The Oxford Handbook of the Neurobiology of Pain
The Oxford Handbook of the Neurobiology of Pain
Contents
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
Basic Organization of Pain Pathways Basic Organization of Pain Pathways
-
Primary Afferent Input to the Spinal and Trigeminal Dorsal Horn Primary Afferent Input to the Spinal and Trigeminal Dorsal Horn
-
Dorsal Horn Interneurons Dorsal Horn Interneurons
-
Classification of Interneurons in Laminae I–III Classification of Interneurons in Laminae I–III
-
Excitatory and Inhibitory Interneurons Excitatory and Inhibitory Interneurons
-
Morphological and Electrophysiological Classification of Interneurons Morphological and Electrophysiological Classification of Interneurons
-
Neurochemical Classification of Interneurons Neurochemical Classification of Interneurons
-
Functions of Dorsal Horn Interneurons Functions of Dorsal Horn Interneurons
-
-
Spinal Projection Neurons and Ascending Tracts Spinal Projection Neurons and Ascending Tracts
-
Neuronal Circuits in the Dorsal Horn Neuronal Circuits in the Dorsal Horn
-
Synaptic Inputs to Projection Neurons Synaptic Inputs to Projection Neurons
-
Synaptic Inputs to Interneurons Synaptic Inputs to Interneurons
-
Synaptic Inputs to Primary Afferents Synaptic Inputs to Primary Afferents
-
Neuropeptide Signaling in the Dorsal Horn Neuropeptide Signaling in the Dorsal Horn
-
-
Supraspinal Pain Pathways Supraspinal Pain Pathways
-
Parallel Pain Processing pathways Parallel Pain Processing pathways
-
The Spinothalamic-Cortical Pain Discriminative Pathway The Spinothalamic-Cortical Pain Discriminative Pathway
-
The Spinoparabrachial-Limbic Pain Affective Pathway The Spinoparabrachial-Limbic Pain Affective Pathway
-
-
Descending Pathways Descending Pathways
-
References References
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
15 Central Nervous System Pain Pathways
Andrew J. Todd, University of Glasgow, UK
Fan Wang, Duke University, USA
-
Published:10 July 2018
Cite
Abstract
Nociceptive primary afferents detect stimuli that are normally perceived as painful, and these afferents form synapses in the dorsal horn of the spinal cord and the spinal trigeminal nucleus. Here they are involved in highly complex neuronal circuits involving projection neurons belonging to the anterolateral tract (ALT) and interneurons, which modulate the incoming sensory information. The ALT neurons convey somatosensory information to a variety of brain regions that are involved in the various aspects of the pain experience. A spinothalamic-cortical pathway provides input to several regions of the cerebral cortex, including the first and second somatosensory areas (S1, S2), the insula and the cingluate cortex. These regions are thought be responsible for the sensory-discriminative aspects of pain (S1), pain-related learning (S2), the autonomic and motivational responses (insula), and the negative affect (cingulate). Another ascending system, The spinoparabrachial-limbic pathway targets a variety of brain regions, including the amygdala, and is likely involved in the affective component of pain. A descending system that includes the limbic system, the periaqueductal gray matter of the midbrain, the locus coeruleus, and the rostral ventral medulla, can suppress pain, and this operates partly through the monoamine transmitters noradrenaline and serotonin which are released in the spinal and trigeminal dorsal horn.
Basic Organization of Pain Pathways
Primary afferent neurons can respond to a variety of stimuli that affect the body surface or internal tissues and organs. Many of these primary afferents are tuned to detect stimuli that cause actual or potential tissue damage, and these are known as nociceptors. Primary afferents from the trunk and limbs enter the spinal cord through the dorsal roots, while those from the head are conveyed via the trigeminal (fifth cranial) nerve to trigeminal nuclei in the brainstem. All of these afferents, which use glutamate as their principal fast transmitter, form excitatory synapses on neurons within the spinal dorsal horn and trigeminal nuclei. Both of these regions contain large numbers of neurons. Most of these are interneurons, with axons that arborize locally, giving rise to complex circuits that process somatosensory information. This information is then conveyed to the brain via projection neurons. These are relatively large cells with axons that enter the white matter and are organized into ascending tracts that terminate in various somatosensory brain regions. In addition to this ascending system, there are also descending pathways, which originate from the brainstem and from cortical regions (primarily somatosensory cortex), and terminate diffusely within the spinal dorsal horn and trigeminal nuclei.
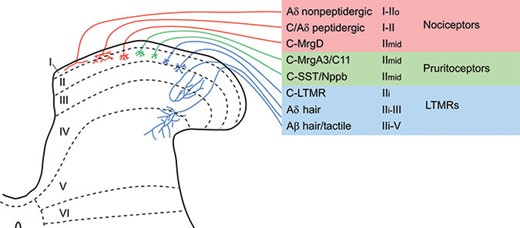
The spinal dorsal horn can be divided into six parallel laminae based on neuronal size and packing density (Rexed, 1952), and this scheme has been used to define the regions targeted by different types of primary afferent (Figure 15.1), as well as to define specific populations of spinal cord neurons. The dorsal horn of the spinal cord is somatotopically organized, with the body surface being mapped in a two-dimensional pattern on the rostrocaudal and mediolateral axes. The dorsoventral axis (i.e., across the laminae) reflects a modality-specific pattern that is determined by the termination of different types of primary afferent (e.g., nociceptors, thermoreceptors, and low-threshold mechanoreceptors). Most nociceptive afferents terminate in the superficial part of the spinal dorsal horn (laminae I and II), or in the most caudal of the trigeminal nuclei, which is known as the spinal trigeminal nucleus caudalis (SpVc). The SpVc nucleus has a similar lamination pattern to the spinal dorsal horn, and is also known as the trigeminal or medullary dorsal horn.
Central projections of different classes of primary afferent. The major classes of primary afferent that have been identified in recent transcriptomic studies (Li et al., 2016; Usoskin et al., 2015) are listed, together with what is currently known about their functions and their central terminations in the spinal dorsal horn. Note that pruritoceptive afferents may also function as nociceptors, and that the C-MrgD nociceptors may act as pruritoceptors. Afferents that respond to innocuous cooling or warming appear to be poorly represented in these studies, and are therefore not included in this scheme.
Over 50 years ago, Melzack and Wall (1965) proposed that neuronal circuits within the dorsal horn could “gate” the sensory information conveyed by nociceptive primary afferents, and thus modulate the perception of pain. More recent studies have revealed that the circuitry within this region is highly complex, and we are still far from a complete understanding of how this is organized. However, it is clear that the spinal dorsal horn and SpVc play a major role in modulating pain in both normal and pathological states. Indeed, much of the information transmitted by projection neurons has already undergone significant processing. The spinal and medullary dorsal horns are likely to provide important targets for new pain therapies, as they contain numerous receptors and signaling pathways. This chapter will summarize what we know about the anatomy of pain pathways from the periphery to higher brain centers, with detailed emphasis on the organization of neuronal populations and circuits in the spinal cord and SpVc.
Primary Afferent Input to the Spinal and Trigeminal Dorsal Horn
The organization of primary afferents is dealt with elsewhere, and here we are concerned with the central projections of these neurons. Early electrophysiological classification schemes for primary afferents have recently been expanded by transcriptomic approaches (Li et al., 2016; Usoskin et al., 2015), which have revealed several distinctive afferent classes, particularly among the fine-diameter (unmyelinated C, and thinly myelinated Aδ) afferents, most of which function as nociceptors, thermoreceptors, or pruritoceptors (“itch detectors”). Although the focus of the chapter is on pain, the low-threshold mechanoreceptors (LTMRs) are also important, because of their likely involvement in touch-evoked pain (tactile allodynia), which is a feature of certain chronic pain conditions (Campbell, Raja, Meyer, & Mackinnon, 1988).
The primary afferent input to the spinal dorsal horn is represented schematically in Figure 15.1. Although our understanding of the central projections of myelinated afferents has come mainly from studies in which individual afferents were labelled in combined electrophysiological/anatomical studies (Brown, 1981), there have been very few studies of this type for unmyelinated (C) fibers (Sugiura, Lee, & Perl, 1986). Much of what we know about these is based on the identification of primary afferent terminals by means of neurochemical markers.
