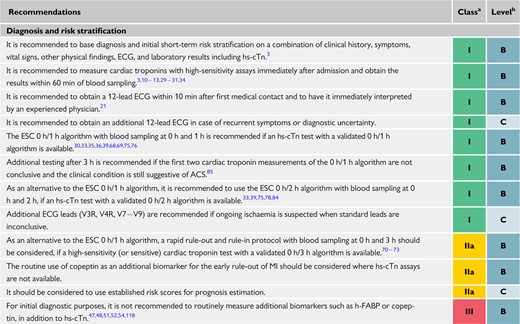 |
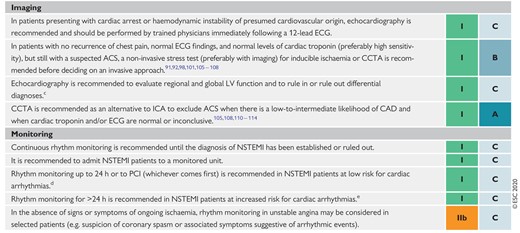 |
 |
 |
0 h = time of first blood test; 1 h, 2 h, 3 h = 1, 2, or 3 h after the first blood test.
ACS = acute coronary syndromes; CAD = coronary artery disease; CCTA = coronary computed tomography angiography; ECG = electrocardiogram/electrocardiography; ESC = European Society of Cardiology; GRACE = Global Registry of Acute Coronary Events; h-FABP = heart-type fatty acid-binding protein; hs-cTn = high-sensitivity cardiac troponin; ICA = invasive coronary angiography; LV = left ventricular; LVEF = left ventricular ejection fraction; NSTEMI = non-ST-segment elevation myocardial infarction; PCI = percutaneous coronary intervention.
Class of recommendation.
Level of evidence.
Does not apply to patients discharged the same day in whom NSTEMI has been ruled out.
If none of the following criteria: haemodynamically unstable, major arrhythmias, LVEF <40%, failed reperfusion, additional critical coronary stenoses of major vessels, complications related to percutaneous revascularization, or GRACE risk score >140 if assessed.
If one or more of the above criteria are present.
Recommendations for diagnosis, risk stratification, imaging, and rhythm monitoring in patients with suspected non-ST-segment elevation acute coronary syndrome
 |
 |
 |
 |
0 h = time of first blood test; 1 h, 2 h, 3 h = 1, 2, or 3 h after the first blood test.
ACS = acute coronary syndromes; CAD = coronary artery disease; CCTA = coronary computed tomography angiography; ECG = electrocardiogram/electrocardiography; ESC = European Society of Cardiology; GRACE = Global Registry of Acute Coronary Events; h-FABP = heart-type fatty acid-binding protein; hs-cTn = high-sensitivity cardiac troponin; ICA = invasive coronary angiography; LV = left ventricular; LVEF = left ventricular ejection fraction; NSTEMI = non-ST-segment elevation myocardial infarction; PCI = percutaneous coronary intervention.
Class of recommendation.
Level of evidence.
Does not apply to patients discharged the same day in whom NSTEMI has been ruled out.
If none of the following criteria: haemodynamically unstable, major arrhythmias, LVEF <40%, failed reperfusion, additional critical coronary stenoses of major vessels, complications related to percutaneous revascularization, or GRACE risk score >140 if assessed.
If one or more of the above criteria are present.
4 Risk assessment and outcomes
4.1 Electrocardiogram indicators (Supplementary Data)
4.2 Biomarkers
Beyond diagnostic utility, initial cardiac troponin levels add prognostic information in terms of short- and long-term mortality to clinical and ECG variables. While hs-cTn T and I have comparable diagnostic accuracy, hs-cTn T has greater prognostic accuracy.38,119 Serial measurements are useful to identify peak levels of cardiac troponin for risk stratification purposes in patients with established MI. The higher the hs-cTn levels, the greater the risk of death.12,76,120 However, evidence is limited regarding the optimal time points of serial hs-cTn measurement. Serum creatinine and eGFR should also be determined in all patients with NSTE-ACS because they affect prognosis and are key elements of the Global Registry of Acute Coronary Events (GRACE) risk score (see section 4.3). Similarly, natriuretic peptides [BNP and N-terminal pro-BNP (NT-proBNP)] provide prognostic information regarding the risk of death, acute heart failure, as well as the development of AF in addition to cardiac troponin.121 In addition, quantifying the presence and severity of haemodynamic stress and heart failure using BNP or NT-proBNP concentrations in patients with left main CAD or three-vessel CAD without NSTE-ACS may help the heart team to select either PCI or CABG as the revascularization strategy of choice.122–124 However, this needs confirmation in randomized trials and has not been tested in NSTE-ACS patients so far. Similarly, natriuretic peptides provide prognostic information on top of cardiac troponin.121,125,126 Other biomarkers, such as high-sensitivity C-reactive protein, mid-regional pro-adrenomedullin, growth differentiation factor 15 (GDF-15), heart-type fatty acid-binding protein (h-FABP), and copeptin may also have some prognostic value.50,118,127–132 However, the assessment of these markers has, so far, not been shown to improve patient management and their added value in risk assessment on top of the GRACE risk calculation and/or BNP/NT-proBNP seems marginal. At the present time, the routine use of these biomarkers for prognostic purposes is not recommended.
4.3 Clinical scores for risk assessment (Supplementary Data)
A number of prognostic models that aim to estimate the future risk of all-cause mortality or the combined risk of all-cause mortality or MI have been developed. These models have been formulated into clinical risk scores and, among these, the GRACE risk score offers the best discriminative performance.133–135 It is important to recognize, however, that there are several GRACE risk scores, and each refers to different patient groups and predicts different outcomes.136–139 The GRACE risk score models have been externally validated using observational data.140 Further information concerning the GRACE risk scores is presented in Supplementary Data section 4.3, Supplementary Table 1, and Supplementary Figure 3. The nomogram to calculate the original GRACE risk score, which estimates the risk of in-hospital death, is shown in Supplementary Figure 3 and online risk calculators are available for other GRACE risk scores: https://www.outcomes-umassmed.org/risk_models_grace_orig.aspx for the GRACE risk score 1.0 and www.outcomes-umassmed.org/grace/acs_risk2/index.html for the GRACE risk score 2.0.
Given that the GRACE risk score predicts clinical outcomes, it is possible to stratify patients according to their estimated risk of future ischaemic events. A GRACE risk score-based risk assessment has been found to be superior to (subjective) physician assessment for the occurrence of death or MI.141,142 Moreover, it is well recognized that the delivery of guideline-directed care is inversely related to the estimated risk of the patient with NSTE-ACS143 – the so called ‘risk-treatment paradox’.144,145 Guideline-directed care is associated with proportionally greater survival gains among those with higher baseline risk, therefore objective risk assessment may help to identify NSTE-ACS patients who would benefit from risk-determined care interventions.144,145 The Australian GRACE Risk score Intervention Study (AGRIS)146 and the ongoing UK GRACE Risk score Intervention Study (UKGRIS)147 have – or are for the first time – investigating the impact of the utilization of the GRACE risk score on outcomes of patients with NSTE-ACS in a randomized manner. The AGRIS cluster-randomized trial failed to demonstrate any add-on value, especially for the guideline-directed treatments with the routine implementation of the GRACE risk score. This was largely explained by better-than-expected performance of the control hospitals. Given temporal improvements in early mortality from NSTE-ACS,148 the prediction of long-term risk is important. Deaths in the early phase following NSTE-ACS are more attributable to ischaemia/thrombosis-related events, whereas in the later phase they are more likely to be associated with the progression of atherosclerosis and non-cardiovascular causes.149–152
Recommendations on biomarker measurements for prognostic stratification
| Recommendations | Classa | Levelb |
| Beyond its diagnostic role, it is recommended to measure hs-cTn serially for the estimation of prognosis.12,13,119,120 | I | B |
| Measuring BNP or NT-proBNP plasma concentrations should be considered to gain prognostic information.121,125,126 | IIa | B |
| The measurement of additional biomarkers, such as mid-regional pro-A-type natriuretic peptide, high-sensitivity C-reactive protein, mid-regional pro-adrenomedullin, GDF-15, copeptin, and h-FABP is not recommended for routine risk or prognosis assessment.50,127,129 | III | B |
| Score to risk stratify in NSTE-ACS | ||
| GRACE risk score models should be considered for estimating prognosis.137–139 | IIa | B |
| The use of risk scores designed to evaluate the benefits and risks of different DAPT durations may be considered.153,154 | IIb | A |
| To estimate bleeding risk, the use of scores may be considered in patients undergoing coronary angiography.155,156 | IIb | B |
| Recommendations | Classa | Levelb |
| Beyond its diagnostic role, it is recommended to measure hs-cTn serially for the estimation of prognosis.12,13,119,120 | I | B |
| Measuring BNP or NT-proBNP plasma concentrations should be considered to gain prognostic information.121,125,126 | IIa | B |
| The measurement of additional biomarkers, such as mid-regional pro-A-type natriuretic peptide, high-sensitivity C-reactive protein, mid-regional pro-adrenomedullin, GDF-15, copeptin, and h-FABP is not recommended for routine risk or prognosis assessment.50,127,129 | III | B |
| Score to risk stratify in NSTE-ACS | ||
| GRACE risk score models should be considered for estimating prognosis.137–139 | IIa | B |
| The use of risk scores designed to evaluate the benefits and risks of different DAPT durations may be considered.153,154 | IIb | A |
| To estimate bleeding risk, the use of scores may be considered in patients undergoing coronary angiography.155,156 | IIb | B |
BNP = B-type natriuretic peptide; DAPT = dual antiplatelet therapy; GDF-15 = growth differentiation factor 15; GRACE = Global Registry of Acute Coronary Events; h-FABP = heart-type fatty acid-binding protein; hs-cTn = high-sensitivity cardiac troponin; NSTE-ACS = non-ST-segment elevation acute coronary syndrome; NT-proBNP = N-terminal pro-B-type natriuretic peptide.
Class of recommendation.
Level of evidence.
