-
PDF
- Split View
-
Views
-
Cite
Cite
Hiroshi Nishiura, Scott B. Halstead, Natural History of Dengue Virus (DENV)—1 and DENV—4 Infections: Reanalysis of Classic Studies, The Journal of Infectious Diseases, Volume 195, Issue 7, 1 April 2007, Pages 1007–1013, https://doi.org/10.1086/511825
Close - Share Icon Share
Abstract
Background. The natural history of wild-type dengue virus (DENV) infections of humans, including incubation and infectious periods, requires further study.
Methods. Two experimental studies in the Philippines of DENV-4 (1924–1925) and DENV-1 (1929–1930) were reexamined. The intrinsic incubation periods were fitted to log-normal distribution using the maximum likelihood method, and the infectious and extrinsic incubation periods were assessed by proportions of successful transmissions causing clinically apparent dengue. Correlations between the intrinsic incubation period and other variables and univariate associations between clinical severity and serotype were also examined.
Results. Mean ± SD incubation periods were 6.0 ± 1.4 and 5.7 ± 1.5 days for DENV-4 and DENV-1, respectively. Significant negative correlations were observed between the incubation period and duration of fever (r = −0.43 and −0.33). Even 1 and 2 days before the onset of fever, 80.0% (95% confidence interval [CI], 44.9%–100%) and 25.0% (CI, 0%–67.4%) of biting experiments caused clinically apparent dengue. DENV-1 infections resulted in a significantly longer duration of fever than DENV-4 infections (P < .01).
Conclusions. Incubation period was negatively correlated with disease severity, potentially reflecting a doseresponse mechanism. The historical data provided useful details concerning serotype differences in the natural history of primary DENV infections.
Dengue fever (DF) is a vectorborne disease caused by 4 closely related dengue viruses (DENV 1–4) [1, 2]. DF is distributed in most tropical and subtropical areas, where Aedes aegypti and/or A. albopictus are abundant [3]. Infection with DENV can also cause dengue hemorrhagic fever (DHF), a syndrome characterized by increased vascular permeability, plasma leakage, hypovolemia, and shock [4, 5]. Although the pathogenesis of DHF is not fully understood, several risks have been reported: secondary infection with heterologous strains [6, 7], primary infection in infants born to dengueimmune mothers [8], differing virulence of the strain [9], and differing human susceptibility according to race or genetic factors [10, 11]. Because DENV infections are often clinically inapparent, it is difficult to clarify the epidemiology of DF and dengue pathophysiology without virological support [12, 13].
The natural history of DF involves the time from infection to onset and recovery from disease. However, although the clinical symptoms of DF and DHF have been relatively well described [14, 15], the incubation and infectious periods for different DENVs have yet to be examined in detail. This is partly attributable to the difficulty in identifying the time of infection after a mosquito bite. Detailed information on the above may assist in quantification of the transmission potential and contribute to an understanding of the epidemiologic processes of this disease [16].
Before World War II, a number of studies were conducted to prove the mode of transmission and further basic knowledge on dengue. These studies were performed by US Army commissions [17] and by Australian [18] and Japanese [19, 20] researchers. Successful studies include those of Ashburn and Craig [21] and Cleland, Bradley, and McDonald [18], the former of which was recently reviewed in the Journal of Infectious Diseases [22]. In the present study, we reanalyzed data from 2 human volunteer experiments in which the DENV etiology was known.
Methods
Brief historical background. Both dengue studies were performed in the Philippines and involved large numbers of human volunteers recruited from US Army personnel. The first study, conducted by Joseph Franklin Siler, MiltonWeston Hall, and Arthur Parker Hitchens took place in 1924–1925, whereas the latter was organized by James Stevens Simmons, Joseph Harold St. John, and Francois Hiie Kari Reynolds in 1929–1930 (the latter study is available at: http://plaza.umin.ac.ip/infepi/simmons1.pdf). Both studies were originally published in the Philippine Journal of Science [23, 24] and were reprinted with appendices by the Bureau of Printing, Manila [25, 26]. In 1971–1972, Halstead obtained blood samples from 4 and 5 volunteers involved in each study and demonstrated specific neutralizing antibodies to DENV-4 and DENV-1, respectively [27]. Following these experimental studies of dengue, J. F. Siler (1875–1960) reported the efficacy of antityphoid vaccines [28], whereas J. S. Simmons (1890–1954) contributed to the advancement of epidemiology during and after World War II, as chief of the Preventive Medicine Service, Office of the Surgeon General and, thereafter, as dean and professor of the Harvard School of Public Health [29].