Myelinated LTMRs (A-LTMRs) arborize in the deeper part of the dorsal horn, in a region that extends from the inner part of lamina II (lamina IIi) to lamina V, and each of the different functional populations has a specific laminar distribution pattern (Abraira & Ginty, 2013). Some C fibers respond to non-noxious hair movement as well as cooling (C-LTMRs), and these terminate in lamina IIi (Seal et al., 2009). Myelinated nociceptors, most of which are Aδ fibers, convey “fast pain.” Many of these have a compact termination zone in lamina I and the outer part of lamina II (lamina IIo) (Light & Perl, 1979), although others appear to arborize diffusely throughout laminae I–V (Boada & Woodbury, 2008; Woodbury & Koerber, 2003). The remaining C fibers consist mainly of nociceptors and thermoreceptors, with some also responding to pruritic stimuli. Although several neurochemically distinct classes of primary afferent have been revealed in transcriptomic studies, we do not yet fully understand how these map onto functionally defined populations. Many C fibers (and some Aδ nociceptors) express neuropeptides, and one well-characterized population consists of cells that contain calcitonin gene-related peptide (CGRP), substance P, and galanin. These are thought to function as nociceptors, and terminate mainly in laminae I and IIo, with some branches penetrating deeply. These afferents express the transient receptor potential channel vanilloid 1 (TRPV1), and are required for perception of heat pain (Cavanaugh et al., 2009). Another major class of C nociceptors is defined by expression of the Mas-related G protein-coupled receptor D (MrgD). These afferents, which lack neuropeptides and do not express TRPV1 in the mouse, have a very compact termination pattern in the middle part of lamina II, and are needed for the normal perception of mechanical pain (Cavanaugh et al., 2009; Zylka, Rice, & Anderson, 2005). Two further groups of C fibers that have been recognized are: (1) those defined by co-expression of the neuropeptides somatostatin and natriuretic polypeptide B (NPPB), and (2) those that express the Mas-related G protein-coupled receptors MrgA3/MrgC11. There is evidence that both of these populations function as pruritoceptors (L. Han et al., 2013; Huang et al., 2018), and both arborize in the middle part of lamina II.
Since all primary afferents are glutamatergic, they require vesicular glutamate transporters (VGLUTs) to enrich the amino acid in their synaptic vesicles. These transporters are differentially expressed among primary afferents. A-LTMRs express VGLUT1, and account for ~60% of the VGLUT1-immunoreactive boutons in the dorsal horn, with the remainder being corticospinal tract terminals (Abraira et al., 2017; Todd et al., 2003). VGLUT2 is expressed by Aδ nociceptors and most C fibers, although the level of VGLUT2 protein that can be detected in their central terminals with immunocytochemistry is generally very low (Brumovsky, Watanabe, & Hokfelt, 2007; Todd et al., 2003). C-LTMRs express VGLUT3, and since they are the major source of this transporter, antibodies against VGLUT3 can be used to reveal their central terminals, which are located in the innermost part of lamina II (Seal et al., 2009).
Central terminals of primary afferents possess a variety of receptors that are likely to be important in regulating their function through presynaptic modulatory mechanisms. These include ionotropic glutamate receptors, GABAA and GABAB receptors, and purinergic (P2X3) receptors. They also express receptors for various neuropeptides, including μ, δ, and κ opioid receptors (Todd & Koerber, 2013).
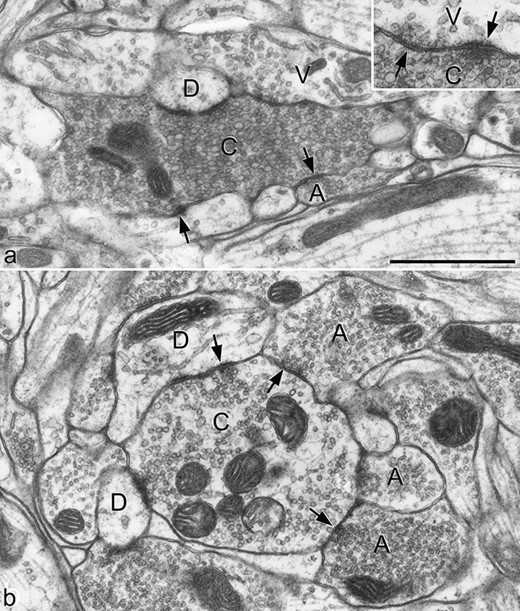
As stated before, all primary afferents give rise to boutons that form glutamatergic synapses on dorsal horn neurons. A characteristic feature of some classes of primary afferent is that their central terminals form complex arrangements known as synaptic glomeruli. These contain a central bouton (the primary afferent terminal), surrounded by several dendritic and/or axonal profiles. The dendritic components may be dendritic spines or shafts, and are postsynaptic to the primary afferent terminal, although they may form reciprocal (axodendritic/dendroaxonic) synaptic arrangements. The peripheral axons in the synaptic glomerulus, which are GABAergic, are presynaptic to the primary afferent terminal at axoaxonic synapses (Todd, 1996), which are the substrate for classical presynaptic inhibition. In addition, there can be triadic arrangements, in which a peripheral axon is presynaptic both to the central (primary afferent) bouton and a dendrite, with the latter also receiving a synapse from the primary afferent. Two distinct types of synaptic glomerulus (types I and II) have been identified in rodent dorsal horn, and these differ in both the appearance of the central (primary afferent) bouton and the arrangement of peripheral profiles (Ribeiro-da-Silva & Coimbra, 1982) (Figure 15.2). These two types are thought to be associated with C-MrgD nociceptors and Aδ D-hair afferents, respectively. Other classes of primary afferent can also be associated with synaptic glomeruli. These include some of the substance P/CGRP-containing afferents, as well as those that express NPPB/somatostatin (Ribeiro-da-Silva, Tagari, & Cuello, 1989; Salio, Ferrini, Muthuraju, & Merighi, 2014). Other primary afferents form simpler synaptic arrangements, but they may still be subject to presynaptic inhibition mediated by axoaxonic synapses.
Two types of synaptic glomerulus in the superficial dorsal horn of the rat. The terminals of some types of primary afferent form central boutons of synaptic glomeruli within the dorsal horn. A: Central axons of type I glomeruli (C) are derived from non-peptidergic C nociceptors, and are typically surrounded by vesicle-containing dendrites (V), dendrites that lack vesicles (D), and peripheral axons (A). The peripheral axon is presynaptic at an axoaxonic synapse with the central bouton. The central bouton forms synapses with both types of dendrite, and can receive dendroaxonic synapses from the vesicle-containing dendrites. In some cases, reciprocal axodendritic synapses are present, as shown in the inset. B: Central axons of type II glomeruli (C) are thought to originate from Aδ D-hair endings, and are presynaptic to dendrites (D), most of which lack vesicles. These glomeruli are often surrounded by several peripheral axons (A), which are presynaptic to the C bouton, and can form triadic synapses involving the C bouton and a dendrite. Arrows in both parts indicate synapses. Scale bar: 1 μm.
Dorsal Horn Interneurons
Interneurons are thought to account for ~99% of all of the nerve cells in the spinal dorsal horn (Abraira & Ginty, 2013; Todd, 2010, 2017). Interneurons are found in all dorsal horn laminae, but here we will focus on those in laminae I–III, as these have been extensively studied and are likely to play important roles in pain mechanisms. All interneurons so far identified appear to give rise to local axonal projections that arborize within the same spinal cord segment that contains the cell body. However, retrograde labelling studies have shown that a significant proportion of the interneurons in this region also have axons that project for several segments (Bice & Beal, 1997). Until recently, the postsynaptic targets of these “propriospinal” axons was unknown, but we have found that at least some of them terminate in the lateral spinal nucleus, a collection of neurons located in the dorsal part of the lateral white matter (Gutierrez-Mecinas, Polgár, Bell, Herau, & Todd, 2018). Spinal interneurons are anatomically and physiologically heterogeneous, and there have been numerous attempts to assign them to specific functional populations. It is essential to be able to define these populations, so that we can investigate their contributions to synaptic circuits and their roles in somatosensory processing.
Classification of Interneurons in Laminae I–III
Excitatory and Inhibitory Interneurons
A basic functional distinction can be made between excitatory interneurons, which use glutamate as their principal neurotransmitter, and inhibitory interneurons, which are GABAergic and/or glycinergic. Immunocytochemistry to reveal GABA and glycine has been used to identify the inhibitory interneurons, but this is technically difficult, and a more convenient way is to reveal the transcription factor Pax2, which is expressed by all of these cells (Foster et al., 2015; Larsson, 2017). Quantitative studies in the mouse reveal that ~25% of neurons in laminae I–II and 40% of those in lamina III are inhibitory, and these are all thought to be interneurons (Polgár, Durrieux, Hughes, & Todd, 2013). The remaining neurons are assumed to be glutamatergic, and these will include both projection neurons (see following discussion) and excitatory interneurons. Axons belonging to either inhibitory or excitatory interneurons can be identified based on their expression of vesicular transporters: the vesicular GABA transporter (VGAT) and VGLUT2, respectively. This method can be applied to neurons that have undergone whole-cell patch-clamp recording if an appropriate tracer (e.g., Neurobiotin) has been included in the recording pipette, and this allows the neurotransmitter phenotype of electrophysiologically characterized neurons to be determined (Yasaka, Tiong, Hughes, Riddell, & Todd, 2010).