Recommendations on biomarker measurements for prognostic stratification
| Recommendations | Classa | Levelb |
| Beyond its diagnostic role, it is recommended to measure hs-cTn serially for the estimation of prognosis.12,13,119,120 | I | B |
| Measuring BNP or NT-proBNP plasma concentrations should be considered to gain prognostic information.121,125,126 | IIa | B |
| The measurement of additional biomarkers, such as mid-regional pro-A-type natriuretic peptide, high-sensitivity C-reactive protein, mid-regional pro-adrenomedullin, GDF-15, copeptin, and h-FABP is not recommended for routine risk or prognosis assessment.50,127,129 | III | B |
| Score to risk stratify in NSTE-ACS | ||
| GRACE risk score models should be considered for estimating prognosis.137–139 | IIa | B |
| The use of risk scores designed to evaluate the benefits and risks of different DAPT durations may be considered.153,154 | IIb | A |
| To estimate bleeding risk, the use of scores may be considered in patients undergoing coronary angiography.155,156 | IIb | B |
| Recommendations | Classa | Levelb |
| Beyond its diagnostic role, it is recommended to measure hs-cTn serially for the estimation of prognosis.12,13,119,120 | I | B |
| Measuring BNP or NT-proBNP plasma concentrations should be considered to gain prognostic information.121,125,126 | IIa | B |
| The measurement of additional biomarkers, such as mid-regional pro-A-type natriuretic peptide, high-sensitivity C-reactive protein, mid-regional pro-adrenomedullin, GDF-15, copeptin, and h-FABP is not recommended for routine risk or prognosis assessment.50,127,129 | III | B |
| Score to risk stratify in NSTE-ACS | ||
| GRACE risk score models should be considered for estimating prognosis.137–139 | IIa | B |
| The use of risk scores designed to evaluate the benefits and risks of different DAPT durations may be considered.153,154 | IIb | A |
| To estimate bleeding risk, the use of scores may be considered in patients undergoing coronary angiography.155,156 | IIb | B |
BNP = B-type natriuretic peptide; DAPT = dual antiplatelet therapy; GDF-15 = growth differentiation factor 15; GRACE = Global Registry of Acute Coronary Events; h-FABP = heart-type fatty acid-binding protein; hs-cTn = high-sensitivity cardiac troponin; NSTE-ACS = non-ST-segment elevation acute coronary syndrome; NT-proBNP = N-terminal pro-B-type natriuretic peptide.
Class of recommendation.
Level of evidence.
4.4 Bleeding risk assessment
Major bleeding events are associated with increased mortality in NSTE-ACS.157 In order to estimate bleeding risk in this setting, scores such as the Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/American Heart Association (AHA) guidelines (CRUSADE; https://www.mdcalc.com/crusade-score-post-mi-bleeding-risk) and the Acute Catheterization and Urgent Intervention Triage strategY (ACUITY) bleeding risk scores have been developed. Overall, the two scores have reasonable predictive value for major bleeding in ACS patients undergoing coronary angiography, with CRUSADE being the most discriminatory.155–157 Changes in interventional practice, such as the use of radial access for coronary angiography and PCI, as well as in antithrombotic treatment, may modify the predictive value of risk scores. In addition, in medically treated patients or those on oral anticoagulants (OACs), the predictive value of these scores has not been established. Given these limitations, the use of the CRUSADE bleeding risk score may be considered in patients undergoing coronary angiography to quantify bleeding risk.
An alternative to these scores may be the assessment of bleeding risk according to the Academic Research Consortium for High Bleeding Risk (ARC-HBR Table 7).158 This consensus definition of patients at high bleeding risk (HBR) was recently developed to provide consistency for clinical trials evaluating the safety and effectiveness of devices and drug regimens for patients undergoing PCI.158 This proposed ARC-HBR represents a pragmatic approach that includes the most recent trials performed in HBR patients, who were previously excluded from clinical trials of dual antiplatelet therapy (DAPT) duration or intensity (Table 7).159–161 However, bleeding risk assessment based on ARC-HBR criteria may be difficult to apply in routine clinical practice as several of the criteria are quite detailed and so far, this score has not been validated.
Major and minor criteria for high bleeding risk according to the Academic Research Consortium for High Bleeding Risk at the time of percutaneous coronary intervention (bleeding risk is high if at least one major or two minor criteria are met)
 |
 |
CKD = chronic kidney disease; DAPT = dual antiplatelet therapy; eGFR = estimated glomerular filtration rate; OAC = oral anticoagulation/anticoagulant; PCI = percutaneous coronary intervention.
This excludes vascular protection doses.162
Baseline thrombocytopenia is defined as thrombocytopenia before PCI.
Active malignancy is defined as diagnosis within 12 months and/or ongoing requirement for treatment (including surgery, chemotherapy, or radiotherapy).
National Institutes of Health Stroke Scale score >5.
Major and minor criteria for high bleeding risk according to the Academic Research Consortium for High Bleeding Risk at the time of percutaneous coronary intervention (bleeding risk is high if at least one major or two minor criteria are met)
 |
 |
CKD = chronic kidney disease; DAPT = dual antiplatelet therapy; eGFR = estimated glomerular filtration rate; OAC = oral anticoagulation/anticoagulant; PCI = percutaneous coronary intervention.
This excludes vascular protection doses.162
Baseline thrombocytopenia is defined as thrombocytopenia before PCI.
Active malignancy is defined as diagnosis within 12 months and/or ongoing requirement for treatment (including surgery, chemotherapy, or radiotherapy).
National Institutes of Health Stroke Scale score >5.
4.5 Integrating ischaemic and bleeding risks
Major bleeding events affect prognosis in a similar way to spontaneous ischaemic complications.163,164 Given the trade-off between ischaemic vs. bleeding risks for any antithrombotic regimen, the use of scores might prove useful to tailor antithrombotic duration, as well as intensity, to maximize ischaemic protection and minimize bleeding risk in the individual patient. Specific risk scores have been developed for patients on DAPT following PCI, in the setting of both CCS as well as ACS. To date, no risk score has been tested in patients requiring long-term anticoagulation. The DAPT and the PREdicting bleeding Complications In patients undergoing Stent implantation and subsEquent Dual Anti Platelet Therapy (PRECISE-DAPT) scores have been designed to guide and inform decision making on DAPT duration.153,154 The applicability of the PRECISE-DAPT score is at patient discharge, while the DAPT score is a bleeding risk estimation to be calculated at 1 year from the index event. The usefulness of the PRECISE-DAPT score was retrospectively assessed within patients randomized to different DAPT durations (n = 10 081) to identify the effect on bleeding and ischaemia of a long (12–24 months) or short (3–6 months) treatment duration in relation to baseline bleeding risk.154 Among HBR patients based on PRECISE-DAPT (i.e. PRECISE-DAPT score ≥25), prolonged DAPT was associated with no ischaemic benefit but a large bleeding burden.154 Conversely, longer treatment in patients without HBR (i.e. PRECISE-DAPT score <25) was associated with no increase in bleeding and a significant reduction in the composite ischaemic endpoint of MI, definite stent thrombosis, stroke, and target vessel revascularization. The findings remained valid in analyses restricted to ACS. However, for the majority of patients in the study, DAPT consisted of aspirin and clopidogrel. An external validation of the PRECISE-DAPT score – in 4424 ACS patients undergoing PCI and treated with prasugrel or ticagrelor – showed a modest predictive value for major bleeding at a median follow-up of 14 months (c-statistic = 0.653).165 In addition, none of these risk prediction models have been prospectively tested in RCTs, therefore, their value in improving patient outcomes remains unclear. The DAPT study has been less well validated, with a retrospective analysis in 1970 patients and a score calculation at a different time point (6 vs. 12 months) than in the derivation cohort used to generate the score.166
5 Pharmacological treatments
5.1 Antithrombotic treatment
Antithrombotic treatment is mandatory in NSTE-ACS patients with and without invasive management. Its choice, the combination, the time point of initiation, and the treatment duration depend on various intrinsic and extrinsic (procedural) factors (Figure 5). Notably, both ischaemic and bleeding complications significantly influence the outcome of NSTE-ACS patients and their overall mortality risk.167 Thus, the choice of treatment should equally reflect the ischaemic and bleeding risk of the patient.
Determinants of antithrombotic treatment in coronary artery disease. Intrinsic (in blue: patient's characteristics, clinical presentation & comorbidities) and extrinsic (in yellow: co-medication & procedural aspects) variables influencing the choice, dosing, and duration of antithrombotic treatment. ACS = acute coronary syndromes; CABG = coronary artery bypass graft(ing); CCS = chronic coronary syndromes; CKD = chronic kidney disease; NSTE-ACS = non-ST-segment elevation acute coronary syndrome; PAD = peripheral artery disease; PCI = percutaneous coronary intervention; STEMI = ST-segment elevation myocardial infarction.
Recommended anticoagulant and antiplatelet drugs and their dosing (for use during and after NSTE-ACS) are summarized in Figure 6 and Table 8.
Antithrombotic treatments in non-ST-segment elevation acute coronary syndrome patients: pharmacological targets. Drugs with oral administration are shown in black letters and drugs with preferred parenteral administration in red. Abciximab (in brackets) is not supplied anymore. ADP = adenosine diphosphate; DAPT = dual antiplatelet therapy; FXa = factor Xa; GP = glycoprotein; TxA2 = thromboxane A2; UFH = unfractionated heparin; VKA = vitamin K antagonist.