The transmission of DENV by arthropods was first demonstrated by Graham, who claimed that Culex quinquefasciatus was the vector [30]. This finding was supported by Ashburn and Craig [21, 22], but not by the Australian researchers Bancroft [31] and Cleland et al. [18], who suggested instead that A. aegypti was the major vector, with very different implications for mosquito control of dengue transmission. In the studies of Ashburn and Craig, mosquitoes were refed on susceptible volunteers within 24 h after viremic blood meals, too short a period for transmission of the virus [21, 22]. Given this knowledge, Siler et al. reexamined DENV transmission by A. aegypti at different extrinsic incubation and human infectious periods (by varying the time interval between feeding and inoculation and the time the mosquitoes were fed on volunteers—i.e., before and after onset of the disease). Simmons et al. extended these investigations to mechanical transmission of DENV through interrupted feeding of C. quinquefasciatus, development of a challenge model to confirm protective immunity, and examination of the possibility that monkeys and other animals (i.e., chickens, lizards, mice, guinea pigs, and rabbits) were capable of being vertebrate hosts of dengue.
Experimental settings and data. The studies by Siler et al. of DENV-4 infections involved 64 volunteers and 111 A. aegypti biting experiments, resulting in 47 clinically apparent cases of DF, whereas that of DENV-1 infections by Simmons et al. included 98 volunteers, 80 of whom developed DF, the majority transmitted by A. aegypti but some by A. albopictus (12 cases) and C. quinquefasciatus (1 case). The number of biting experiments in the latter study was not documented in the original publication. All volunteers were white male US Army personnel. Each volunteer was required to meet 3 conditions to determine that they had not been exposed to DENV: (1) short residence in the tropics (preferably <3 months), (2) previous residence in the United States in geographical areas where DF had never occurred, and (3) no record of admission to the hospital for DF and a negative medical history suggestive of a previous attack.
Siler's group propagated A. aegypti in a screw-top, glass fruit jar of 1000 cm3 capacity. A. aegypti used in the experiments had emerged 2–7 days earlier. Variable numbers of female mosquitoes were used for each experiment (range, 2–36; median [25%–75% quartile], 10 [6–18]). Extrinsic incubation periods also varied, ranging from 2 to 75 days (median [25%–75% quartile], 20 days [17–29 days]), including experiments that failed to result in clinically apparent infections. The time of mosquito feeding on infected volunteers ranged from 52 h before to 114 h after the onset of fever (median [25%–75% quartile], 18 h [6–32 h] after onset); based on Siler's pilot study, mosquitoes were fed on volunteers mainly during the first day of disease. Mosquitoes were placed in a cage specifically designed to permit biting (approximate internal dimensions, 35 cm × 60 cm × 35 cm), which was applied to one leg of the volunteers. The experimental series was initiated by exposing mosquitoes to patients with naturally occurring DF during the first 3 days after onset of fever. Successful chains of transmission by A. aegypti reasonably excluded the possibility of other directly transmitted diseases with a similar clinical appearance (and, thereafter, the serological study of Halstead showed that chikungunya and St. Louis encephalitis virus infections had not occurred [27]). At the outset of the experiments, Siler also allowed himself to be bitten by infected mosquitoes; he developed DF and then proceeded to infect volunteers from September 1924 to March 1925.