Morphological and Electrophysiological Classification of Interneurons
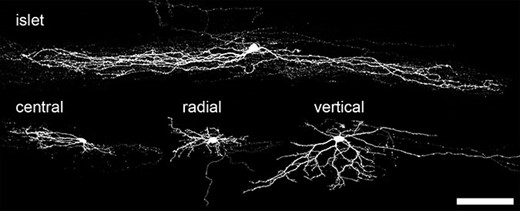
In many parts of the central nervous system (CNS), the dendritic morphology of neurons is closely related to their function; therefore, numerous studies have attempted to identify morphological classes among the interneurons in laminae I–III. Initially, these were carried out with Golgi staining, but more recently they have been performed on neurons that were labelled during whole-cell patch-clamp recording experiments, thus allowing correlations between structure and function to be investigated. The most widely accepted classification scheme based on these approaches has been that of Grudt and Perl (2002), who identified four major types of neuron in lamina II: islet, vertical, radial, and central cells (Figure 15.3). Islet cells have axonal and dendritic trees that are highly elongated along the rostrocaudal axis; vertical cells have a conical dendritic tree that extends ventrally from the soma; radial cells have radiating dendrites that form a relatively compact dendritic tree; central cells are similar in appearance to islet cells but with much shorter dendritic trees. There were also physiological differences between these cells, since radial and vertical cells often showed delayed action potential firing in response to injection of depolarizing current, whereas islet cells fired continuously (tonically) throughout the current injection. Central cells were further subdivided, based on their firing pattern and the presence or absence of an A-type potassium current (IA current). However, although other studies have identified cells of these morphological types, a limitation of this scheme is that a significant proportion of cells cannot be assigned to any of these classes.
Morphological classification of lamina II neurons. Four main morphological types of interneuron have been identified in lamina II: islet cells, central cells, radial cells, and vertical cells. Examples of each type are shown here (scale bar: 100 μm).
Yasaka et al. (2010) recorded from a large sample of lamina II interneurons and compared morphology and firing patterns with neurotransmitter phenotypes. They reported that all islet cells were inhibitory, whereas radial cells and most vertical cells were excitatory. However neurons that were classified as central cells could be either inhibitory or excitatory, and there were many “morphologically unclassified” neurons among both transmitter types. They also reported that firing pattern was related to function, since most excitatory cells showed firing patterns associated with IA current (delayed, gap, or reluctant firing), whereas these were seldom seen in inhibitory neurons. These findings suggest that, although there is a correlation between morphology and function, neither morphology nor firing pattern can provide a comprehensive classification scheme for interneurons in lamina II. Even less is known about interneurons in other laminae, but it seems likely that a similar degree of caution is needed when defining populations based on these parameters.
Neurochemical Classification of Interneurons
The dorsal horn contains a wide variety of neuropeptides, neuropeptide receptors, and other proteins that are differentially expressed by populations of neurons in laminae I–III. Some of these molecules are preferentially expressed by either excitatory or inhibitory interneurons (Todd, 2017; Todd & Spike, 1993). For example, several neuropeptides, including somatostatin, substance P, gastrin-releasing peptide (GRP), neurotensin, neurokinin B (NKB), and cholecystokinin, are found mainly or exclusively in excitatory neurons. In contrast, neuropeptide Y (NPY), galanin, and nociceptin are restricted to inhibitory interneurons, while the opioid peptides enkephalin and dynorphin are found in both types of interneuron. Calcium-binding proteins are also highly expressed in this region, with calbindin and calretinin being found predominantly in excitatory neurons, and parvalbumin mainly in inhibitory cells.
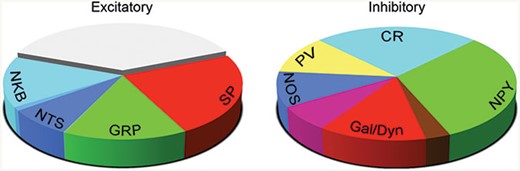
These neurochemical markers often show a non-uniform laminar distribution, which suggests that they may be expressed by specific subpopulations among the interneurons in laminae I–III. We have recently confirmed this by showing that non-overlapping neurochemical populations can be found within both major classes (Boyle et al., 2017; Gutierrez-Mecinas et al., 2017; Gutierrez-Mecinas, Furuta, Watanabe, & Todd, 2016). In laminae I–III of the rat dorsal horn, we identified four largely separate classes of inhibitory interneuron, based on expression of NPY, galanin, parvalbumin, and the neuronal form of nitric oxide synthase (nNOS) (Polgár, Sardella, et al., 2013). The galanin-containing cells also express dynorphin, although this is also found in some excitatory interneurons. We have since found a very similar pattern in the mouse dorsal horn, although there is significant overlap between the neurons that express nNOS and those with galanin/dynorphin. Between them, we found that these four populations accounted for ~75% of the inhibitory interneurons in laminae I–II of the mouse (Boyle et al., 2017), and the remaining 25% appear to correspond to the inhibitory interneurons that express the calcium-binding protein calretinin (Smith et al., 2015). Among the excitatory interneurons, we have recently shown that four neuropeptides—substance P, GRP, neurotensin, and NKB—are expressed in largely separate subpopulations, which account for nearly two-thirds of the excitatory neurons in laminae I–II (Gutierrez-Mecinas et al., 2017). Another peptide, somatostatin, is also restricted to excitatory interneurons, but this is far more widely expressed. The excitatory interneurons that contain neurotensin or NKB largely correspond to a previously defined class of neurons: those that express the γ isoform of protein kinase C (PKCγ). The relative sizes of the different neurochemical populations of excitatory and inhibitory interneurons within the superficial laminae are shown in Figure 15.4.
Neurochemically defined populations among the excitatory and inhibitory interneurons in the superficial dorsal horn of the mouse spinal cord. The pie charts show the relative sizes of the neurochemical populations among the excitatory and inhibitory interneurons in laminae I–II of the mouse dorsal horn. For the inhibitory interneurons, the unmarked wedges indicate overlap between the NOS and Galanin/Dynorphin populations (purple wedge) and between the Galanin/Dynorphin and NPY populations (brown wedge). Note that a substantial proportion of the excitatory interneurons is not accounted for by the expression of the four neuropeptides shown. NKB: neurokinin B, NTS: neurotensin; GRP: gastrin-releasing peptide; SP: substance P; CR: calretinin; NPY: neuropeptide Y; Gal: galanin; Dyn: dynorphin; NOS: neuronal nitric oxide synthase; PV: parvalbumin.
There is some evidence relating neurochemistry to morphology. Among the inhibitory interneurons, those that contain parvalbumin or calretinin often show islet morphology (Hughes et al., 2012; Smith et al., 2015), whereas those that express NPY, galanin/dynorphin, or nNOS are never islet cells and are morphologically heterogeneous (Todd, 2017). Less is known about the morphology of neurochemically defined excitatory interneuron populations, although both the PKCγ cells (which include neurotensin and NKB populations) and the GRP cells appear to have central or “unclassified” morphology. We have recently found that many of the substance P-expressing neurons correspond to radial cells, but interestingly, none of these four populations (substance P-, GRP-, neurotensin-, or NKB-expressing cells) seem to include vertical cells (Bell, Dickie, Iwagaki, Polgár, & Todd, unpublished data).