Dose regimen of antiplatelet and anticoagulant drugs in non-ST-segment elevation acute coronary syndrome patientsa
| I. Antiplatelet drugs | |
| Aspirin | LD of 150–300 mg orally or 75–250 mg i.v. if oral ingestion is not possible, followed by oral MD of 75–100 mg o.d. |
| P2Y12 receptor inhibitors (oral or i.v.) | |
| Clopidogrel | LD of 300–600 mg orally, followed by a MD of 75 mg o.d., no specific dose adjustment in CKD patients. |
| Prasugrel | LD of 60 mg orally, followed by a MD of 10 mg o.d. In patients with body weight <60 kg, a MD of 5 mg o.d. is recommended. In patients aged ≥75 years, prasugrel should be used with caution, but a dose of 5 mg o.d. should be used if treatment is deemed necessary. No specific dose adjustment in CKD patients. Prior stroke is a contraindication for prasugrel. |
| Ticagrelor | LD of 180 mg orally, followed by a MD of 90 mg b.i.d., no specific dose adjustment in CKD patients. |
| Cangrelor | Bolus of 30 µg/kg i.v. followed by 4 µg/kg/min infusion for at least 2 h or the duration of the procedure (whichever is longer). |
| GP IIb/IIIa receptor inhibitors (i.v.) | |
| Abciximab | Bolus of 0.25 mg/kg i.v. and 0.125 μg/kg/min infusion (maximum 10 μg/min) for 12 h (drug is not supplied anymore). |
| Eptifibatide | Double bolus of 180 μg/kg i.v. (given at a 10-min interval) followed by an infusion of 2.0 μg/kg/min for up to18 h. |
| Tirofiban | Bolus of 25 μg/kg i.v. over 3 min, followed by an infusion of 0.15 μg/kg/min for up to 18 h. |
| II. Anticoagulant drugs (for use before and during PCI) | |
| UFH | 70–100 U/kg i.v. bolus when no GP IIb/IIIa inhibitor is planned followed up by an IV infusion until the invasive procedure. 50–70 U/kg i.v. bolus with GP IIb/IIIa inhibitors. |
| Enoxaparin | 0.5 mg/kg i.v. bolus. |
| Bivalirudin | 0.75 mg/kg i.v. bolus followed by i.v. infusion of 1.75 mg/kg/h for up to 4 h after the procedure as clinically warranted. |
| Fondaparinux | 2.5 mg/d subcutaneously (only before PCI). |
| III. Oral anticoagulant drugsb | |
| Rivaroxaban | Very low MD of 2.5 mg b.i.d. (in combination with aspirin) for long-term extended antithrombotic treatment in a secondary prevention setting of CAD patients. |
| I. Antiplatelet drugs | |
| Aspirin | LD of 150–300 mg orally or 75–250 mg i.v. if oral ingestion is not possible, followed by oral MD of 75–100 mg o.d. |
| P2Y12 receptor inhibitors (oral or i.v.) | |
| Clopidogrel | LD of 300–600 mg orally, followed by a MD of 75 mg o.d., no specific dose adjustment in CKD patients. |
| Prasugrel | LD of 60 mg orally, followed by a MD of 10 mg o.d. In patients with body weight <60 kg, a MD of 5 mg o.d. is recommended. In patients aged ≥75 years, prasugrel should be used with caution, but a dose of 5 mg o.d. should be used if treatment is deemed necessary. No specific dose adjustment in CKD patients. Prior stroke is a contraindication for prasugrel. |
| Ticagrelor | LD of 180 mg orally, followed by a MD of 90 mg b.i.d., no specific dose adjustment in CKD patients. |
| Cangrelor | Bolus of 30 µg/kg i.v. followed by 4 µg/kg/min infusion for at least 2 h or the duration of the procedure (whichever is longer). |
| GP IIb/IIIa receptor inhibitors (i.v.) | |
| Abciximab | Bolus of 0.25 mg/kg i.v. and 0.125 μg/kg/min infusion (maximum 10 μg/min) for 12 h (drug is not supplied anymore). |
| Eptifibatide | Double bolus of 180 μg/kg i.v. (given at a 10-min interval) followed by an infusion of 2.0 μg/kg/min for up to18 h. |
| Tirofiban | Bolus of 25 μg/kg i.v. over 3 min, followed by an infusion of 0.15 μg/kg/min for up to 18 h. |
| II. Anticoagulant drugs (for use before and during PCI) | |
| UFH | 70–100 U/kg i.v. bolus when no GP IIb/IIIa inhibitor is planned followed up by an IV infusion until the invasive procedure. 50–70 U/kg i.v. bolus with GP IIb/IIIa inhibitors. |
| Enoxaparin | 0.5 mg/kg i.v. bolus. |
| Bivalirudin | 0.75 mg/kg i.v. bolus followed by i.v. infusion of 1.75 mg/kg/h for up to 4 h after the procedure as clinically warranted. |
| Fondaparinux | 2.5 mg/d subcutaneously (only before PCI). |
| III. Oral anticoagulant drugsb | |
| Rivaroxaban | Very low MD of 2.5 mg b.i.d. (in combination with aspirin) for long-term extended antithrombotic treatment in a secondary prevention setting of CAD patients. |
AF = atrial fibrillation; b.i.d. = bis in die (twice a day); CAD = coronary artery disease; CKD = chronic kidney disease; GP = glycoprotein; i.v. = intravenous; MD = maintenance dose; LD = loading dose; NOAC = non-vitamin K antagonist oral anticoagulant; OAC = oral anticoagulation/anticoagulant; o.d. = once daily; PCI = percutaneous coronary intervention; UFH = unfractionated heparin; VKA = vitamin K antagonist.
All dosing regimens refer to doses given for the respective drugs for protection against thrombosis within the arterial system.
Section III lists the dosing for rivaroxaban in a secondary prevention setting in CAD patients. For a comprehensive summary on dosing of OACs (NOACs and VKAs) in a setting of full-dose anticoagulation please see: The 2018 European Heart Rhythm Association Practical Guide on the use of NOACs in patients with AF.168
Dose regimen of antiplatelet and anticoagulant drugs in non-ST-segment elevation acute coronary syndrome patientsa
| I. Antiplatelet drugs | |
| Aspirin | LD of 150–300 mg orally or 75–250 mg i.v. if oral ingestion is not possible, followed by oral MD of 75–100 mg o.d. |
| P2Y12 receptor inhibitors (oral or i.v.) | |
| Clopidogrel | LD of 300–600 mg orally, followed by a MD of 75 mg o.d., no specific dose adjustment in CKD patients. |
| Prasugrel | LD of 60 mg orally, followed by a MD of 10 mg o.d. In patients with body weight <60 kg, a MD of 5 mg o.d. is recommended. In patients aged ≥75 years, prasugrel should be used with caution, but a dose of 5 mg o.d. should be used if treatment is deemed necessary. No specific dose adjustment in CKD patients. Prior stroke is a contraindication for prasugrel. |
| Ticagrelor | LD of 180 mg orally, followed by a MD of 90 mg b.i.d., no specific dose adjustment in CKD patients. |
| Cangrelor | Bolus of 30 µg/kg i.v. followed by 4 µg/kg/min infusion for at least 2 h or the duration of the procedure (whichever is longer). |
| GP IIb/IIIa receptor inhibitors (i.v.) | |
| Abciximab | Bolus of 0.25 mg/kg i.v. and 0.125 μg/kg/min infusion (maximum 10 μg/min) for 12 h (drug is not supplied anymore). |
| Eptifibatide | Double bolus of 180 μg/kg i.v. (given at a 10-min interval) followed by an infusion of 2.0 μg/kg/min for up to18 h. |
| Tirofiban | Bolus of 25 μg/kg i.v. over 3 min, followed by an infusion of 0.15 μg/kg/min for up to 18 h. |
| II. Anticoagulant drugs (for use before and during PCI) | |
| UFH | 70–100 U/kg i.v. bolus when no GP IIb/IIIa inhibitor is planned followed up by an IV infusion until the invasive procedure. 50–70 U/kg i.v. bolus with GP IIb/IIIa inhibitors. |
| Enoxaparin | 0.5 mg/kg i.v. bolus. |
| Bivalirudin | 0.75 mg/kg i.v. bolus followed by i.v. infusion of 1.75 mg/kg/h for up to 4 h after the procedure as clinically warranted. |
| Fondaparinux | 2.5 mg/d subcutaneously (only before PCI). |
| III. Oral anticoagulant drugsb | |
| Rivaroxaban | Very low MD of 2.5 mg b.i.d. (in combination with aspirin) for long-term extended antithrombotic treatment in a secondary prevention setting of CAD patients. |
| I. Antiplatelet drugs | |
| Aspirin | LD of 150–300 mg orally or 75–250 mg i.v. if oral ingestion is not possible, followed by oral MD of 75–100 mg o.d. |
| P2Y12 receptor inhibitors (oral or i.v.) | |
| Clopidogrel | LD of 300–600 mg orally, followed by a MD of 75 mg o.d., no specific dose adjustment in CKD patients. |
| Prasugrel | LD of 60 mg orally, followed by a MD of 10 mg o.d. In patients with body weight <60 kg, a MD of 5 mg o.d. is recommended. In patients aged ≥75 years, prasugrel should be used with caution, but a dose of 5 mg o.d. should be used if treatment is deemed necessary. No specific dose adjustment in CKD patients. Prior stroke is a contraindication for prasugrel. |
| Ticagrelor | LD of 180 mg orally, followed by a MD of 90 mg b.i.d., no specific dose adjustment in CKD patients. |
| Cangrelor | Bolus of 30 µg/kg i.v. followed by 4 µg/kg/min infusion for at least 2 h or the duration of the procedure (whichever is longer). |
| GP IIb/IIIa receptor inhibitors (i.v.) | |
| Abciximab | Bolus of 0.25 mg/kg i.v. and 0.125 μg/kg/min infusion (maximum 10 μg/min) for 12 h (drug is not supplied anymore). |
| Eptifibatide | Double bolus of 180 μg/kg i.v. (given at a 10-min interval) followed by an infusion of 2.0 μg/kg/min for up to18 h. |
| Tirofiban | Bolus of 25 μg/kg i.v. over 3 min, followed by an infusion of 0.15 μg/kg/min for up to 18 h. |
| II. Anticoagulant drugs (for use before and during PCI) | |
| UFH | 70–100 U/kg i.v. bolus when no GP IIb/IIIa inhibitor is planned followed up by an IV infusion until the invasive procedure. 50–70 U/kg i.v. bolus with GP IIb/IIIa inhibitors. |
| Enoxaparin | 0.5 mg/kg i.v. bolus. |
| Bivalirudin | 0.75 mg/kg i.v. bolus followed by i.v. infusion of 1.75 mg/kg/h for up to 4 h after the procedure as clinically warranted. |
| Fondaparinux | 2.5 mg/d subcutaneously (only before PCI). |
| III. Oral anticoagulant drugsb | |
| Rivaroxaban | Very low MD of 2.5 mg b.i.d. (in combination with aspirin) for long-term extended antithrombotic treatment in a secondary prevention setting of CAD patients. |
AF = atrial fibrillation; b.i.d. = bis in die (twice a day); CAD = coronary artery disease; CKD = chronic kidney disease; GP = glycoprotein; i.v. = intravenous; MD = maintenance dose; LD = loading dose; NOAC = non-vitamin K antagonist oral anticoagulant; OAC = oral anticoagulation/anticoagulant; o.d. = once daily; PCI = percutaneous coronary intervention; UFH = unfractionated heparin; VKA = vitamin K antagonist.