The experimental conditions of the study by Simmons et al. of DENV-1 were similar to those of Siler et al., but the number of mosquitoes used for each experiment varied widely from 1 to 150 (median [25%–75% quartile], 22 [10–56]). Experimental infections were conducted from December 1928 to March 1930. In both experiments, all DF cases survived. Further details are given in the original literature (e.g., the Simmons et al. study is available at: http://plaza.umin.ac.ip/infepi/simmons1.pdf.
Consequently, information on 47 DENV-4 and 80 DENV-1 cases was compiled, including the name and age of the volunteers, the number of mosquitoes used, the extrinsic incubation period, dates of inoculation and onset, and duration of fever. The intrinsic incubation period (i.e., incubation period in humans) was calculated from the difference between the date of onset (i.e., fever) and mosquito bite. In the study of DENV-4, the infectious period in humans was assessed by the proportion of successful transmissions (i.e., inoculation resulting in clinically apparent infection) by disease-age (n = 62 available experiments for disease-ages ranging from −3 to 5 days); disease-age is defined as the time since onset of initial symptom (i.e., 0 days indicates disease-age at onset of fever, and −1 day indicates 1 day before onset of fever). In the same way, using only the study of DENV-4, the extrinsic incubation period was determined from the proportion of successful transmissions with different intervals between feeding of A. aegypti and inoculation (n = 19 experiments for <16 days after feeding). In the DENV-1 study, tabulated individual data included minimum white blood cell (WBC) counts, duration of leukopenia, and appearance of a primary and secondary rash. In both studies, common signs and symptoms were recorded in groups (i.e., presented separately from other individual data) for 48 (47 experiments and 1 pilot study) and 60 cases of DENV-4 and DENV-1 infections, respectively. In the DENV-4 case summaries, some signs and symptoms were recorded as “not noted”; this notation was deemed equivalent to “absent” in our analyses.

Second, differences in the above parameters between experiments were examined using the F test followed by Student t test. Because the intrinsic incubation periods were skewed to the right, this variable was analyzed after logarithmic transformation (including the following analyses of correlations).Comparisons of each sign and symptom between experiments were performed using the χ2 or Fisher's exact test.
Furthermore, comparisons between the intrinsic incubation period and other variables were also performed. Age, duration of fever, extrinsic incubation period, and the number of mosquitoes used were recorded in both experiments, and the remaining variables—minimum WBC counts, duration of leukopenia, and appearance of primary and secondary rash—were available only for the DENV-1 study. Because primary and secondary rash data are dichotomous variables, we used univariate logistic regression to assess the association with the incubation period. For the remaining variables, correlations with the incubation period were assessed by Pearson's correlation coefficients. The level of statistical significance was set at α = 0.05. All statistical data were analyzed using the statistical software JMP IN (version 5.1; SAS Institute).
Results
Descriptive characteristics. Among those who developed clinically apparent DF, mean ± SD ages were 22.5 ± 4.0 and 22.0 ± 3.4 years in the DENV-4 and DENV-1 studies, respectively. Age was not significantly different between studies (P = .97). The mean ± SD durations of fever were 3.6 ± 1.2 and 4.8 ± 1.2 days, respectively. The febrile period of DENV-1 infection was significantly longer than that of DENV-4 (P < .01). In the DENV-1 study, the mean ± SD values for minimum WBC count and duration of leukopenia were 3.01 × 109 ± 0.78 × 109cells/L and 6.1 ± 2.0 days, respectively, and 37.5% (95% CI, 27.7%–48.5%) and 68.8% (95% CI, 57.9%–77.8%) of diseased individuals exhibited a primary or secondary rash, respectively.