Functions of Dorsal Horn Interneurons
Early insight into the role of inhibitory interneurons came from studies involving spinal administration of GABAA and glycine receptor antagonists (Sivilotti & Woolf, 1994; Yaksh, 1989), since these interneurons are the main source of GABAergic and glycinergic synapses within the dorsal horn. These studies showed that blocking inhibitory synaptic transmission resulted in exaggerated pain responses, and led to the view that inhibitory interneurons are involved in suppressing both acute and pathological pain (Sandkuhler, 2009; Zeilhofer, Wildner, & Yevenes, 2012). More recently, molecular-genetic approaches have been used to target specific populations of interneurons and reveal their function. Foster et al. (2015) used intraspinal injection of viral vectors to manipulate the activity of glycinergic neurons, based on selective expression of the neuronal glycine transporter (GlyT2) by these cells. GlyT2-expressing cells account for a large proportion of dorsal horn inhibitory interneurons: ~20% of those in laminae I–II and ~90% of those in deeper laminae. Ablation or inactivation of these cells resulted in hypersensitivity to both mechanical and thermal stimuli, together with apparent signs of spontaneous itching. In contrast, activation of the cells suppressed the responses to acute noxious stimuli, as well as reducing signs of neuropathic pain, and itch behaviors following injection of pruritogens. Other studies have investigated the roles of smaller populations of inhibitory interneurons. Parvalbumin-expressing cells are likely to have a role in regulating tactile input, since they receive synapses from A-LTMRs, and their axons give rise to axoaxonic synapses on these afferents (Hughes et al., 2012). Consistent with this suggestion, Petitjean et al. (2015) showed that ablation of parvalbumin-expressing dorsal horn interneurons caused mechanical allodynia, whereas activating them increased withdrawal thresholds for mechanical (but not thermal) noxious stimuli and reduced mechanical allodynia after peripheral nerve injury. There is conflicting evidence concerning the role of the dynorphin-expressing inhibitory interneurons. Kardon et al. (2014) reported that these cells, together with those that expressed nNOS, were selectively lost in mice lacking the transcription factor Bhlhb5. Since these knockout mice develop exaggerated itch (Ross et al., 2010), they suggested that the dynorphin/galanin and/or nNOS populations may play a role in suppressing pruritogen-evoked itch. However, Duan et al. (2014) ablated dynorphin cells and observed an increase in response to noxious mechanical (but not thermal) stimuli and no change in itch behavior. We have recently shown that chemogenetic activation of dynorphin cells suppresses itch, whereas activation of the nNOS cells increased the thresholds for both mechanical and heat pain (Huang et al., 2018). This suggests that the dynorphin neurons are anti-pruritic, while the nNOS cells are anti-nociceptive. Cui et al. (2016) identified a population of inhibitory interneurons that were mainly located in lamina III, and were defined by the early expression of the receptor tyrosine kinase RET. They showed that ablating these cells resulted in mechanical allodynia and increased responses to mechanical and thermal noxious stimuli, as well as increased hyperalgesia in inflammatory and neuropathic models. In contrast, activating them reduced acute pain and hyperalgesia. François et al. (2017) reported that Penk1+ (enkephalin expressing) GABAergic interneurons gate nociceptive sensory transmission through coordinated GABA- and enkephalin-mediated presynaptic inhibition of sensory afferents.
Several studies have investigated the functions of excitatory interneurons. Mice lacking PKCγ show normal acute pain thresholds but fail to develop mechanical or thermal allodynia after nerve injury (Malmberg, Chen, Tonegawa, & Basbaum, 1997), which led to the suggestion that the PKCγ-expressing excitatory interneurons in laminae II–III are required for neuropathic pain. Duan et al. (2014) ablated cells that expressed somatostatin, and found that this dramatically reduced acute mechanical pain, as well as the mechanical allodynia seen in both neuropathic and inflammatory models. Consistent with these findings, Christensen et al. (2016) showed that activating somatostatin cells resulted in pain-like behavior, and confirmed that inactivating them reduced mechanical pain and allodynia, although they also saw a slight increase in latency of withdrawal to noxious heat. Somatostatin appears to be expressed by a large and heterogeneous population of excitatory interneurons in laminae I–II, and these findings demonstrate an important role for these cells in pain evoked by mechanical stimuli. Peirs et al. (2015) showed that transient expression of VGLUT3 defined a large population of excitatory interneurons in lamina III, which overlapped with PKCγ-expressing neurons. They showed that deletion of VGLUT3 from these cells attenuated acute mechanical pain and reduced mechanical allodynia caused by nerve injury or inflammation, whereas chemogenetic activation of the cells evoked mechanical allodynia but no change in thermal pain thresholds. The receptor for GRP (GRPR) is expressed by a population of excitatory interneurons in laminae I–IIo (Sun & Chen, 2007), and there are several lines of evidence that implicate these cells in the itch elicited by injection of pruritogens (Sun & Chen, 2007; Sun et al., 2009). Mice lacking GRPR, and those in which the GRPR-expressing cells have been ablated, show reduced responses to pruritogens, while intrathecal GRPR agonists evoke itch behaviors.
Taken together, the results of these studies indicate that several populations of dorsal horn interneurons are involved in pain and itch mechanisms, and that these are likely to have complex and overlapping functions.
Spinal Projection Neurons and Ascending Tracts
Spinal cord neurons with axons that reach the brain (projection neurons) can be revealed in anatomical studies by injection of retrograde tracers into regions of the brain to which they project (Figure 15.5). Major targets of dorsal horn projection neurons include the thalamus, the periaqueductal gray (PAG) matter of the midbrain, the lateral parabrachial area (LPb) in the pons, and various nuclei in the medulla. Dorsal horn neurons projecting to these brain regions are concentrated in lamina I and scattered throughout the deep dorsal horn (III–VI), but are largely absent from lamina II. Anatomical and electrophysiological studies have shown that there is extensive collateralization of axons, with many cells sending axons to several different brain nuclei (McMahon & Wall, 1985; Spike, Puskar, Andrew, & Todd, 2003). The projection is mainly to the contralateral side, although some dorsal horn neurons appear to project symmetrically to both sides of the brain (Spike et al., 2003), especially the projection from trigeminal SpVc neurons to LPb, which appears to be bilateral (Saito et al., 2017). Axons of dorsal horn projection neurons cross the midline and ascend in the white matter of the lateral or ventral funiculus. Although this system is often referred to as the anterolateral tract (ALT), based on its location in the anterolateral white matter in the human spinal cord, in rodents at least some of it occupies the dorsal part of the lateral funiculus (Gutierrez-Mecinas et al., 2018; McMahon & Wall, 1983). This difference in position may result from the presence of a large lateral corticospinal tract in humans, but not rodents, which would displace the ALT ventrally.
Quantitative studies in the lumbar enlargement of the rat spinal cord have shown that nearly all of the ALT neurons in lamina I send axons to the LPb, with ~30% targeting the PAG, but surprisingly only ~5% of these cells have axons that reach the thalamus (Todd, 2010). The situation is very different in the cervical enlargement, where the lamina I spinothalamic component is much larger (~40% of all projection neurons) (Polgár, Wright, & Todd, 2010), and this appears to be similar to the relative size of the spinothalamic projection from lamina I in both lumbar and cervical enlargements of the cat and monkey (Zhang & Craig, 1997; Zhang, Han, & Craig, 1996). The anatomy and the functions of the spinoparabrachial and spinothalamic pathways are discussed later in this chapter.
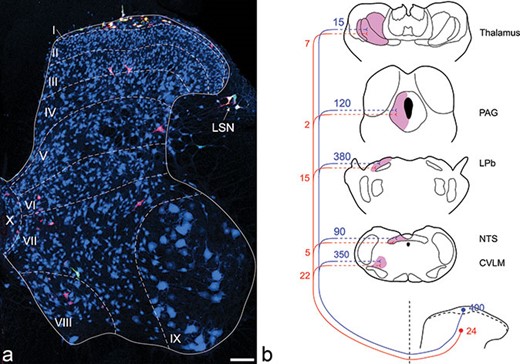
Anterolateral tract (ALT) projection neurons in the rat lumbar enlargement. (A) shows a transverse section from the L4 segment of a rat that had received injections of cholera toxin B subunit (CTb) into the caudal ventrolateral medulla, and of Fluorogold into the lateral parabrachial area. The section was immunostained to reveal CTb (red), Fluorogold (green), and the neuronal marker NeuN (blue). The locations of Rexed’s laminae are indicated. Tracer injections into these two sites can label virtually all of the projection neurons in lamina I, as well as scattered cells throughout the deep dorsal horn (laminae III–VI). Note that some cells have taken up both tracers, and therefore appear yellow. Scale bar = 100 μm. (B) Quantitative data showing the approximate numbers of neurons in laminae I and III in the rat L4 spinal segment that can be retrogradely labeled from various brain regions. LSN: lateral spinal nucleus; PAG: periaqueductal grey matter; LPb: lateral parabrachial area; NTS: nucleus of the solitary tract; CVLM: caudal ventrolateral medulla.