All dosing regimens refer to doses given for the respective drugs for protection against thrombosis within the arterial system.
Section III lists the dosing for rivaroxaban in a secondary prevention setting in CAD patients. For a comprehensive summary on dosing of OACs (NOACs and VKAs) in a setting of full-dose anticoagulation please see: The 2018 European Heart Rhythm Association Practical Guide on the use of NOACs in patients with AF.168
5.1.1 Antiplatelet drugs and pre-treatment
5.1.1.1 Antiplatelet drugs and dual antiplatelet therapy
Activation of blood platelets and the coagulation cascade play a key role in the initial phase and evolution of NSTE-ACS. Hence, sufficient platelet inhibition and (temporary) anticoagulation is essential in NSTE-ACS patients, especially in those undergoing myocardial revascularization by PCI. Aspirin is considered to be the cornerstone of treatment for inhibition of thromboxane A2 generation (Figure 6), which is normally complete with a dose ≥75 mg/d. Aspirin treatment is started with a loading dose (LD) followed by maintenance treatment (Table 8). Current evidence supports a maintenance dose (MD) of 75–100 mg once daily (o.d.).169 Based on the results of the phase III PLATelet inhibition and patient Outcomes (PLATO) and TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel–Thrombolysis In Myocardial Infarction 38 (TRITON-TIMI 38) trials,170,171 DAPT including aspirin and a potent P2Y12 receptor inhibitor (ticagrelor or prasugrel) is the recommended standard treatment for NSTE-ACS patients. Clopidogrel, characterized by less potent and variable platelet inhibition,172,173 should only be used when prasugrel or ticagrelor are contraindicated, not available, or cannot be tolerated due to an unacceptable HBR. P2Y12 receptor inhibitors differ with respect to their pharmacokinetic and pharmacodynamic properties. Table 9 summarizes the essential features of the available oral and intravenous (i.v.) drugs. For further details on recent DAPT trials, please refer to the 2017 ESC focused update on DAPT in CAD.169
Trial data on the head-to-head comparison of prasugrel vs. ticagrelor became available with the open-label randomized Intracoronary stenting and Antithrombotic regimen–Rapid Early Action for Coronary Treatment (ISAR-REACT) 5 trial.174 This study was conducted in 4018 ACS patients (NSTE-ACS and STEMI) for whom an invasive evaluation was planned. The trial demonstrated that treatment with prasugrel vs. ticagrelor significantly reduced the composite rate of death, MI, or stroke (6.9 vs. 9.3%, P=0.006) without any increase in bleeding complications (4.8 vs. 5.4%, P=0.46). Limitations of the study, amongst multiple others, include its open-label design and the limited data on medically managed or CABG-treated patients, which were more prominent in the PLATO trial.170 Ticagrelor also led to more patients stopping medication because of side effects. The actual treatment strategy was PCI in >80% of randomized patients and, consequently, prasugrel should be considered the preferred P2Y12 receptor inhibitor for NSTE-ACS patients who proceed to PCI. The possible benefit of prasugrel, in comparison with ticagrelor or clopidogrel, may be related to improved endothelial function.175 Recommended treatment algorithms and treatment durations, as well as options for extended treatment (>12 months) in NSTE-ACS patients, are shown in Figure 7.
P2Y12 receptor inhibitors for use in non-ST-segment elevation acute coronary syndrome patients
| . | Oral administration . | i.v. administration . | ||
|---|---|---|---|---|
| . | Clopidogrel . | Prasugrel . | Ticagrelor . | Cangrelor . |
| Drug class | Thienopyridine | Thienopyridine | Cyclopentyl-triazolopyrimidine | Adenosine triphosphate analogue |
| Reversibility | Irreversible | Irreversible | Reversible | Reversible |
| Bioactivation | Yes (pro-drug, CYP dependent, 2 steps) | Yes (pro-drug, CYP dependent, 1 step) | Noa | No |
| (Pretreatment)-Dose | 600 mg LD, 75 mg MD | 60 mg LD, 10 (5) mg MD | 180 mg LD, 2 × 90 (60) mg MD | 30 µg/kg i.v. bolus, 4 µg/kg/min i.v. infusion for PCI |
| Onset of effect | Delayed: 2–6 h | Rapid: 0.5–4 h | Rapid: 0.5–2 h | Immediate: 2 min |
| Offset of effect | 3–10 days | 5–10 days | 3–4 days | 30–60 min |
| Delay to surgery | 5 days | 7 days | 5 days | No significant delay |
| Kidney failure | No dose adjustment | No dose adjustment | No dose adjustment | No dose adjustment |
| Dialysis or CrCl <15 mL/min | Limited data | Limited data | Limited data | Limited data |
| . | Oral administration . | i.v. administration . | ||
|---|---|---|---|---|
| . | Clopidogrel . | Prasugrel . | Ticagrelor . | Cangrelor . |
| Drug class | Thienopyridine | Thienopyridine | Cyclopentyl-triazolopyrimidine | Adenosine triphosphate analogue |
| Reversibility | Irreversible | Irreversible | Reversible | Reversible |
| Bioactivation | Yes (pro-drug, CYP dependent, 2 steps) | Yes (pro-drug, CYP dependent, 1 step) | Noa | No |
| (Pretreatment)-Dose | 600 mg LD, 75 mg MD | 60 mg LD, 10 (5) mg MD | 180 mg LD, 2 × 90 (60) mg MD | 30 µg/kg i.v. bolus, 4 µg/kg/min i.v. infusion for PCI |
| Onset of effect | Delayed: 2–6 h | Rapid: 0.5–4 h | Rapid: 0.5–2 h | Immediate: 2 min |
| Offset of effect | 3–10 days | 5–10 days | 3–4 days | 30–60 min |
| Delay to surgery | 5 days | 7 days | 5 days | No significant delay |
| Kidney failure | No dose adjustment | No dose adjustment | No dose adjustment | No dose adjustment |
| Dialysis or CrCl <15 mL/min | Limited data | Limited data | Limited data | Limited data |
CrCl = creatine clearance; CYP = cytochrome P450; i.v. = intravenous; LD = loading dose, MD = maintenance dose, PCI = percutaneous coronary intervention.
Following intestinal absorption, ticagrelor does not need to be metabolized to inhibit platelets. Of note, a metabolite (AR-C124910XX) of ticagrelor is also active.
P2Y12 receptor inhibitors for use in non-ST-segment elevation acute coronary syndrome patients
| . | Oral administration . | i.v. administration . | ||
|---|---|---|---|---|
| . | Clopidogrel . | Prasugrel . | Ticagrelor . | Cangrelor . |
| Drug class | Thienopyridine | Thienopyridine | Cyclopentyl-triazolopyrimidine | Adenosine triphosphate analogue |
| Reversibility | Irreversible | Irreversible | Reversible | Reversible |
| Bioactivation | Yes (pro-drug, CYP dependent, 2 steps) | Yes (pro-drug, CYP dependent, 1 step) | Noa | No |
| (Pretreatment)-Dose | 600 mg LD, 75 mg MD | 60 mg LD, 10 (5) mg MD | 180 mg LD, 2 × 90 (60) mg MD | 30 µg/kg i.v. bolus, 4 µg/kg/min i.v. infusion for PCI |
| Onset of effect | Delayed: 2–6 h | Rapid: 0.5–4 h | Rapid: 0.5–2 h | Immediate: 2 min |
| Offset of effect | 3–10 days | 5–10 days | 3–4 days | 30–60 min |
| Delay to surgery | 5 days | 7 days | 5 days | No significant delay |
| Kidney failure | No dose adjustment | No dose adjustment | No dose adjustment | No dose adjustment |
| Dialysis or CrCl <15 mL/min | Limited data | Limited data | Limited data | Limited data |
| . | Oral administration . | i.v. administration . | ||
|---|---|---|---|---|
| . | Clopidogrel . | Prasugrel . | Ticagrelor . | Cangrelor . |
| Drug class | Thienopyridine | Thienopyridine | Cyclopentyl-triazolopyrimidine | Adenosine triphosphate analogue |
| Reversibility | Irreversible | Irreversible | Reversible | Reversible |
| Bioactivation | Yes (pro-drug, CYP dependent, 2 steps) | Yes (pro-drug, CYP dependent, 1 step) | Noa | No |
| (Pretreatment)-Dose | 600 mg LD, 75 mg MD | 60 mg LD, 10 (5) mg MD | 180 mg LD, 2 × 90 (60) mg MD | 30 µg/kg i.v. bolus, 4 µg/kg/min i.v. infusion for PCI |
| Onset of effect | Delayed: 2–6 h | Rapid: 0.5–4 h | Rapid: 0.5–2 h | Immediate: 2 min |
| Offset of effect | 3–10 days | 5–10 days | 3–4 days | 30–60 min |
| Delay to surgery | 5 days | 7 days | 5 days | No significant delay |
| Kidney failure | No dose adjustment | No dose adjustment | No dose adjustment | No dose adjustment |
| Dialysis or CrCl <15 mL/min | Limited data | Limited data | Limited data | Limited data |
CrCl = creatine clearance; CYP = cytochrome P450; i.v. = intravenous; LD = loading dose, MD = maintenance dose, PCI = percutaneous coronary intervention.
Following intestinal absorption, ticagrelor does not need to be metabolized to inhibit platelets. Of note, a metabolite (AR-C124910XX) of ticagrelor is also active.