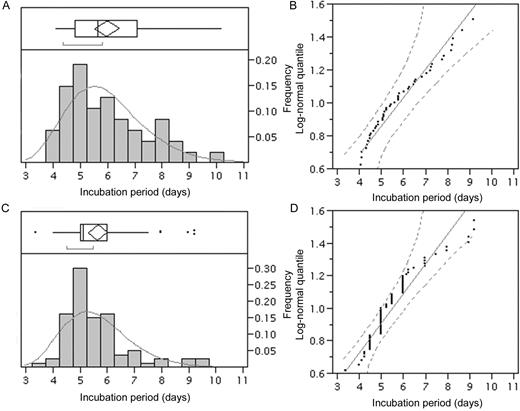
Distributions of the intrinsic incubation period are shown in figure 1. The mean ± SD values were 6.0 ± 1.4 days (95% CI, 5.6–6.4 days) and 5.7 ± 1.5 days (95% CI, 5.3–6.0 days) in the DENV-4 and DENV-1 studies, respectively, yielding maximum likelihood estimates of the scale and shape parameters of 1.76 and 0.24 and 1.71 and 0.22, respectively. Figure 1B–figure 1D shows quantile plots for the hypothesized log-normal distributions. The majority of the data points and quantiles were relatively close in both distributions. Although the points snaked over the line for DENV-1 (figure 1D), only a few cases out of 80 were outside the 95% CI. The Kolmogorov-Smirnov test revealed no significant deviation in the DENV-4 study (D = 0.11; P = .15) and only a slight deviation in the DENV-1 study (D = 0.17; P = .01) between the observed and expected distributions. The incubation period was not significantly different between the 2 viruses (P = .15).
Frequency distributions of the intrinsic incubation periods of dengue fever cases. Distributions of the incubation periods of dengue fever with dengue virus (DENV)—4 (A; n = 47) and DENV-1 (C; n = 80) infections based on maximum likelihood estimations assuming log-normal distributions. Observed (bars) and predicted (solid lines) frequencies are shown. The whiskers extend from the ends of each box to the outermost data points that fall within the distances computed. The bracket along the edge of each box identifies the shortest half, which represents the highest density (50%) of the observations. Log-normal quantile plots of the incubation periods of DENV-4 (B) and DENV-1 (D) infections are also given. The diagonal reference lines indicate the line of fit, and the 2 dashed lines denote the 95% confidence interval (0.001–0.99).
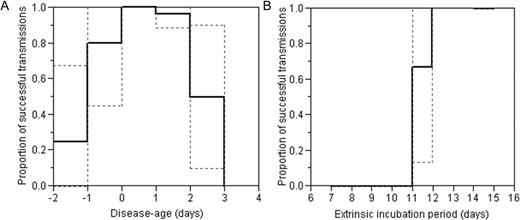
The infectious period and extrinsic incubation period in the DENV-4 study are summarized in figure 2 as the proportion of successful dengue transmissions. Even 1 and 2 days before the onset of fever, 80.0% (95% CI, 44.9%–100%) and 25.0% (95% CI, 0%–67.4%) of biting experiments resulted in clinically apparent DF. At 2 days after the onset of fever, 50.0% (95% CI, 10.0%–90.0%) successfully caused clinically apparent DF, but no successful transmission was achieved thereafter. As for the extrinsic incubation period in the DENV-4 study, 66.7% (95% CI, 27.2%–100%) of biting experiments succeeded in transmission at 11 days. No experiments successfully caused clinically apparent DF at 10 days or earlier, but all did so at 12 days or after.
Infectious period and extrinsic incubation period of dengue fever caused by dengue virus (DENV)—4. Both infectious period (A; n = 62) and extrinsic incubation period (B; n = 19) were measured as the proportion of successful transmissions that resulted in clinically apparent dengue fever. Thus, it should be noted that the proportions given in each panel are probably underestimated because of the substantial fraction of clinically inapparent infections. The broken lines show 95% confidence intervals, and the solid lines show the expected value. The horizontal axis in panel A (the disease-age) is 0 at the onset of fever.