Many of the lamina I ALT neurons (80% in rat, 90% in mouse) express the neurokinin 1 receptor (NK1r), which is a target for substance P released from nociceptive primary afferents and some excitatory interneurons (Cameron et al., 2015; Mantyh et al., 1995; Todd, 2010). These cells are known to be densely innervated by substance P-containing primary afferents (Todd et al., 2002), indicating that they can be activated both by glutamatergic synaptic input and by substance P acting through volume transmission. Not all lamina I ALT neurons express the NK1r, and among those that lack the receptor, there is a sparse population of very large neurons (giant cells) that appear to lack significant primary afferent input (Polgár, Al-Khater, Shehab, Watanabe, & Todd, 2008).
Both electrophysiological studies and anatomical studies involving the activity-dependent transcription factor Fos have shown that lamina I projection neurons respond to a variety of noxious and pruritic stimuli (Akiyama, Curtis, Nguyen, Carstens, & Carstens, 2016; Andrew, 2009; Bester, Chapman, Besson, & Bernard, 2000; Moser & Giesler, 2014; Todd et al., 2002). Less is known about ALT projection neurons in deeper laminae, although one type has been characterized in some detail. This consists of large neurons in lamina III or IV that have long dorsal dendrites extending into lamina I (Todd, 2010). These cells are also densely innervated by substance P-expressing nociceptive afferents, and in the rat (but not the mouse), they all express the NK1r (Cameron et al., 2015). They have also been shown to respond to noxious stimuli (Polgár, Campbell, MacIntyre, Watanabe, & Todd, 2007). Not surprisingly, ablation of NK1r-expressing neurons, including ALT cells in laminae I and III–IV, led to a substantial reduction in hyperalgesia in both neuropathic and inflammatory pain models (Nichols et al., 1999), although acute pain thresholds were apparently unchanged. This suggests that the NK1r-expressing ALT projection neurons are involved in pathological pain, but are not required for detection of acute pain.
Based on the findings in patients with anterolateral cordotomy, it is thought that the ALT is responsible for perception of stimuli perceived as pain and itch, as well as temperature sensation. However, it is not the only ascending system to originate from the dorsal horn. Two additional pathways have been identified in other mammalian species: the postsynaptic dorsal column pathway (PSDC) and the spinocervical tract. Both of these have been shown to originate from cells in the deeper laminae of the dorsal horn, but much less is known about their functions (Abraira & Ginty, 2013; Brown, 1981).
Neuronal Circuits in the Dorsal Horn
Our knowledge about the synaptic connections between different neuronal components in the dorsal horn has come from two types of experimental approach. Firstly, immunocytochemical studies have been able to demonstrate contacts (and in some cases synapses) between axons and cell bodies or dendrites of neurochemically defined neuronal populations. Secondly, whole-cell patch-clamp recordings either made from pairs of neurons, or else combined with dorsal root stimulation, have been used to identify monosynaptic connections. Here, we will restrict ourselves to the synaptic circuits in which both components are well characterized. A diagram illustrating some of the circuits described here is shown in Figure 15.6.
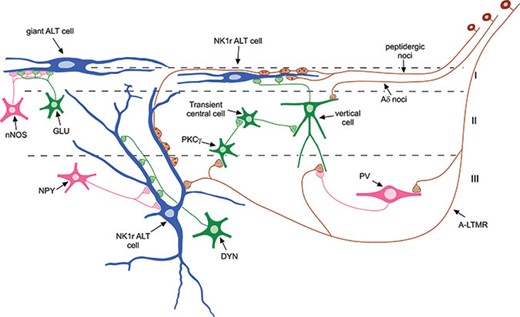
A diagram showing some of the synaptic circuits that have been identified in the spinal dorsal horn. In this diagram, inhibitory interneurons are shown in pink, excitatory interneurons in green, projection neurons in blue, and primary afferents in brown. Three ALT projection neurons are shown: cells in lamina I and III that express the neurokinin 1 receptor (NK1r) and a giant lamina I cell. Peptidergic nociceptors (noci) densely innervate the NK1r+ ALT neurons, and the lamina III cells also receive input from A-LTMRs. The lamina III ALT neurons are selectively innervated by NPY+ inhibitory interneurons and dynorphin+ (DYN) excitatory interneurons. The giant ALT cells apparently do not receive primary afferent input, but are densely innervated by nNOS+ inhibitory interneurons and by unknown class(es) of excitatory (glutamatergic) interneuron (GLU). A circuit involving three types of excitatory interneuron (PKCγ+, transient central, and vertical cells) is thought to convey input from A-LTMRs to lamina I ALT neurons. Relatively little is known about the primary afferent input to the vertical cells, but this is thought to include Aδ nociceptors and probably A-LTMRs. Inhibitory interneurons that express parvalbumin (PV) are innervated by A-LTMRs and generate axoaxonic synapses on these afferents (note that for simplicity, only one of these axoaxonic synapses is shown).
Synaptic Inputs to Projection Neurons
As we noted, NK1r-expressing ALT neurons in lamina I, as well as those in laminae III–IV, receive a dense synaptic input from substance P-expressing primary afferents, which are likely to correspond to peptidergic C fibers. It has been estimated that this input accounts for over half of the excitatory synaptic input to these neurons (Polgár, Al Ghamdi, & Todd, 2010). This presumably represents a powerful monosynaptic input from nociceptive primary afferents. The lamina III–IV ALT neurons receive some direct input from myelinated primary afferents in the deeper laminae, and these are thought to be A-LTMRs. However, despite having dendrites that pass through the superficial laminae, they do not seem to receive significant input from non-peptidergic C nociceptors (Todd, 2010). This indicates that synaptic connectivity is selectively organized, and does not depend only on the proximity to specific types of axon.
All of the ALT neurons receive numerous contacts from boutons with strong VGLUT2 immunoreactivity, which are thought to originate from local excitatory interneurons. In the case of lamina I giant cells, these appear to represent the main (or exclusive) source of excitatory synaptic input (Polgár et al., 2008). At present, we have limited information about the types of excitatory interneuron that are presynaptic to projection neurons, but these are likely to include vertical cells in lamina II (Cordero-Erausquin et al., 2009; Lu & Perl, 2005). It has been shown that 60% of the VGLUT2-immunoreactive boutons that synapse on lamina III ALT cells in the rat contain preprodynorphhin, which is only present in ~5% of all VGLUT2 boutons in this region (Baseer et al., 2012). This therefore suggests that these cells receive a highly selective input from dynorphin-expressing excitatory interneurons.
ALT projection neurons also receive synapses from local inhibitory interneurons, and again there is some evidence that this is organized in a selective manner (Todd, 2010). The lamina III ALT cells are densely innervated by axons that contain high levels of NPY, and these may well originate from a specific subset of NPY-expressing GABAergic interneurons (Polgár, Sardella, Watanabe, & Todd, 2011). In contrast, the lamina I giant cells are densely innervated by axons that originate from nNOS-expressing inhibitory interneurons (Ganley et al., 2015).
Synaptic Inputs to Interneurons
It is very likely that all dorsal horn interneurons receive direct synaptic input from primary afferents, but relatively little is known about the precise organization of these inputs. Excitatory interneurons of the vertical and radial types are innervated by both Aδ and C afferents, including TRPV1-expressing nociceptors (Grudt & Perl, 2002; Uta et al., 2010; Yasaka et al., 2007). Although much of the Aδ input to vertical cells is likely to originate from nociceptors, some may be from A-LTMRs (Aδ D-hair afferents), since the dendrites of these cells often extend ventrally into lamina III, and have been shown to receive contacts from myelinated primary afferents in this region (Yasaka et al., 2014). PKCγ-expressing neurons in laminae IIi and III are innervated by Aβ afferents, which are presumably A-LTMRs (Lu et al., 2013). Among the inhibitory interneurons, islet cells have been shown to receive monosynaptic input from C fibers, but not from myelinated afferents.
Interestingly, recording studies show that only around 10% of randomly recorded pairs of interneurons are synaptically linked, which suggests that synapses between different types of interneuron are also arranged according to specific rules (Lu & Perl, 2005). Much of what we know about the synaptic connections between interneurons is based on a series of elegant studies by Perl, Lu, and colleagues (Lu et al., 2013; Lu & Perl, 2003, 2005; Zheng, Lu, & Perl, 2010). They reported that islet cells were reciprocally connected with another population of inhibitory interneurons that were identified by the presence of green fluorescent protein (GFP) in mice in which GFP was expressed under control of the Prion promoter (PrP). These PrP-GFP cells, which were subsequently shown to correspond to the nNOS and galanin/dynorphin populations (Iwagaki, Garzillo, Polgár, Riddell, & Todd, 2013), were presynaptic to vertical cells. Both islet cells (inhibitory) and PKCγ neurons (excitatory) were shown to be presynaptic to a class of interneurons named “transient central cells,” and these formed excitatory synapses on vertical cells. Among the various synaptic connections described here, a serial circuit involving excitatory synapses between Aβ afferents, PKCγ cells, transient central cells, vertical cells, and lamina I projection neurons was identified (Figure 15.6). It was suggested that this pathway, which was normally under powerful feed-forward inhibitory control, provided a route through which A-LTMR input could activate nociceptive projection neurons, and thus contributed to tactile allodynia (Lu et al., 2013). However, since vertical cells may also receive direct input from A-LTMRs (see preceding discussion), it is likely that more than one mechanism contributes to allodynia.