5.1.1.2 Pre-treatment
Pre-treatment defines a strategy according to which antiplatelet drugs, usually a P2Y12 receptor inhibitor, are given before coronary angiography and when the coronary anatomy is unknown.176 Although a rationale for pre-treatment in NSTE-ACS may seem obvious, for achieving sufficient platelet inhibition at the time of PCI, large-scale randomized trials supporting a routine pre-treatment strategy with either clopidogrel or the potent P2Y12 receptor inhibitors – prasugrel and ticagrelor – are lacking. The randomized Comparison of Prasugrel at the Time of Percutaneous Coronary Intervention or as Pretreatment at the Time of Diagnosis in Patients with Non-ST Elevation Myocardial Infarction (ACCOAST) trial177 demonstrated a lack of any ischaemic benefit for pre-treatment in NSTE-ACS patients, but instead, a substantially higher bleeding risk with prasugrel pre-treatment. In line with these results, observational data on pre-treatment with ticagrelor, prasugrel, and clopidogrel were reported from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR) in 64 857 NSTE-ACS patients.178 In this large dataset on pre-treatment, the authors reported that P2Y12 receptor inhibitor pre-treatment in NSTE-ACS patients was not associated with improved ischaemic outcomes, but instead, with a significantly increased risk of bleeding events. With respect to pre-treatment data for ticagrelor, the recently published ISAR-REACT 5 trial showed that a prasugrel-based strategy with deferred loading after knowledge of coronary anatomy in NSTE-ACS patients was superior to a ticagrelor-based strategy that implied a routine pre-treatment strategy.174 Importantly, there was no apparent benefit of a pre-treatment strategy (that utilized ticagrelor) in that study.
Based upon the available evidence,174,177 it is not recommended to administer routine pre-treatment with a P2Y12 receptor inhibitor in NSTE-ACS patients in whom coronary anatomy is not known and an early invasive management is planned. For patients with a delayed invasive management, pre-treatment with a P2Y12 receptor inhibitor may be considered in selected cases and according to the bleeding risk of the patient.
Fortunately, the recommended standard treatment with potent P2Y12 receptor inhibitors (ticagrelor or prasugrel) exhibits a fast onset of action (Table 9), thereby allowing LD administration after diagnostic coronary angiography and directly before PCI. Of note, a routine pre-treatment strategy may be deleterious for a relevant proportion of patients with diagnoses other than NSTE-ACS (e.g. aortic dissection or bleeding complications including intracranial bleeding) and may increase bleeding risk or delay procedures in patients scheduled for CABG after diagnostic angiography.
Algorithm for antithrombotic therapy in non-ST-segment elevation acute coronary syndrome patients without atrial fibrillation undergoing percutaneous coronary intervention. HBR is considered as an increased risk of spontaneous bleeding during DAPT (e.g. PRECISE-DAPT score ≥25 or ARC-HBR158). Colour-coding refers to the ESC classes of recommendations (green = class I; yellow = IIa; orange = Class IIb). Very HBR is defined as recent bleeding in the past month and/or not deferrable planned surgery. A = aspirin; ARC-HBR = Academic Research Consortium – High Bleeding Risk; C = clopidogrel; DAPT = dual antiplatelet therapy; DAT = dual antithrombotic therapy (here: aspirin + rivaroxaban); eGFR = estimated glomerular filtration rate; ESC = European Society of Cardiology; HBR = high bleeding risk; NSTE-ACS = non-ST-segment elevation acute coronary syndrome; P = prasugrel; PCI = percutaneous coronary intervention; PRECISE-DAPT = PREdicting bleeding Complications In patients undergoing Stent implantation and subsEquent Dual Anti Platelet Therapy; R = rivaroxaban; T = ticagrelor; UFH = unfractionated heparin. aClopidogrel during 12 months DAPT if patient is not eligible for treatment with prasugrel or ticagrelor or in a setting of DAPT de-escalation with a switch to clopidogrel (class IIb). bClopidogrel or prasugrel if patient is not eligible for treatment with ticagrelor. cClass IIa indication for DAT or DAPT >12 months in patients at high risk for ischaemic events (see Table 9 for definitions) and without increased risk of major bleeding (= prior history of intracranial haemorrhage or ischaemic stroke, history of other intracranial pathology, recent gastrointestinal bleeding or anaemia due to possible gastrointestinal blood loss, other gastrointestinal pathology associated with increased bleeding risk, liver failure, bleeding diathesis or coagulopathy, extreme old age or frailty, renal failure requiring dialysis, or with eGFR <15 mL/min/1.73 m2); Class IIb indication for DAT or DAPT >12 months in patients with moderately increased risk of ischaemic events (see Table 11 for definitions) and without increased risk of major bleeding. Listen to the audio guide of this figure online.
Recommendations for antithrombotic treatment in non-ST-segment elevation acute coronary syndrome patients without atrial fibrillation undergoing percutaneous coronary intervention
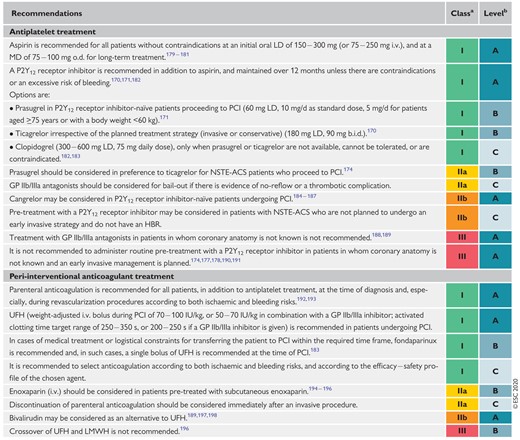 |
 |
b.i.d. = bis in die (twice a day); GP = glycoprotein; HBR = high bleeding risk; i.v. = intravenous; LD = loading dose; LMWH = low-molecular-weight heparin; MD = maintenance dose; NSTE-ACS = non-ST-segment elevation acute coronary syndrome; o.d. = once daily; PCI = percutaneous coronary intervention; UFH = unfractionated heparin.
Class of recommendation.
Level of evidence.
Recommendations for antithrombotic treatment in non-ST-segment elevation acute coronary syndrome patients without atrial fibrillation undergoing percutaneous coronary intervention
 |
 |
b.i.d. = bis in die (twice a day); GP = glycoprotein; HBR = high bleeding risk; i.v. = intravenous; LD = loading dose; LMWH = low-molecular-weight heparin; MD = maintenance dose; NSTE-ACS = non-ST-segment elevation acute coronary syndrome; o.d. = once daily; PCI = percutaneous coronary intervention; UFH = unfractionated heparin.
Class of recommendation.
Level of evidence.
5.1.2 Peri-interventional anticoagulant treatment
Peri-interventional treatment for NSTE-ACS patients consists of anticoagulation to inhibit thrombin generation and thrombin activity (Figure 6). Anticoagulation is recommended for all patients in addition to antiplatelet therapy during invasive management for NSTE-ACS.192 Table 8 provides an overview of the relevant drugs and their dosing in NSTE-ACS patients. Unfractionated heparin (UFH) is the standard of care for NSTE-ACS patients due to its favourable risk-benefit profile. In general, a crossover between anticoagulants should be avoided [especially between UFH and low-molecular-weight heparin (LMWH)], with the exception of adding UFH to fondaparinux when a patient proceeds to PCI after fondaparinux treatment.196,199 The respective drugs should be discontinued immediately after PCI, except in specific clinical settings such as the confirmed presence of LV aneurysm with thrombus formation or AF requiring anticoagulation, which is usually done with UFH in (per)-acute settings.
Adjunctive treatment [e.g. glycoprotein (GP) IIb/IIIa inhibitors] and procedural aspects (radial vs. femoral access) have been subject to change in recent years. In contrast to older studies, recent and contemporary trials have pursued a balanced and more selective use of GP IIb/IIIa inhibitors, with both bivalirudin and UFH. These trials have been reviewed extensively in a number of meta-analyses.200–203 A recent meta-analysis, which included the Minimizing Adverse Haemorrhagic Events by TRansradial Access Site and Systemic Implementation of angioX (MATRIX) trial,197 showed no significant benefit of bivalirudin vs. UFH for ischaemic outcomes.202 Bivalirudin was associated with a significant increase in the risk of stent thrombosis and a significant decrease in bleeding risk. Bleeding risk reduction was linked to unbalanced use of GP IIb/IIIa inhibitors, predominantly with UFH. Recently, the Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies (VALIDATE-SWEDEHEART) study204 compared UFH vs. bivalirudin on a background of radial access and limited use of GP IIb/IIIa inhibitors. The study demonstrated similar risks for both ischaemia and bleeding when comparing the two drugs. Another meta-analysis, updated with the results of the VALIDATE-SWEDEHEART study, confirmed that bivalirudin vs. UFH was associated with a similar incidence of all-cause death and ischaemic events after PCI in ACS.203 A significant association between bivalirudin and decreased risk of bleeding was only found with unbalanced use of GP IIb/IIIa inhibitors in conjunction with UFH.
In summary, and based on the aforementioned trials, UFH is primarily recommended as an anticoagulant for PCI. Due to its short half-life and favourable results in some of the studies, bivalirudin may be considered as an alternative to UFH in selected cases. For a more detailed description and a historical summary of the older clinical trials (with unbalanced use of GP IIb/IIIa inhibitors) comparing UFH with bivalirudin, please refer to the 2018 ESC/EACTS Guidelines on myocardial revascularization.205
Patients may undergo cardiac catheterization after a conservative treatment phase and these patients might be treated with fondaparinux during this period. This regimen is based on the Fifth Organization to Assess Strategies in Acute Ischemic Syndromes (OASIS-5) trial.206 Of note, catheter thrombus formation was an issue with fondaparinux and, therefore, full-dose UFH must be added to prevent thrombus formation when the patient proceeds to PCI.
Enoxaparin, a LMWH with a predictable dose-effect relationship and a lower risk for heparin-induced thrombocytopenia (HIT) compared to UFH, should be considered as an anticoagulant for PCI in patients pre-treated with subcutaneous enoxaparin. A benefit of enoxaparin over UFH –reduced mortality and bleeding complications – was reported in a meta-analysis that included NSTE-ACS patients,194 but dedicated large-scale trials comparing enoxaparin vs. UFH in NSTE-ACS are lacking.
