-
PDF
- Split View
-
Views
-
Cite
Cite
Kurt T. Barnhart, Adriana Izquierdo, E. Scott Pretorius, David M. Shera, Mayadah Shabbout, Alka Shaunik, Baseline dimensions of the human vagina, Human Reproduction, Volume 21, Issue 6, June 2006, Pages 1618–1622, https://doi.org/10.1093/humrep/del022
Close - Share Icon Share
Abstract
BACKGROUND: Vaginal anatomy has been poorly studied. This study aimed to measure baseline dimensions of the undistended vagina of women of reproductive age. METHODS: We combined baseline information collected from five clinical trials using magnetic resonance imaging (MRI) to quantify distribution of a vaginal gel. Seventy-seven MRI scans were performed on 28 women before gel application to establish baseline vaginal measurements. Average dimensions were calculated for each woman and for the population. The influence of potential covariates (age, height, weight and parity) on these dimensions was assessed. RESULTS: MRI measurements are reproducible. The SD surrounding the mean at each anatomical site, and with summary measurements, was significantly smaller with each subject compared with the population. Mean vaginal length from cervix to introitus was 62.7 mm. Vaginal width was largest in the proximal vagina (32.5 mm), decreased as it passed through the pelvic diaphragm (27.8 mm) and smallest at the introitus (26.2 mm). Significant positive associations were parity with vaginal fornix length, age with pelvic flexure width and the height with width at the pelvic flexure. CONCLUSION: No one description characterized the shape of the human vagina. Although there is variation among women, variables such as parity, age and height are positively associated with differences in baseline dimensions.
Introduction
In comparison with other female pelvic organs, the anatomy of the vagina has been relatively poorly studied. Our knowledge of female pelvic anatomy is based on old descriptions derived from the dissection of a small number of female cadavers. These studies depict the vagina as a straight hollow tube extending vertically upwards towards the sacral promontory (Grant, 1943; Eycleshymer and Schoemaker, 1983; Sultan et al., 1993). In the late 19th century, Hadra in ‘Lesions of the vagina and pelvic floor’, discussed the possibility of differences in anatomy for a living patient, also noting that the vaginal axis is different in the upper and lower vagina (defined as the sections of the vagina above and below the pelvic diaphragm) (Hadra, 1888).
New research on pelvic imaging has focused on paediatric, premenopausal and post-menopausal groups; or disease entities like utero-vaginal anomalies, prolapse, fistula or pelvic malignancies. As the focus of past research has been mainly curative, limited study has been conducted on the normal anatomical variations of the vagina of young, healthy and sexually active women (Hafez and Evans, 1978).
The literature, to date, describes the relaxed vagina as a fibro-muscular tube that exists as a collapsed potential space. The shape of the tube is not symmetrical or similar to any known geometric shape. Rather, the vaginal lumen is a potential space with walls that are easily distensible. The overall shape and stretching of the vaginal canal are constrained by the elasticity of the vaginal wall and its relationship to other pelvic organs. The cross section of the relaxed vagina at the level of the cervical os has been classically characterized as an ‘H’ shape. More recent data suggest that the shape may instead resemble a ‘W’ (Barnhart et al., 2004a).
Studies have utilized casts to visualize the vagina in three dimensions and to compare vaginal shape, dimensions and surface contact in various ethnic populations. The casts consisted of wax, rapidly solidifying dental impression paste, polyvinyl siloxane, etc. (Morgan, 1961; Richter, 1967; Pendergrass et al., 1996). These studies were limited by the abnormal distension of the vagina; however, they suggested differences among vaginal shapes and dimensions in African-American, Caucasian and Hispanic women (Richter, 1967; Pendergrass et al., 2000). These studies have also suggested a uniform ‘size’ of all the different shapes of the vagina, and hence have supported the development of the ‘one size fits all’ vaginal product, formulation or microbicide (Pendergrass et al., 2003).
The goal of this project was to define baseline, nondistended dimensions of the vagina of women of reproductive age using noninvasive imaging. Magnetic resonance imaging (MRI) is the optimal imaging modality for female pelvic organs because the images have excellent spatial resolution and inherently high soft tissue contrast (McCarthy and Vaqueno, 1986; Aronson et al., 1990; Barnhart et al., 2001, 2004b). A secondary goal was to explore the importance of the potential covariates to the dimensions of the human vagina including the impact of age, height, weight, gravity and parity. This information may help researchers optimize vaginal products and drug delivery.