These studies have provided major insights into the synaptic circuitry of the dorsal horn. However, certain caveats should be borne in mind. For example, the interneuron populations described in these studies may not be completely homogeneous. In addition, it is likely that both the synaptic inputs and outputs for each class of neuron are more extensive than those that have so far been identified.
Synaptic Inputs to Primary Afferents
As we noted, primary afferent boutons can receive axoaxonic synapses, which mediate GABAergic presynaptic inhibition. Although in most cases, the source of these synapses is not known, Hughes et al. (2012) demonstrated that parvalbumin-expressing inhibitory interneurons form axoaxonic synapses on the central terminals of presumed Aδ D-hair afferents. This input was highly selective, because ~75% of the parvalbumin-immunoreactive boutons in lamina IIi were involved in axoaxonic synapses. François et al. (2017) showed that Penk1+ (enkephalinergic) and also GABAergic interneurons provide presynaptic inhibitory inputs to primary afferents. However, the relationship between parvalbumin+ and the Penk1+ interneurons is unclear (Francois et al., 2017). Zhang et al. (2015) have suggested that primary afferents also receive direct descending GABAergic/enkephalinergic inputs.
Neuropeptide Signaling in the Dorsal Horn
Fast synaptic transmission by glutamate, GABA, and glycine is likely to be responsible for most of the somatosensory information processing in the dorsal horn. However, the presence of numerous peptides and their receptors indicates that neuropeptide signaling is also likely to be important.
Substance P, which can be released from nociceptive primary afferents (and to a lesser extent, from excitatory interneurons), will act on NK1 receptors that are mainly located on ALT projection neurons, resulting in depolarization of these cells. This mechanism is likely to contribute to pain perception, because mice lacking either the neuropeptide or its receptor show reductions in pain behavior (Cao et al., 1998; De Felipe et al., 1998). Somatostatin will also be released by both primary afferents and excitatory interneurons, and will act on the somatostatin 2a (sst2A) receptor, which is expressed on some primary afferent terminals and on many inhibitory interneurons, including the nNOS and galanin/dynorphin populations (Todd, 2017). Somatostatin will cause hyperpolarization of these interneurons, and it has been proposed that the resulting disinhibition of the nNOS and/or dynorphin/galanin cells contributes to the itch evoked by intrathecal administration of somatostatin or its analogues (Huang et al., 2018; Kardon et al., 2014). As described here, GRP released by a population of excitatory interneurons is also thought to play an important role in itch. Neurotensin and NKB are also expressed by excitatory interneurons, but, although the corresponding receptors are present in the dorsal horn, we know little about the functions of these peptides.
NPY and galanin are both expressed by inhibitory interneurons. Galanin is also present in some nociceptive primary afferents, while NPY is upregulated in A-LTMRs following peripheral nerve injury (Wakisaka, Kajander, & Bennett, 1991). Receptors for both peptides are expressed by large numbers of excitatory dorsal horn neurons, and in each case, release of the peptide is likely to suppress nociceptive transmission (Todd, 2017). At least in the case of NPY, there is evidence that signaling may be more important in pathological pain states (Solway, Bose, Corder, Donahue, & Taylor, 2011). The opioid peptides dynorphin and enkephalin are found in both inhibitory and excitatory cells, and all three classes of opioid receptor (μ, δ, κ) are expressed by primary afferents, with μ and κ receptors also being present on some dorsal horn neurons. The δ and μ receptors are thought to have complementary roles in anti-nociception (Scherrer et al., 2009), while the κ receptor appears to have an anti-pruritic action (Huang et al., 2018; Kardon et al., 2014).
Supraspinal Pain Pathways
Parallel Pain Processing pathways
As we mentioned, the second-order spinal ALT neurons or trigeminal medullary projection neurons send information to multiple higher brain areas such that temperature, itch, and pain sensation are processed by a large, distributed neural network. Although the exact connectivity of this large network remains poorly understood, it is generally recognized that there are two main pain processing streams: the spinothalamo-cortical pathway that processes the discriminative aspects of pain, and the spinoparabrachial-limbic pathway that processes the affective-motivational aspects of pain (summarized in Figure 15.7) (Gauriau & Bernard, 2002; Hunt & Mantyh, 2001). However, it should be noted that such a separation is an oversimplified view, since the medial and central thalamic nuclei that receive inputs from spinothalamic neurons are connected to limbic cortical and subcortical regions, and several cortical areas receive inputs from both the spinothalamic and the spinoparabrachial pathways. Furthermore, the two processing streams interact with each other through cortical–cortical and cortical–limbic connections. In addition, second-order ALT neurons also have projections to various brainstem nuclei such as the PAG and reticular formation (Figure 15.7). Next, we discuss the two main pathways in more detail.
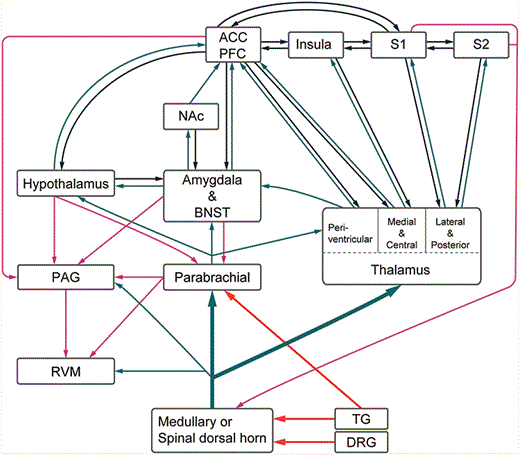
Schematic diagram of neural pain pathways. ACC, anterior cingulate cortex; PFC, prefrontal cortex; S1, primary somatosensory cortex; S2, secondary somatosensory cortex; NAc, nucleus accumbens; BNST, bed nucleus stria terminalis; PAG, periaqueductal gray; RVM, rostroventral medulla; TG, trigeminal ganglion; DRG, dorsal root ganglion.
The Spinothalamic-Cortical Pain Discriminative Pathway
Early extensive studies using anterograde and retrograde neuronal tracers in primates showed that many axons of the ALT nociceptive projection neurons terminate in multiple nuclei in thalamus, including the ventral posterior complex (with VPL receiving projections from spinal dorsal horn, and VPM receiving inputs from trigeminal SpVc), the central nucleus (both centrolateral or CL, and centromedial or CM), the medial dorsal nucleus (MD), and posterior nuclear group (Po) (Boivie, 1979; Friedman & Murray, 1986; Gingold, Greenspan, & Apkarian, 1991). Note that Po is not a well-defined region in primates. In rodents, another area, called PoT (posterior triangular nucleus), which is located in the caudalmost part of Po, should be added as one of the main targets for ALT nociceptive axons (Al-Khater, Kerr, & Todd, 2008; Gauriau & Bernard, 2004). Neurons of these thalamic nuclei in turn project to several cortical areas, including the primary somatosensory (S1), the secondary somatosensory (S2), the insula, and the cingulate cortex. The main axonal target of VPM and VPL neurons is S1. Neurons in Po project to S1, S2, and the insular cortex. CL and CM send axons to S1 and the cingulate cortex, whereas MD projects to both the insular and cingulate cortex (Burton & Jones, 1976; Craig, 2014; Friedman & Murray, 1986; Gingold et al., 1991). Generally speaking, S1 is critically involved in detecting the location, type, and intensity of pain. This is the main reason that this spinothalamic-cortical pathway is regarded as the sensory-discrimination pathway. On the other hand, S2 is thought to play a role in recognizing, learning, and remembering painful events; the insular cortex seems to be important for the autonomous and motivational responses during pain, and also for learning; while the cingulate cortex is linked to perceiving the negative affect of pain, and overall integration of pain perception and action selection (Schnitzler & Ploner, 2000). Thus, this spinothalamic pathway also contributes to pain learning and pain affect.