5.1.3 Peri-interventional antiplatelet treatment
Drugs for peri-interventional i.v. antiplatelet treatment include cangrelor and GP IIb/IIIa inhibitors (abciximab, eptifibatide, and tirofiban). Most of the trials evaluating GP IIb/IIIa inhibitors in PCI-treated ACS patients predated the era of routine DAPT with early DAPT initiation including a P2Y12 receptor inhibitor LD.205,207 Today, with routine and potent oral P2Y12 receptor inhibitors, there is no compelling evidence for an additional benefit of routine upstream use of GP IIb/IIIa inhibitors in NSTE-ACS patients scheduled for coronary angiography.188,189 Even more so, in a setting of potent platelet inhibition with ticagrelor or prasugrel, where randomized data on GP IIb/IIIa use is limited, routine use of these agents cannot be recommended. Nevertheless, use should be considered for bail-out situations or thrombotic complications and may be considered for high-risk PCI in patients without pre-treatment with P2Y12 receptor inhibitors (see 2018 ESC/EACTS Guidelines on myocardial revascularization for more details).205
Cangrelor is a direct reversible, short-acting P2Y12 receptor inhibitor that has been evaluated during PCI for stable CCS and ACS in clinical trials comparing cangrelor with clopidogrel, administered before PCI [Cangrelor versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition (CHAMPION)] or after PCI (CHAMPION PLATFORM and CHAMPION PHOENIX).185–187 A meta-analysis of these trials showed a benefit with respect to major ischaemic endpoints that was counter-balanced by an increase in minor bleeding complications.184 Moreover, the benefit of cangrelor with respect to ischaemic endpoints was attenuated in CHAMPION PCI with upfront administration of clopidogrel, while data for its use in conjunction with ticagrelor or prasugrel treatment are limited. Due to its proven efficacy in preventing intra-procedural and post-procedural stent thrombosis in P2Y12 receptor inhibitor-naïve patients, cangrelor may be considered on a case-by-case basis in P2Y12 receptor inhibitor-naïve NSTE-ACS patients undergoing PCI (see 2018 ESC/EACTS Guidelines on myocardial revascularization for more details).205
5.1.4 Post-interventional and maintenance treatment
Following PCI for NSTE-ACS, DAPT consisting of a potent P2Y12 receptor inhibitor in addition to aspirin is generally recommended for 12 months, irrespective of the stent type, unless there are contraindications.170,171,182 In specific clinical scenarios, DAPT duration can be shortened (<12 months), extended (>12 months, see Figure 7 and Tables 10 and 11), or modified (switching DAPT, DAPT de-escalation) and these decisions depend on individual clinical judgement being driven by the patient’s ischaemic and bleeding risk, the occurrence of adverse events, comorbidities, co-medications, and the availability of the respective drugs. For a detailed description of the pertinent and numerous trials that have compared different DAPT treatment durations (especially 3–6 vs. 12 months in NSTE-ACS patients), please refer to the 2017 ESC focused update on DAPT in CAD169 and recent trial publications.208,209 In patients with NSTE-ACS and stent implantation who are at high risk of bleeding (e.g. PRECISE-DAPT ≥25 or ARC-HBR criteria met), discontinuation of P2Y12 receptor inhibitor therapy after 3–6 months should be considered.154 In patients at very high risk of bleeding, defined as a recent bleeding episode in the past month or planned, not deferrable surgery in the near future, 1 month of aspirin and clopidogrel should be considered.
Four recent trials (n = 29 089) have explored the benefit of a shortened DAPT duration of 1–3 months.208–211 Low-to-intermediate ischaemic risk and low bleeding risk patients were included and early monotherapy with clopidogrel/ticagrelor was used. All bleeding events were reduced, with a favourable trend towards less ischaemic events including MI. Importantly, more than 50% had ACS as an inclusion criterion. In particular, the Ticagrelor With Aspirin or Alone in High-Risk Patients After Coronary Intervention (TWILIGHT) trial211 examined the effect of ticagrelor alone vs. ticagrelor plus aspirin with regard to clinically relevant bleeding among patients at high risk for bleeding or ischaemic events who had undergone PCI, according to the inclusion criteria. However, these patients were not at HBR according to current HBR criteria and event rates at follow-up. Based on this, these patients were more a low bleeding and ischaemic risk cohort even though more than two thirds had an ACS. After 3 months of treatment with ticagrelor plus aspirin, patients who did not have a major bleeding or ischaemic event continued to take ticagrelor and were randomly assigned to receive aspirin or placebo for 1 year. The primary endpoint of Bleeding Academic Research Consortium (BARC) type 2, 3, or 5 bleeding was significantly reduced by omitting aspirin (4.0 vs. 7.1%; HR 0.56, 95% CI 0.45–0.68, P<0.001), with a significant interaction according to ACS at presentation. The trial was not powered for the composite endpoint of death from any cause, non-fatal MI, or non-fatal stroke. However, in exploratory non-inferiority hypothesis testing, there was no signal of increased ischaemic risk.211 It should be acknowledged that the actual ischaemic event rate in TWILIGHT was low compared to other trials for deemed high-risk PCI patients.
Contrary to this, and based on the results of the DAPT and Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin-Thrombolysis in Myocardial Infarction (PEGASUS-TIMI) 54 trials, in patients with ACS who have tolerated DAPT without a bleeding complication, a prolonged DAPT course >12 months should be considered in those with high thrombotic risk and without an increased risk for major or life-threatening bleeding, and may be considered in patients with moderately elevated thrombotic risk (see Figure 7 and Tables 10 and 11).212,213 Of note, the 60 mg bis in die [b.i.d. (twice a day)] dose for ticagrelor was better tolerated than the 90 mg b.i.d dose214,215 and this dose is now approved in many (albeit not all) countries for this indication.
Switching between oral P2Y12 receptor inhibitors is common and triggers may include bleeding complications (or concerns for bleeding), non-bleeding side effects (e.g. dyspnoea on ticagrelor, allergic reactions), as well as socio-economic factors.216,217 Switching between oral P2Y12 receptor inhibitors may be considered in selected cases, and for a more detailed description on switching antiplatelet drugs, please refer to the International Expert Consensus on Switching Platelet P2Y12 Receptor-Inhibiting Therapies217 and the 2017 ESC DAPT focused update.169
DAPT de-escalation (switch from potent drugs like prasugrel or ticagrelor to clopidogrel) in NSTE-ACS patients may be considered as an alternative treatment regimen.216,217 However, it is important to note that there is a potential for increased ischaemic risk with a uniform de-escalation of P2Y12 receptor inhibiting therapy after PCI, particularly if performed early (<30 days) after the index event. Indeed, dedicated large-scale trials on a uniform and unguided DAPT de-escalation are lacking and the available data on uniform de-escalation are conflicting.218,219 Based on the results of the Testing Responsiveness to Platelet Inhibition on Chronic Antiplatelet Treatment for Acute Coronary Syndromes (TROPICAL-ACS) and POPULAR Genetics trials,220,221 an approach of DAPT de-escalation guided by either platelet function testing (TROPICAL-ACS: NSTE-ACS and STEMI patients) or CYP2C19-directed genotyping (POPULAR Genetics: STEMI patients) may be considered in selected NSTE-ACS patients as an alternative to 12 months of potent platelet inhibition, especially for patients deemed unsuitable for maintained potent platelet inhibition. For further details, please refer to the updated expert consensus statement on platelet function and genetic testing for guiding P2Y12 receptor inhibitor treatment in PCI.222
Recently, data on a novel strategy of dual antithrombotic therapy (DAT) consisting of factor-Xa inhibition with a very low dose of rivaroxaban (2.5 mg b.i.d.) plus aspirin has emerged, and such a regimen should be considered as a treatment option for maintenance treatment beyond 12 months post ACS PCI. In a secondary prevention setting, the Cardiovascular OutcoMes for People using Anticoagulation StrategieS (COMPASS) trial162,223 investigated very low-dose rivaroxaban (2.5 mg b.i.d.) in combination with aspirin vs. aspirin alone or rivaroxaban 5 mg b.i.d. alone. Rivaroxaban 2.5 mg b.i.d. plus aspirin 100 mg o.d. reduced the risk of the combined ischaemic endpoint, overall mortality (without reaching the threshold P-value according to the Hochberg procedure), and cardiovascular mortality alone, while this combination increased the risk for major bleeding complications without a significant increase in the risk of fatal, intracranial, or critical organ bleeding events. Greater absolute risk reductions were seen in high-risk patients, including those with diabetes or polyvascular disease [CAD plus peripheral artery disease (PAD)]. Thus, rivaroxaban (2.5 mg b.i.d.) should be considered, in addition to aspirin 75 − 100 mg/d in patients at high thrombotic risk and without an increased risk for major or life-threatening bleeding, and may be considered in patients with moderately elevated thrombotic risk (see Figure 7 and Tables 10 and 11 for selection criteria and for ischaemic and bleeding risk definitions).
Rivaroxaban has also been studied in the Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Standard Therapy in Subjects with Acute Coronary Syndrome 2–Thrombolysis In Myocardial Infarction 51 (ATLAS ACS 2–TIMI 51) trial on a background of clopidogrel treatment. The study showed a reduction of ischaemic events and cardiovascular mortality along with a higher risk for bleeding.224 However, data are lacking on a background of ticagrelor or prasugrel treatment and it is therefore difficult to extrapolate trial results to contemporary practice including the use of potent P2Y12 receptor inhibitors.