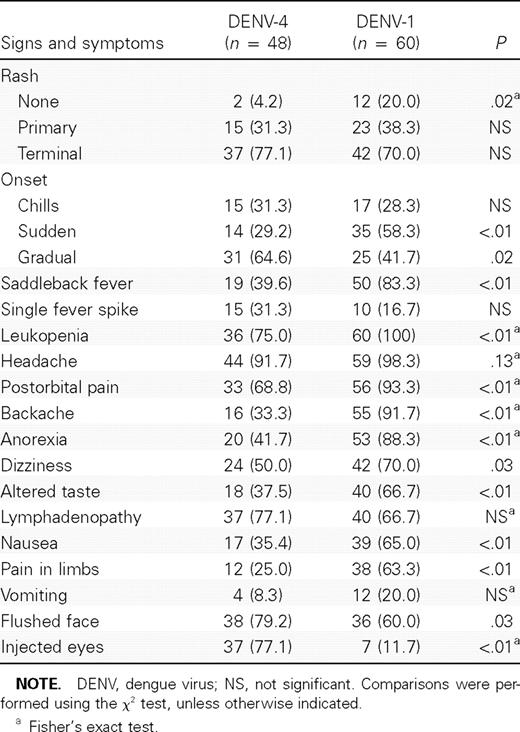
Comparison of signs and symptoms.Table 1 compares signs and symptoms in the 2 studies. Whereas the proportion of those showing a rash was significantly higher with DENV-4 (P = .02), the appearance of a primary and terminal rash did not differ (P = .44 and P = .41, respectively). Sudden onset was more frequently observed with DENV-1 infections (P < .01), and a gradual onset was more common with DENV-4 (P = .02). Saddleback (biphasic) fever was more frequently observed with DENV-1 infections than DENV-4 infections (P < .01). Moreover, the DENV-1 study revealed more-severe signs and symptoms than the DENV-4 study with regard to the following: proportion of leukopenia, complaints of several kinds of pain, variations in appetite, dizziness, a flushed face, and injected eyes.
Comparison of the clinical findings of 2 experimental studies on dengue fever.
Incubation period versus other variables. In both studies, age (P = .94 and P = .99, for DENV-4 and DENV-1, respectively), extrinsic incubation period (P = .23 and P = .20, for DENV-4 and DENV-1, respectively) and the number of mosquitoes (P = .45 and P = .77, for DENV-4 and DENV-1, respectively) did not show any significant correlations with intrinsic incubation period. Figure 3 shows the relationship between the incubation period and duration of fever. Significant negative correlations were observed between these 2 variables in both studies (r = −0.43 [P < .01] and r = −0.33 [P < .01], respectively). In the DENV-1 study, the incubation period was also compared with other variables: minimum WBC counts (P = .28) and the duration of leukopenia (P = .55) did not reveal significant correlations with the incubation period, and no association was found between the incubation period and frequency of primary and secondary rash (P = .81 and P = .86, respectively).
Comparisons between the incubation period and duration of fever in the 2 experimental studies of dengue fever. Results with dengue virus (DENV)—4 (A; n = 47; r = −0.43 [P < .01]) and DENV-1 (B; n = 80; r = −0.33 [P < .01]) infection are shown. The density ellipse contains the specified mass of points as determined by 95% of expected variation.
Discussion
This study examined several aspects of the natural history of DF based on 2 rigorous historical experiments. These studies are unique in that the experimental details for primary DENV infection were provided and because the etiology was known [27]. These records are the most comprehensive of their kind with regard to the intrinsic and extrinsic incubation and infectious periods. Moreover, it was possible for us to compare signs and symptoms as well as other epidemiologic parameters between serotypes. Three findings were notable.