Among the thalamic nuclei, VPM/VPL and Po nuclei have been most extensively characterized. VPM/VPL and Po receive both innocuous tactile inputs (through the dorsal column pathway) and nociceptive inputs (through the spinothalamic component of the ALT). Studies in primates have found that neurons in VPM/VPL that responded to tactile or painful stimuli are anatomically segregated into different clusters, and express different calcium-binding proteins (Rausell, Bae, Vinuela, Huntley, & Jones, 1992; Rausell & Jones, 1991). The same anatomical segregation of touch- and pain-processing neurons may also exist in the primate Po nucleus. Furthermore, the tactile inputs are relayed to middle layers in S1 by VPM/VPL/Po touch neurons, whereas the noxious inputs from the spinothalamic tract are relayed to layer 1 in S1 by nociceptive VPM/VPL/Po neurons. In rodents, VPM/VPL also contain neurons that respond either to innocuous tactile or to noxious stimuli. The majority of neurons specifically activated in response to noxious stimuli are found in the medial part of Po (PoM) and in PoT (Frangeul et al., 2014; Gauriau & Bernard, 2004; Masri et al., 2009). Tactile-responsive rodent VPM/VPL axons target Layer 4 of S1, whereas noci-responsive axons innervate Layer 1 and Layer 5a of S1 (Pouchelon et al., 2014). Thus, both in primates and in rodents, tactile and pain information are processed by different layers in S1. Both PoM and PoT neurons also project axons to multiple layers (including Layer 4) of S2 and insular cortex (Gauriau & Bernard, 2004; Pouchelon et al., 2014). The central and medial thalamic nuclei (CL, CM, MD) contain predominantly nociceptive neurons, and many of them receive bilateral inputs from wide areas of the body (Dong, Ryu, & Wagman, 1978; Iwata et al., 2011).
Recognition of the role of S1 in processing the sensory discriminative aspect of pain initially came from human studies. Patients with S1 surgically removed or injured had problems in localizing pain. Patients with S1 lesions reported no sensation of pain but intact perception of the negative affect and unpleasantness of pain (Ploner, Freund, & Schnitzler, 1999). The primate S1 is subdivided into Brodmann’s areas 3a, 3b, 1, and 2. Nociceptive neurons are found in areas 3a, 3b, and 1, and are interspersed among neurons that respond solely to non-noxious touch stimuli. Area 2 appears to process tactile and proprioceptive information. Nociceptive S1 neurons in 3b/1 mediate the “first pain,” i.e., the well localized sensation evoked by activation of Aδ nociceptors. Nociceptive neurons in area 3a mediate second pain evoked by slowly conducting C fibers. It is believed that the interactions between 3a and 3b/1 collectively encode the intensity, duration, and type of pain (Vierck, Whitsel, Favorov, Brown, & Tommerdahl, 2013).
The Spinoparabrachial-Limbic Pain Affective Pathway
The LPb is another major target of spinal ALT projection neurons. Electrophysiological recordings revealed that noxious-responsive LPb neurons are driven by Aδ and C-nociceptive inputs and generally have receptive fields covering a large area of the body. Many LPb neurons respond to both cutaneous and visceral noxious stimuli (Gauriau & Bernard, 2002). In other words, the spinoparabrachial pathway does not encode precise locations of noxious stimuli. A subset of LPb neurons respond to warmth, cooling, or itch.
The nociceptive LPb neurons send ascending projections to the central nucleus of amygdala (CeA), the lateral bed nucleus of stria terminalis (BNST), the midline paraventricular nucleus of the thalamus (PVT), and the lateral and periventricular region of hypothalamus (Hyp) (Rodriguez et al., 2017). CeA and BNST neurons are known to project to the nucleus accumbens (NAc) and to the cingulate and prefrontal cortex. PVT provides input to the cingulate and insular cortex. CeA, BNST, and the cingulate cortex (especially the anterior cingulate cortex, ACC) are known to process affective emotional responses. The lateral-capsular region of CeA is dubbed the “nociceptive amygdala,” as this region receives purely nociceptive inputs from LPb (Neugebauer, 2015). Activation of the axons from CGRP-expressing LPb neurons in CeA is sufficient to cause fear/threat learning (Han, Soleiman, Soden, Zweifel, & Palmiter, 2015). In various preclinical pain models, it has been found that there was enhanced excitatory transmission at LPb–CeA synapses, along with increased background and evoked activity, and increased expression of neuroactivity markers such as Fos and phosphorylated extracellular signal-regulated kinase (pERK) in CeA (Thompson & Neugebauer, 2017). Interestingly, such neuroplastic changes in inflammatory or neuropathic pain models were only observed in the right, but not the left, CeA (Thompson & Neugebauer, 2017), although the mechanisms underlying such lateralization remain unclear.
The cingulate cortex receives inputs from amygdala (both the central amygdala as well as the lateral and basolateral amygdala). Patients with cingulotomy reported that they could still perceive pain sensations, but such sensation was less unpleasant, and they were less bothered by the pain (Foltz & White, 1962). These observations have led to the notion that the main function of this spinoparabrachial pathway is to process pain affect. However, as mentioned previously, the midline thalamic nuclei also project to cingulate cortex, and hence the spinothalamo-cortical pathway is also involved in the affective pain component. The noci-responsive neurons in PVT, Hyp, and the insular are probably involved in processing the autonomic and motivational aspects of pain.
Notably, trigeminal nociceptive sensory neurons innervating the face and head region send axon collaterals that synapse directly with nociceptive LPb neurons (Figure 15.7), and this is in addition to the canonical projection of these primary afferents to the medullary SpVc nucleus. Specifically, stimulation of this trigemino-parabrachial monosynaptic pathway drives robust aversive behavior and distress calls, whereas silencing this direct pathway reduces craniofacial pain-related behaviors, but not those from elsewhere in the body (Rodriguez et al., 2017). Thus, noxious stimuli experienced by the head and face region engage dual pathways (directly or indirectly via SpVc) to activate the limbic system, which may underlie the heightened affective response to face/head pain compared to body pain.
LPb neurons also send descending projections to PAG, the rostral ventral medulla (RVM), and reticular regions of the medulla. We will discuss the potential functions of these projections later, with other descending pathways.
Descending Pathways
All pain-associated cortical areas mentioned here send descending axons that terminate in multiple brainstem regions, including the PAG, the pons, the midline raphe nuclei, and various medullary reticular nuclei. Corticospinal and corticobulbar neurons also terminate in the spinal and trigeminal dorsal horn. These cortical descending projections are collectively referred to as the corticofugal pathway. Accumulating evidence indicates that descending cortical projections (from S1, cingulate and insular cortex) facilitate sensory transmission. Pain-induced plasticity in these descending cortical neurons plays important roles in facilitating pain hypersensitivity and/or maintaining chronic pain state (Chen et al., 2014; Cichon, Blanck, Gan, & Yang, 2017; Kuner & Flor, 2016; Tan et al., 2017), although much of the detailed circuitry and the underlying mechanisms remain to be discovered.
The CeA and hypothalamic nuclei send descending projections to LPb, PAG, and the locus coeruleus (LC, where norepinephrinergic neurons reside). Neurons in LPb innervate PAG and RVM, including the raphe nucleus, which contains serotonergic neurons (Krukoff, Harris, & Jhamandas, 1993). PAG also provide inputs to RVM (Heinricher, Tavares, Leith, & Lumb, 2009). The LC norepinephrinergic and the RVM serotonergic neurons project diffusely to the dorsal horn, including its superficial part. Both monoamines are thought to operate mainly through volume transmission, and therefore the distribution of monoamine receptors is likely to be the main factor determining their targets. However, we still know relatively little about the expression of these receptors by specific types of dorsal horn neuron and primary afferent. In addition to descending monoaminergic axons, there is also a GABAergic projection from RVM (Antal, Petko, Polgar, Heizmann, & Storm-Mathisen, 1996). Some of the RVM GABAergic descending neurons co-express Penk1, and they appear to play an inhibitory role in attenuating pain transmission; whereas other RVM GABAergic descending neurons that do not express Penk1 seem to facilitate pain transmission (Francois et al., 2017; Zhang et al., 2015). The pathway from CeA via PAG to RVM pain-inhibitory neurons is considered the brain’s main descending pain-suppressing system. The main descending pathways are shown with red arrows in Figure 15.7.