Recommendations for post-interventional and maintenance treatment in patients with non-ST-segment elevation acute coronary syndrome
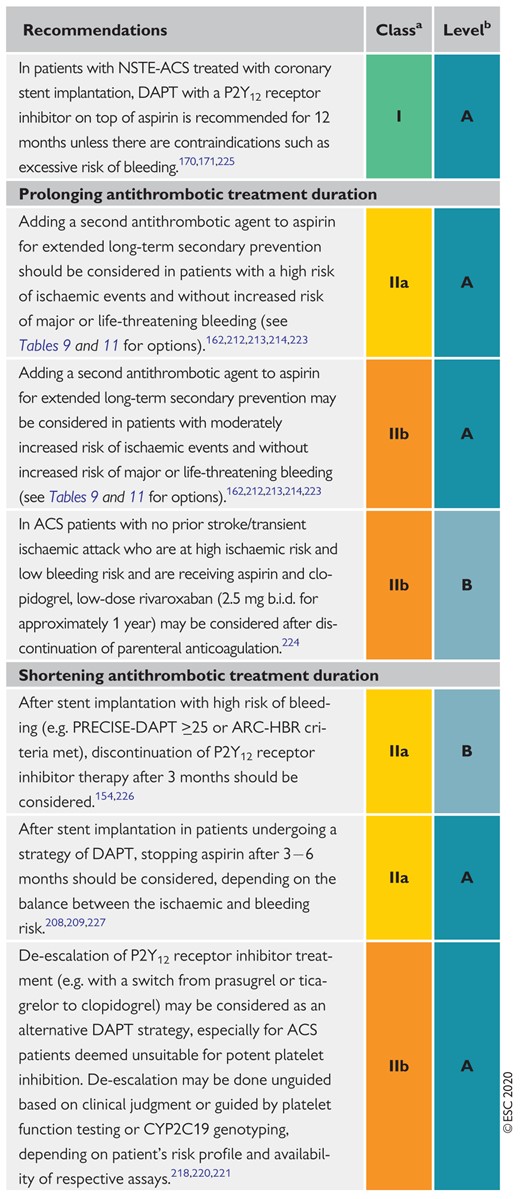 |
 |
ACS = acute coronary syndromes; ARC-HBR = Academic Research Consortium – High Bleeding Risk; b.i.d. = bis in die (twice a day); DAPT = dual antiplatelet therapy; NSTE-ACS = non-ST-segment elevation acute coronary syndrome; PRECISE-DAPT = PREdicting bleeding Complications In patients undergoing Stent implantation and subsEquent Dual Anti Platelet Therapy.
Class of recommendation.
Level of evidence.
Recommendations for post-interventional and maintenance treatment in patients with non-ST-segment elevation acute coronary syndrome
 |
 |
ACS = acute coronary syndromes; ARC-HBR = Academic Research Consortium – High Bleeding Risk; b.i.d. = bis in die (twice a day); DAPT = dual antiplatelet therapy; NSTE-ACS = non-ST-segment elevation acute coronary syndrome; PRECISE-DAPT = PREdicting bleeding Complications In patients undergoing Stent implantation and subsEquent Dual Anti Platelet Therapy.
Class of recommendation.
Level of evidence.
Treatment options for extended dual antithrombotic or antiplatelet therapies
| Drug . | Dose . | Indication . | NNT (ischaemic outcomes) . | NNH (bleeding outcomes) . |
|---|---|---|---|---|
| DAT regimens for extended treatment (including aspirin 75–100 mg o.d.) | ||||
| Rivaroxaban (COMPASS trial) | 2.5 mg b.i.d. | Patients with CAD or symptomatic PAD at high risk of ischaemic events | 77 | 84 |
| DAPT regimens for extended treatment (including aspirin 75–100 mg o.d.) | ||||
| Clopidogrel (DAPT trial) | 75 mg/d | Post MI in patients who have tolerated DAPT for 1 year | 63 | 105 |
| Prasugrel (DAPT trial) | 10 mg/d (5 mg/d if body weight <60 kg or age >75 years) | Post PCI for MI in patients who have tolerated DAPT for 1 year | 63 | 105 |
| Ticagrelor (PEGASUS-TIMI 54) | 60/90 mg b.i.d. | Post MI in patients who have tolerated DAPT for 1 year | 84 | 81 |
| Drug . | Dose . | Indication . | NNT (ischaemic outcomes) . | NNH (bleeding outcomes) . |
|---|---|---|---|---|
| DAT regimens for extended treatment (including aspirin 75–100 mg o.d.) | ||||
| Rivaroxaban (COMPASS trial) | 2.5 mg b.i.d. | Patients with CAD or symptomatic PAD at high risk of ischaemic events | 77 | 84 |
| DAPT regimens for extended treatment (including aspirin 75–100 mg o.d.) | ||||
| Clopidogrel (DAPT trial) | 75 mg/d | Post MI in patients who have tolerated DAPT for 1 year | 63 | 105 |
| Prasugrel (DAPT trial) | 10 mg/d (5 mg/d if body weight <60 kg or age >75 years) | Post PCI for MI in patients who have tolerated DAPT for 1 year | 63 | 105 |
| Ticagrelor (PEGASUS-TIMI 54) | 60/90 mg b.i.d. | Post MI in patients who have tolerated DAPT for 1 year | 84 | 81 |
Drugs (in addition to aspirin 75–100 mg/d) for extended DAPT treatment options are in alphabetical order. For indications and definitions for high/moderately increased risk and bleeding risk see Table 9 and Figure 7. NNT refers to the primary ischaemic endpoints of the respective trials and NNH refers to the key safety (bleeding) endpoints. NNT and NNH numbers from the DAPT trial are pooled numbers for clopidogrel and prasugrel.
b.i.d. = bis in die (twice a day); CAD = coronary artery disease; COMPASS = Cardiovascular OutcoMes for People using Anticoagulation StrategieS; DAPT = dual antiplatelet therapy; DAT = dual antithrombotic therapy; MI = myocardial infarction; NNH = number needed to harm; NNT = number needed to treat; o.d. = once daily; PAD = peripheral artery disease; PCI = percutaneous coronary intervention; PEGASUS-TIMI 54 = Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin-Thrombolysis In Myocardial Infarction 54.
Treatment options for extended dual antithrombotic or antiplatelet therapies
| Drug . | Dose . | Indication . | NNT (ischaemic outcomes) . | NNH (bleeding outcomes) . |
|---|---|---|---|---|
| DAT regimens for extended treatment (including aspirin 75–100 mg o.d.) | ||||
| Rivaroxaban (COMPASS trial) | 2.5 mg b.i.d. | Patients with CAD or symptomatic PAD at high risk of ischaemic events | 77 | 84 |
| DAPT regimens for extended treatment (including aspirin 75–100 mg o.d.) | ||||
| Clopidogrel (DAPT trial) | 75 mg/d | Post MI in patients who have tolerated DAPT for 1 year | 63 | 105 |
| Prasugrel (DAPT trial) | 10 mg/d (5 mg/d if body weight <60 kg or age >75 years) | Post PCI for MI in patients who have tolerated DAPT for 1 year | 63 | 105 |
| Ticagrelor (PEGASUS-TIMI 54) | 60/90 mg b.i.d. | Post MI in patients who have tolerated DAPT for 1 year | 84 | 81 |
| Drug . | Dose . | Indication . | NNT (ischaemic outcomes) . | NNH (bleeding outcomes) . |
|---|---|---|---|---|
| DAT regimens for extended treatment (including aspirin 75–100 mg o.d.) | ||||
| Rivaroxaban (COMPASS trial) | 2.5 mg b.i.d. | Patients with CAD or symptomatic PAD at high risk of ischaemic events | 77 | 84 |
| DAPT regimens for extended treatment (including aspirin 75–100 mg o.d.) | ||||
| Clopidogrel (DAPT trial) | 75 mg/d | Post MI in patients who have tolerated DAPT for 1 year | 63 | 105 |
| Prasugrel (DAPT trial) | 10 mg/d (5 mg/d if body weight <60 kg or age >75 years) | Post PCI for MI in patients who have tolerated DAPT for 1 year | 63 | 105 |
| Ticagrelor (PEGASUS-TIMI 54) | 60/90 mg b.i.d. | Post MI in patients who have tolerated DAPT for 1 year | 84 | 81 |
Drugs (in addition to aspirin 75–100 mg/d) for extended DAPT treatment options are in alphabetical order. For indications and definitions for high/moderately increased risk and bleeding risk see Table 9 and Figure 7. NNT refers to the primary ischaemic endpoints of the respective trials and NNH refers to the key safety (bleeding) endpoints. NNT and NNH numbers from the DAPT trial are pooled numbers for clopidogrel and prasugrel.
b.i.d. = bis in die (twice a day); CAD = coronary artery disease; COMPASS = Cardiovascular OutcoMes for People using Anticoagulation StrategieS; DAPT = dual antiplatelet therapy; DAT = dual antithrombotic therapy; MI = myocardial infarction; NNH = number needed to harm; NNT = number needed to treat; o.d. = once daily; PAD = peripheral artery disease; PCI = percutaneous coronary intervention; PEGASUS-TIMI 54 = Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin-Thrombolysis In Myocardial Infarction 54.
Risk criteria for extended treatment with a second antithrombotic agent
| High thrombotic risk (Class IIa) . | Moderate thrombotic risk (Class IIb) . |
|---|---|
| Complex CAD and at least 1 criterion | Non-complex CAD and at least 1 criterion |
| Risk enhancers | |
| Diabetes mellitus requiring medication | Diabetes mellitus requiring medication |
| History of recurrent MI | History of recurrent MI |
| Any multivessel CAD | Polyvascular disease (CAD plus PAD) |
| Polyvascular disease (CAD plus PAD) | CKD with eGFR 15–59 mL/min/1.73 m2 |
| Premature (<45 years) or accelerated (new lesion within a 2-year time frame) CAD | |
| Concomitant systemic inflammatory disease (e.g. human immunodeficiency virus, systemic lupus erythematosus, chronic arthritis) | |
| CKD with eGFR 15–59 mL/min/1.73 m2 | |
| Technical aspects | |
| At least 3 stents implanted | |
| At least 3 lesions treated | |
| Total stent length >60 mm | |
| History of complex revascularization (left main, bifurcation stenting with ≥2 stents implanted, chronic total occlusion, stenting of last patent vessel) | |
| History of stent thrombosis on antiplatelet treatment | |
| High thrombotic risk (Class IIa) . | Moderate thrombotic risk (Class IIb) . |
|---|---|
| Complex CAD and at least 1 criterion | Non-complex CAD and at least 1 criterion |
| Risk enhancers | |
| Diabetes mellitus requiring medication | Diabetes mellitus requiring medication |
| History of recurrent MI | History of recurrent MI |
| Any multivessel CAD | Polyvascular disease (CAD plus PAD) |
| Polyvascular disease (CAD plus PAD) | CKD with eGFR 15–59 mL/min/1.73 m2 |
| Premature (<45 years) or accelerated (new lesion within a 2-year time frame) CAD | |
| Concomitant systemic inflammatory disease (e.g. human immunodeficiency virus, systemic lupus erythematosus, chronic arthritis) | |
| CKD with eGFR 15–59 mL/min/1.73 m2 | |
| Technical aspects | |
| At least 3 stents implanted | |
| At least 3 lesions treated | |
| Total stent length >60 mm | |
| History of complex revascularization (left main, bifurcation stenting with ≥2 stents implanted, chronic total occlusion, stenting of last patent vessel) | |
| History of stent thrombosis on antiplatelet treatment | |
In line with guideline recommendations, CAD patients are stratified into two different risk groups (high vs. moderately increased thrombotic or ischaemic risk). Stratification of patients towards complex vs. non-complex CAD is based on individual clinical judgement with knowledge of patients’ cardiovascular history and/or coronary anatomy. Selection and composition of risk-enhancing factors are based on the combined evidence of clinical trials on extended antithrombotic treatment in CAD patients162,212,214 and on data from related registries.228–230
CAD = coronary artery disease; CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate; MI = myocardial infarction; PAD = peripheral artery disease.