First, this study is the first, to our knowledge, to characterize statistical details of the incubation period of DF. Usually, it is extremely difficult to identify the time of infection for vectorborne diseases. For this reason, the incubation period is conveniently extrapolated from experimental inoculation data, travel histories of cases, or incidence data during the point source outbreak, which offers the time of exposure [33]. Similar data to ours (i.e., inoculation data) are available as a result of widespread use of malaria therapy [34]. We observed that the incubation period was negatively correlated with the duration of fever. Assuming that the duration of fever reasonably reflects the severity of disease, the most likely explanation for this negative correlation is a dose-response phenomenon. Other variables (e.g., minimum WBC count and the duration of leukopenia) were not significantly correlated with the incubation period and were difficult to directly interpret because of limited time precision and unclear laboratory methods. A dose-response relationship in DENV infection is consistent with descriptions from experimental studies of humans infected with DENV-2 intracutaneously [17, 35]. In these studies, an inoculation of 10 MID/mL produced classical dengue fever, whereas an inoculation of 1 MID/mL reportedly caused milder disease. An experimental study of dengue in intracerebrally infected mice also demonstrated that incubation period was negatively correlated with the dose of inoculum [36]. Although there might be potential confounders (e.g., underlying host-related factors regulating susceptibility [37]), and despite the fact the sample sizes were limited, the 2 experimental studies presented suggest that the incubation period is an important predictor of subsequent disease severity.
Second, based on the DENV-4 study, we found that DF cases could be infectious to the mosquitoes even 2 days before the onset of fever. It should be noted that we assessed this information using the proportions causing clinically apparent DF; that is, even though the historical studies recruited susceptible volunteers under strict enrollment conditions, the proportions obtained in figure 2 might have been underestimated (i.e., more inoculations may have successfully caused silent infections and the infectious period could start slightly earlier than is shown here). However, this does not influence our finding suggesting that the infectious period starts at least a few days earlier than the symptomatic period. As for the extrinsic incubation period, successful transmissions were observed 11 days or more after mosquitoes took blood meals. Although, historically, this point improved our overall understanding, adding to the findings of Ashburn and Craig [21, 22], it has to be noted that the experiments were performed from September to March in the Philippines. The extrinsic incubation period can be influenced by temperature and other environmental factors [38, 39], and moreover, the population dynamics of A. aegypti also vary seasonally [40].
Third, comparisons revealed that the severity of DF differed between DENV-4 and DENV-1. The duration of fever, which roughly corresponds to the severity, was significantly longer for DENV-1 than for DENV-4 infections. In addition, other signs and symptoms also supported the view that DENV-1 infection results in a more-severe clinical appearance than infection with DENV-4. Although it is a widely held view that primary DENV infections in adults more often result in overt disease than primary DENV infections in children [41, 42], our findings are consistent with those of recent studies that suggest that primary DENV-4 infection results in relatively mild illness [42, 43] and that primary infections with DENV-1 result in fairly severe manifestations [44, 45]. Most clinical studies of DF cases attributed to DENV-4 are accompanied by secondary infections in children, and, thus, the present study helped explicitly confirm that primary DENV-4 infections in adults tend to be relatively mild.
In conclusion, this study reexamined 2 historical experiments, revealing details of the incubation and infectious periods of dengue as well as serotype-specific clinical characteristics. We believe this knowledge will significantly enhance the overall understanding of the natural history of DF during primary infection.




![Comparisons between the incubation period and duration of fever in the 2 experimental studies of dengue fever. Results with dengue virus (DENV)—4 (A; n = 47; r = −0.43 [P < .01]) and DENV-1 (B; n = 80; r = −0.33 [P < .01]) infection are shown. The density ellipse contains the specified mass of points as determined by 95% of expected variation.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/195/7/10.1086_511825/2/m_195-7-1007-fig003.jpeg?Expires=1716345632&Signature=C74k2ke58rp6dkuuII7rL24qZv~mzovRntNrCtr8CQ6LZkUkbD9yXcevv4LMRJy35YEnP4X1DqsvjFQkpQkEm0bbqJ~FjR3yjAqTGz399webuM8EqkIkMBzNPpPwsuGJUpzLaV~2MVB-zotr6fGTBKVxfQOH4U-YYl~UfQoCkMaS4wXK81g99NhApVS9J3HpzSh6GkUsCvcCFw3GOz46yA-XoxdnjNUpmSu5skYPw7opYGk3IdE6i4uGx4OATlDBwoWbr4alWHQ-9QZFkw95mtkye0Royc-33AdkwV6-PnegxgFbmGdOsHoWX-dbGaqaNADfr5I7U9tiwEoQpi4YFg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