Materials and methods
This study was reviewed and approved by the Institutional Review Board of the University of Pennsylvania (Protocol CSA-03-333). This study is an analysis of the baseline MRI at entry in five clinical trials evaluating the distribution of a vaginal product. Data from the following five experimental protocols were utilized for this study:
spread of 3 ml of KY jelly and Replens with and without ambulation (unpublished data);
comparison of two volumes of cellulose sulphate (2.5 ml versus 3.5 ml) with and without ambulation (Barnhart et al., 2005a);
effect of volume (3.0 ml versus 5.0 ml) and ambulation on gel spread, and a substudy on the effect of simulated intercourse on the spread of gel in the vagina (Barnhart et al., 2004b);
study of spread of Savvy gel in the vagina (Barnhart et al., 2005b) and
vaginal distribution of miconazole nitrate suspension from administration of a single vaginal insert (Barnhart et al., 2004c).
As part of these trials, an MRI was performed prior to use of any study product to serve as a baseline for comparison after gel insertion. The trials then quantified the spread of the gel depending on time from application, ambulation of the volunteer, sexual activity of the subject, gel volume and formulation. Other data that quantified the spread of the gel were not included. The analysis for this study is confined to the MRI examinations of these volunteers before using the experimental product to assess the baseline vaginal dimensions of women of reproductive age.
All subjects were aged 18–45 years, not at risk for pregnancy (using reliable contraception or abstinence), menstruated regularly and had a normal Papanicolaou smear.
MRI examinations were performed on a dedicated research GE 1.5 Tesla Signa scanner with the assistance of a phased array surface coil centred on the pelvis, to allow small fields of view and to increase signal to noise ratio. We used Sun Ultra workstation, GE Advantage Windows 3.1 software, electronic callipers and digitally stored images to make measurements. The specific details of MR techniques have been published previously (Barnhart et al., 2001, 2004b, 2005a; Pretorius et al., 2002).
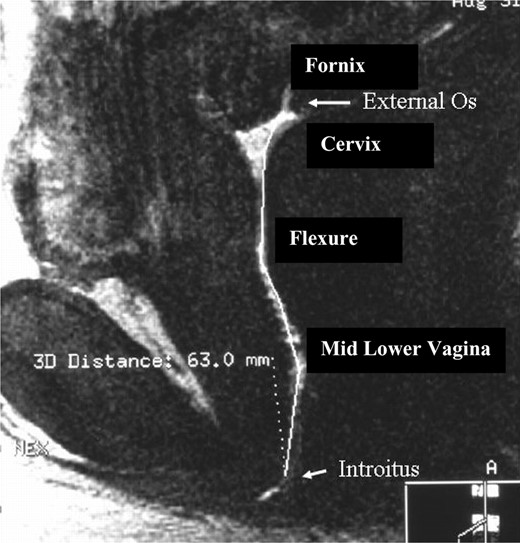
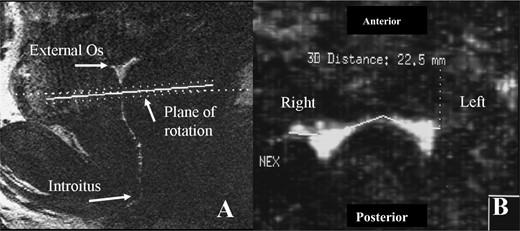
The ‘linear length’ of the vagina was the measurement in the sagittal plane from the external cervical os to the introitus (approximately at the level of the hymeneal ring) (Figure 1). Measurements were also taken at the following anatomic structures in millimetres: (i) two measurements of the posterior vaginal fornix—width and length in the anterior posterior (AP) and sagittal planes; (ii) transverse measurement of upper vagina (1 cm below the cervix); (iii) pelvic flexure width—transverse measurement of the flexure of the vagina as it passes through the pelvic diaphragm; (iv) transverse measurement of the lower vagina (3 cm above the introitus); (v) at the level of the introitus (transverse measurement of the vagina 1 cm above the introitus). An example of a measurement in the transverse plane is demonstrated in Figure 2. Measurements at all five demarcated areas were assessed as demonstrated in Figure 2. Surface contact is a summary measurement of the dimensions of the vagina and is calculated by the summation of length of the fornix (AP plane) and the transverse measurement at the four other demarcated sites (Pretorius et al., 2002).
This figure represents a sagittal image of the human vagina. For illustration purposes this is an image that contains gel mixed with gadolinium contrast (white) to demarcate the vaginal canal. The outline of the vagina can be seen from the cervix and linearly to the introitus. The length of the ‘curved’ line in the vertical plane is the linear length of the vagina. In this case, the measurement was 63 mm.