Risk criteria for extended treatment with a second antithrombotic agent
| High thrombotic risk (Class IIa) . | Moderate thrombotic risk (Class IIb) . |
|---|---|
| Complex CAD and at least 1 criterion | Non-complex CAD and at least 1 criterion |
| Risk enhancers | |
| Diabetes mellitus requiring medication | Diabetes mellitus requiring medication |
| History of recurrent MI | History of recurrent MI |
| Any multivessel CAD | Polyvascular disease (CAD plus PAD) |
| Polyvascular disease (CAD plus PAD) | CKD with eGFR 15–59 mL/min/1.73 m2 |
| Premature (<45 years) or accelerated (new lesion within a 2-year time frame) CAD | |
| Concomitant systemic inflammatory disease (e.g. human immunodeficiency virus, systemic lupus erythematosus, chronic arthritis) | |
| CKD with eGFR 15–59 mL/min/1.73 m2 | |
| Technical aspects | |
| At least 3 stents implanted | |
| At least 3 lesions treated | |
| Total stent length >60 mm | |
| History of complex revascularization (left main, bifurcation stenting with ≥2 stents implanted, chronic total occlusion, stenting of last patent vessel) | |
| History of stent thrombosis on antiplatelet treatment | |
| High thrombotic risk (Class IIa) . | Moderate thrombotic risk (Class IIb) . |
|---|---|
| Complex CAD and at least 1 criterion | Non-complex CAD and at least 1 criterion |
| Risk enhancers | |
| Diabetes mellitus requiring medication | Diabetes mellitus requiring medication |
| History of recurrent MI | History of recurrent MI |
| Any multivessel CAD | Polyvascular disease (CAD plus PAD) |
| Polyvascular disease (CAD plus PAD) | CKD with eGFR 15–59 mL/min/1.73 m2 |
| Premature (<45 years) or accelerated (new lesion within a 2-year time frame) CAD | |
| Concomitant systemic inflammatory disease (e.g. human immunodeficiency virus, systemic lupus erythematosus, chronic arthritis) | |
| CKD with eGFR 15–59 mL/min/1.73 m2 | |
| Technical aspects | |
| At least 3 stents implanted | |
| At least 3 lesions treated | |
| Total stent length >60 mm | |
| History of complex revascularization (left main, bifurcation stenting with ≥2 stents implanted, chronic total occlusion, stenting of last patent vessel) | |
| History of stent thrombosis on antiplatelet treatment | |
In line with guideline recommendations, CAD patients are stratified into two different risk groups (high vs. moderately increased thrombotic or ischaemic risk). Stratification of patients towards complex vs. non-complex CAD is based on individual clinical judgement with knowledge of patients’ cardiovascular history and/or coronary anatomy. Selection and composition of risk-enhancing factors are based on the combined evidence of clinical trials on extended antithrombotic treatment in CAD patients162,212,214 and on data from related registries.228–230
CAD = coronary artery disease; CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate; MI = myocardial infarction; PAD = peripheral artery disease.
5.2 Pharmacological treatment of ischaemia (Supplementary Data)
5.2.1 Supportive pharmacological treatment (Supplementary Data)
5.2.2 Nitrates and beta-blockers (Supplementary Data)
Recommendations for anti-ischaemic drugs in the acute phase of non-ST-segment elevation acute coronary syndrome
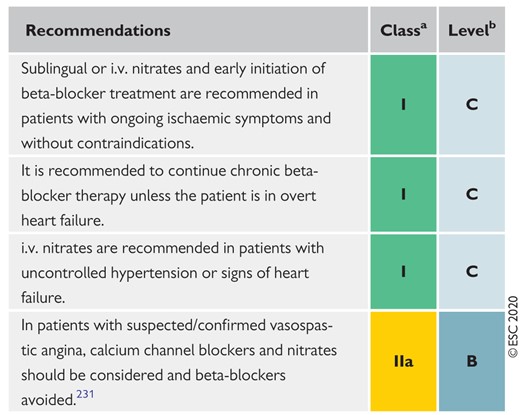 |
 |
i.v. = intravenous.
Class of recommendation.
Level of evidence.
Recommendations for anti-ischaemic drugs in the acute phase of non-ST-segment elevation acute coronary syndrome
 |
 |
i.v. = intravenous.
Class of recommendation.
Level of evidence.
5.3 Managing oral antiplatelet agents in patients requiring long-term oral anticoagulants
5.3.1 Patients with atrial fibrillation without mechanical prosthetic heart valves or moderate-to-severe mitral stenosis undergoing percutaneous coronary intervention or managed medically (Supplementary Data)
In 6–8% of patients undergoing PCI, long-term OAC is indicated and should also be continued during the procedure because its interruption and bridging with parenteral anticoagulants may lead to increased thromboembolic episodes and bleeds.232–234 In patients undergoing PCI, it is unknown whether it is safe to bridge non-vitamin K antagonist (VKA) OACs (NOACs) with parenteral anticoagulants or continue NOACs without additional parenteral anticoagulation, while no parenteral anticoagulation is needed if the international normalized ratio (INR) is >2.5 in VKA-treated patients.235–237 Strategies to minimize PCI-related complications in patients on OACs are listed in Table 12.
Suggested strategies to reduce bleeding risk related to percutaneous coronary intervention
|
|
|
|
|
|
|
|
|
|
|
|
DAPT = dual antiplatelet therapy; GP = glycoprotein; INR = international normalized ratio; i.v. = intravenous; NOAC = non-vitamin K antagonist oral anticoagulant; OAC = oral anticoagulation/anticoagulant; PCI = percutaneous coronary intervention; UFH = unfractionated heparin; VKA = vitamin K antagonist.
Suggested strategies to reduce bleeding risk related to percutaneous coronary intervention
|
|
|
|
|
|
|
|
|
|
|
|
DAPT = dual antiplatelet therapy; GP = glycoprotein; INR = international normalized ratio; i.v. = intravenous; NOAC = non-vitamin K antagonist oral anticoagulant; OAC = oral anticoagulation/anticoagulant; PCI = percutaneous coronary intervention; UFH = unfractionated heparin; VKA = vitamin K antagonist.
In NSTE-ACS patients, evidence on the management of patients undergoing PCI requiring long-term OAC is derived from subgroups of RCTs (see Table 13 and Supplementary Data section 5.3.1).238–242
Overall, in patients with AF without mechanical prosthetic valves or moderate-to-severe mitral stenosis, the evidence supports the use of NOACs over VKA in terms of safety (i.e. lower bleeding risk). DAT with a NOAC at the recommended dose for stroke prevention and single antiplatelet therapy (SAPT preferably clopidogrel, chosen in more than 90% of cases in available trials) is recommended as the default strategy up to 12 months after a short period (up to 1 week) of triple antithrombotic therapy (TAT with NOAC and DAPT Figure 8). Although none of the available RCTs were designed to detect subtle differences in ischaemic events, the numerically higher risk of stent thrombosis or MIs observed in some trials might have been offset by the higher risk of bleeding, resulting in a neutral effect on major adverse cardiovascular events (MACE) or overall death.243,244 At variance with the default strategy, in patients with HBR, DAT should be shortened to 6 months by withdrawing the ongoing antiplatelet therapy; while in patients with high coronary ischaemic risk, TAT should be prolonged up to 1 month, followed by DAT for up to 12 months. There is currently limited evidence to support the use of OACs with ticagrelor or prasugrel as dual therapy after PCI as an alternative to TAT.241,245 Following coronary stenting, DAPT with aspirin and ticagrelor or prasugrel, without OAC, may be considered as an alternative to TAT in patients with high ischaemic risk NSTE-ACS and AF and one non-sex stroke risk factor within the first 4 weeks. Regarding the need to continue with any antiplatelet agent beyond 12 months, the AFIRE trial randomized 2236 AF patients treated with PCI or CABG more than 1 year earlier or with documented CAD to receive either monotherapy with rivaroxaban or combination therapy with rivaroxaban plus a single antiplatelet agent.246 Rivaroxaban monotherapy (15 mg o.d. or 10 mg o.d. with creatinine clearance (CrCl) 15–49 mL/min) was non-inferior to combination therapy for the primary efficacy composite endpoint of stroke, systemic embolism, MI, unstable angina requiring revascularization, or overall death (HR 0.72, 95% CI 0.55–0.95). Rivaroxaban monotherapy was superior for the primary safety endpoint of major bleeding (HR 0.59, 95% CI 0.39–0.89).
In NSTE-ACS patients managed medically, available data support DAT over TAT, with a single antiplatelet agent (most commonly clopidogrel) for at least 6 months.247 In a registry, bleeding risk was increased on TAT compared to VKA plus a single antiplatelet agent at 90 days, but not at 1 year, without differences in ischaemic events.248 In addition, warfarin plus clopidogrel resulted in a non-significant reduction in major bleeds compared with TAT, with a non-significant reduction in MI or cardiovascular death.249 In the randomized Antithrombotic Therapy after Acute Coronary Syndrome or PCI in Atrial Fibrillation (AUGUSTUS) trial,241 approximately 23% of enrolled patients presented with medically managed ACS. In these patients, apixaban significantly reduced bleeding events vs. VKA (HR 0.44, 95% CI 0.28–0.68) and death or hospitalization (HR 0.71, 95% CI 0.54–0.92), while no significant differences were observed in death or ischaemic events (HR 0.71, 95% CI 0.46–1.09]). Aspirin vs. place