References
Abraira, V. E., & Ginty, D. D. (
Abraira, V. E., Kuehn, E. D., Chirila, A. M., Springel, M. W., Toliver, A. A., Zimmerman, A. L., … Ginty, D. D. (
Akiyama, T., Curtis, E., Nguyen, T., Carstens, M. I., & Carstens, E. (
Al-Khater, K. M., Kerr, R., & Todd, A. J. (
Andrew, D. (
Antal, M., Petko, M., Polgar, E., Heizmann, C. W., & Storm-Mathisen, J. (
Baseer, N., Polgar, E., Watanabe, M., Furuta, T., Kaneko, T., & Todd, A. J. (
Bester, H., Chapman, V., Besson, J. M., & Bernard, J. F. (
Bice, T. N., & Beal, J. A. (
Boada, M. D., & Woodbury, C. J. (
Boivie, J. (
Boyle, K. A., Gutierrez-Mecinas, M., Polgar, E., Mooney, N., O’Connor, E., Furuta, T., … Todd, A. J. (
Brown, A. G. (
Brumovsky, P., Watanabe, M., & Hokfelt, T. (
Burton, H., & Jones, E. G. (
Cameron, D., Polgar, E., Gutierrez-Mecinas, M., Gomez-Lima, M., Watanabe, M., & Todd, A. J. (
Campbell, J. N., Raja, S. N., Meyer, R. A., & Mackinnon, S. E. (
Cao, Y. Q., Mantyh, P. W., Carlson, E. J., Gillespie, A. M., Epstein, C. J., & Basbaum, A. I. (
Cavanaugh, D. J., Lee, H., Lo, L., Shields, S. D., Zylka, M. J., Basbaum, A. I., & Anderson, D. J. (
Chen, T., Koga, K., Descalzi, G., Qiu, S., Wang, J., Zhang, L. S., … Zhuo, M. (
Christensen, A. J., Iyer, S. M., Francois, A., Vyas, S., Ramakrishnan, C., Vesuna, S., … Delp, S. L. (
Cichon, J., Blanck, T. J. J., Gan, W. B., & Yang, G. (
Cordero-Erausquin, M., Allard, S., Dolique, T., Bachand, K., Ribeiro-da-Silva, A., & De Koninck, Y. (
Craig, A. D. (
Cui, L., Miao, X., Liang, L., Abdus-Saboor, I., Olson, W., Fleming, M. S., … Luo, W. (
De Felipe, C., Herrero, J. F., O’Brien, J. A., Palmer, J. A., Doyle, C. A., Smith, A. J., … Hunt, S. P. (
Dong, W. K., Ryu, H., & Wagman, I. H. (
Duan, B., Cheng, L., Bourane, S., Britz, O., Padilla, C., Garcia-Campmany, L., … Ma, Q. (
Foltz, E. L., & White, L. E., Jr. (
Foster, E., Wildner, H., Tudeau, L., Haueter, S., Ralvenius, W. T., Jegen, M., … Zeilhofer, H. U. (
Francois, A., Low, S. A., Sypek, E. I., Christensen, A. J., Sotoudeh, C., Beier, K. T., … Scherrer, G. (
Frangeul, L., Porrero, C., Garcia-Amado, M., Maimone, B., Maniglier, M., Clasca, F., & Jabaudon, D. (
Friedman, D. P., & Murray, E. A. (
Ganley, R. P., Iwagaki, N., Del Rio, P., Baseer, N., Dickie, A. C., Boyle, K. A., … Todd, A. J. (
Gauriau, C., & Bernard, J. F. (
Gauriau, C., & Bernard, J. F. (
Gingold, S. I., Greenspan, J. D., & Apkarian, A. V. (
Grudt, T. J., & Perl, E. R. (
Gutierrez-Mecinas, M., Bell, A. M., Marin, A., Taylor, R., Boyle, K. A., Furuta, T., … Todd, A. J. (
Gutierrez-Mecinas, M., Furuta, T., Watanabe, M., & Todd, A. J. (
Gutierrez-Mecinas, M., Polgár, E., Bell, A. M., Herau, M., & Todd, A. J. (
Han, L., Ma, C., Liu, Q., Weng, H. J., Cui, Y., Tang, Z., … Dong, X. (
Han, S., Soleiman, M. T., Soden, M. E., Zweifel, L. S., & Palmiter, R. D. (
Heinricher, M. M., Tavares, I., Leith, J. L., & Lumb, B. M. (
Huang, J., Polgár, E., Solinski, H. J., Mishra, S., Tseng, P.-Y., Iwagaki, N., … Hoon, M. A. (
Hughes, D. I., Sikander, S., Kinnon, C. M., Boyle, K. A., Watanabe, M., Callister, R. J., & Graham, B. A. (
Hunt, S. P., & Mantyh, P. W. (
Iwagaki, N., Garzillo, F., Polgár, E., Riddell, J. S., & Todd, A. J. (
Iwata, K., Miyachi, S., Imanishi, M., Tsuboi, Y., Kitagawa, J., Teramoto, K., … Takada, M. (
Kardon, A. P., Polgár, E., Hachisuka, J., Snyder, L. M., Cameron, D., Savage, S., … Ross, S. E. (
Krukoff, T. L., Harris, K. H., & Jhamandas, J. H. (
Kuner, R., & Flor, H. (
Larsson, M. (
Li, C. L., Li, K. C., Wu, D., Chen, Y., Luo, H., Zhao, J. R., … Zhang, X. (
Light, A. R., & Perl, E. R. (
Lu, Y., Dong, H., Gao, Y., Gong, Y., Ren, Y., Gu, N., … Xiong, L. (
Lu, Y., & Perl, E. R. (
Lu, Y., & Perl, E. R. (
Malmberg, A. B., Chen, C., Tonegawa, S., & Basbaum, A. I. (
Mantyh, P. W., DeMaster, E., Malhotra, A., Ghilardi, J. R., Rogers, S. D., Mantyh, C. R., … et al. (
Masri, R., Quiton, R. L., Lucas, J. M., Murray, P. D., Thompson, S. M., & Keller, A. (
McMahon, S. B., & Wall, P. D. (
McMahon, S. B., & Wall, P. D. (
Melzack, R., & Wall, P. D. (
Moser, H. R., & Giesler, G. J., Jr. (
Neugebauer, V. (
Nichols, M. L., Allen, B. J., Rogers, S. D., Ghilardi, J. R., Honore, P., Luger, N. M., … Mantyh, P. W. (
Peirs, C., Williams, S. P., Zhao, X., Walsh, C. E., Gedeon, J. Y., Cagle, N. E., … Seal, R. P. (
Petitjean, H., Pawlowski, S. A., Fraine, S. L., Sharif, B., Hamad, D., Fatima, T., … Sharif-Naeini, R. (
Ploner, M., Freund, H. J., & Schnitzler, A. (
Polgár, E., Al-Khater, K. M., Shehab, S., Watanabe, M., & Todd, A. J. (
Polgár, E., Al Ghamdi, K. S., & Todd, A. J. (
Polgár, E., Campbell, A. D., MacIntyre, L. M., Watanabe, M., & Todd, A. J. (
Polgár, E., Durrieux, C., Hughes, D. I., & Todd, A. J. (
Polgár, E., Sardella, T. C., Tiong, S. Y., Locke, S., Watanabe, M., & Todd, A. J. (
Polgár, E., Sardella, T. C., Watanabe, M., & Todd, A. J. (
Polgár, E., Wright, L. L., & Todd, A. J. (
Pouchelon, G., Gambino, F., Bellone, C., Telley, L., Vitali, I., Luscher, C., … Jabaudon, D. (
Rausell, E., Bae, C. S., Vinuela, A., Huntley, G. W., & Jones, E. G. (
Rausell, E., & Jones, E. G. (
Rexed, B. (
Ribeiro-da-Silva, A., & Coimbra, A. (
Ribeiro-da-Silva, A., Tagari, P., & Cuello, A. C. (
Rodriguez, E., Sakurai, K., Xu, J., Chen, Y., Toda, K., Zhao, S., … Wang, F. (
Ross, S. E., Mardinly, A. R., McCord, A. E., Zurawski, J., Cohen, S., Jung, C., … Greenberg, M. E. (
Saito, H., Katagiri, A., Okada, S., Mikuzuki, L., Kubo, A., Suzuki, T., … Iwata, K. (
Salio, C., Ferrini, F., Muthuraju, S., & Merighi, A. (
Sandkuhler, J. (
Scherrer, G., Imamachi, N., Cao, Y. Q., Contet, C., Mennicken, F., O’Donnell, D., … Basbaum, A. I. (
Schnitzler, A., & Ploner, M. (
Seal, R. P., Wang, X., Guan, Y., Raja, S. N., Woodbury, C. J., Basbaum, A. I., & Edwards, R. H. (
Sivilotti, L., & Woolf, C. J. (