Panel A represents a sagittal image of the human vagina. For illustration purposes these images contain gel mixed with gadolinium contrast (white) to demarcate the vaginal canal. The outline of the vagina can be seen from the cervix (above the dotted line) and linearly in the vertical plane to the introitus. The lateral dimensions of the vagina are measured in the transverse plane as depicted by the dotted line in Panel A. The image is ‘90°’ from the vertical plane. Panel B represents complete cross section of the vagina in the transverse plane (1 cm below the cervical os), as the vagina can be seen contiguously from left to right. In this instance, the measurement was 22.5 mm. Surface contact is the sum of the transverse measurements at five demarcated sites in the vagina.
The data were manually checked for any discrepancies resulting from illegal values, extreme outliers and suspicious combinations. We explored various analytical techniques, statistical methods and experimental designs to determine the optimal use of MRI to study the baseline vaginal dimensions. Comparisons using Wilcoxon signed rank tests were made at each distinct measurement within the vagina. SAS (Cary, NC, USA) software was used for statistical analysis.
Multivariate statistical methods were used to summarize the measurements derived from images in the most efficient manner. Repeated analysis of variance and related methods such as mixed effects models were used. Standard data reduction techniques such as principal components and factor analyses were used to form a reduced set of variables. For dichotomous and discrete variables we used appropriate methods such as logistic regression or generalized estimating equation (GEE). All P-values were produced using Fischer’s exact tests methods. The association of baseline vaginal dimensions with age, race, gravity, parity, height and weight were determined.
Results
Data from 28 volunteers were included in the analysis. The average age of the participants was 29.2 ± 5.8 years with a range of 18–39 years. The average height was 1.66 ± 0.05 m with a range of 1.5–1.7 m. The average weight was 70.13 ± 12.6 kg with a range of 49.9–95.3 kg. The ethnic distribution of the participants was as follows: 17 were Caucasians, eight African-Americans, two Hispanic and one Asian/Pacific Islander. Of the participants, 14 women were nulliparous and 14 were parous.
Most women (23) participated in one trial; one woman participated in all five. Seventy-seven MRI measurements were performed at baseline (minimum one and maximum twelve per subject). Thirteen of the 28 women had more than one baseline MRI either in the same study or in a second study.
The mean dimensions of the resting human vagina are presented in Table I. These represent the average of the mean values of each of the 28 women. In other words, the average dimensions (and SD) for each woman was calculated if she had more than one MRI. Average dimensions and SD were then calculated for all 28 women. Also presented in Table I are data about the SD of the mean for each woman (within subjects) and the SD of the mean for the population of 28 women (between subjects). The SD within subjects was noted to be significantly less than the SD of the mean for the population of 28 for all individual and summary measurements.
Dimensions of the human vagina with comparison of inter- and intra-person standard deviation
| . | Mean (MM) . | Range (MM) . | SD between subjects . | SD within subjects . | P-valuea . |
|---|---|---|---|---|---|
| Fornix width | 41.9 | 26–82.8 | 10.34 | 0.85 | <0.001 |
| Fornix length | 29.2 | 12.5–48.4 | 8.01 | 2.4 | <0.001 |
| Cervical os | 32.5 | 21.7–55 | 7.13 | 1.55 | <0.001 |
| Flexure width | 27.9 | 19.3–39.1 | 5.47 | 1.99 | <0.001 |
| Mid-lower vagina | 27.2 | 15.8–36.7 | 4.79 | 1.21 | <0.001 |
| Introitus width | 26.1 | 18.7–37 | 4.41 | 2.09 | <0.001 |
| Surface contactb | 143 | 106.8–185 | 16.14 | 5.16 | <0.001 |
| Linear lengthb | 62.7 | 40.8–95 | 11.25 | 1.23 | <0.001 |
| . | Mean (MM) . | Range (MM) . | SD between subjects . | SD within subjects . | P-valuea . |
|---|---|---|---|---|---|
| Fornix width | 41.9 | 26–82.8 | 10.34 | 0.85 | <0.001 |
| Fornix length | 29.2 | 12.5–48.4 | 8.01 | 2.4 | <0.001 |
| Cervical os | 32.5 | 21.7–55 | 7.13 | 1.55 | <0.001 |
| Flexure width | 27.9 | 19.3–39.1 | 5.47 | 1.99 | <0.001 |
| Mid-lower vagina | 27.2 | 15.8–36.7 | 4.79 | 1.21 | <0.001 |
| Introitus width | 26.1 | 18.7–37 | 4.41 | 2.09 | <0.001 |
| Surface contactb | 143 | 106.8–185 | 16.14 | 5.16 | <0.001 |
| Linear lengthb | 62.7 | 40.8–95 | 11.25 | 1.23 | <0.001 |
Comparison is the SD between and within subjects.
Similar data already published.
Fornix width: transverse measurement in the posterior vaginal fornix; fornix length: longitudinal measurement in the posterior vaginal fornix (external os to most ‘superior’ aspect of vagina in the same plane); cervical os: transverse measurement at the level of 1 cm below the cervix; flexion width: transverse measurement as the vagina passes through the pelvic diaphragm; mid-lower vagina: transverse measurement 3 cm above the level of the introitus; introitus width: transverse measurement 1 cm above the level of the introitus; surface contact: sum of transverse measurements at the four demarcated sites in the upper and lower vagina and of longitudinal measurement in the vaginal fornices; linear spread of gel: total length along the long axis of the vagina from the external cervical os to the introitus.
Dimensions of the human vagina with comparison of inter- and intra-person standard deviation
| . | Mean (MM) . | Range (MM) . | SD between subjects . | SD within subjects . | P-valuea . |
|---|---|---|---|---|---|
| Fornix width | 41.9 | 26–82.8 | 10.34 | 0.85 | <0.001 |
| Fornix length | 29.2 | 12.5–48.4 | 8.01 | 2.4 | <0.001 |
| Cervical os | 32.5 | 21.7–55 | 7.13 | 1.55 | <0.001 |
| Flexure width | 27.9 | 19.3–39.1 | 5.47 | 1.99 | <0.001 |
| Mid-lower vagina | 27.2 | 15.8–36.7 | 4.79 | 1.21 | <0.001 |
| Introitus width | 26.1 | 18.7–37 | 4.41 | 2.09 | <0.001 |
| Surface contactb | 143 | 106.8–185 | 16.14 | 5.16 | <0.001 |
| Linear lengthb | 62.7 | 40.8–95 | 11.25 | 1.23 | <0.001 |
| . | Mean (MM) . | Range (MM) . | SD between subjects . | SD within subjects . | P-valuea . |
|---|---|---|---|---|---|
| Fornix width | 41.9 | 26–82.8 | 10.34 | 0.85 | <0.001 |
| Fornix length | 29.2 | 12.5–48.4 | 8.01 | 2.4 | <0.001 |
| Cervical os | 32.5 | 21.7–55 | 7.13 | 1.55 | <0.001 |
| Flexure width | 27.9 | 19.3–39.1 | 5.47 | 1.99 | <0.001 |
| Mid-lower vagina | 27.2 | 15.8–36.7 | 4.79 | 1.21 | <0.001 |
| Introitus width | 26.1 | 18.7–37 | 4.41 | 2.09 | <0.001 |
| Surface contactb | 143 | 106.8–185 | 16.14 | 5.16 | <0.001 |
| Linear lengthb | 62.7 | 40.8–95 | 11.25 | 1.23 | <0.001 |
Comparison is the SD between and within subjects.
Similar data already published.
Fornix width: transverse measurement in the posterior vaginal fornix; fornix length: longitudinal measurement in the posterior vaginal fornix (external os to most ‘superior’ aspect of vagina in the same plane); cervical os: transverse measurement at the level of 1 cm below the cervix; flexion width: transverse measurement as the vagina passes through the pelvic diaphragm; mid-lower vagina: transverse measurement 3 cm above the level of the introitus; introitus width: transverse measurement 1 cm above the level of the introitus; surface contact: sum of transverse measurements at the four demarcated sites in the upper and lower vagina and of longitudinal measurement in the vaginal fornices; linear spread of gel: total length along the long axis of the vagina from the external cervical os to the introitus.
The average linear length of the vagina was 62.7 mm with a relatively large range (40.8–95 mm). It was noted that the width of the vagina varies throughout its length. The transverse diameter of the vagina is the highest at the level of the vaginal fornices (41.87 mm). The transverse diameter then progressively decreases from the cervical os (32.52 mm) to the pelvic flexure (27.97 mm), mid-lower vagina (27.21 mm), to the narrowest part of the vagina at the level of the vaginal introitus (26.15 mm).
Table II gives results of evaluation of the relationship between baseline vaginal dimensions and covariates of age, weight, height and parity. Surprisingly, there were very few statistically significant associations noted with multivariable analysis. Race was not associated with any differences in measurements of vaginal dimensions. Parity was more predictive than gravidity; hence it was used (and gravidity was eliminated) in the final models. Associations noted were between (i) parity and length of vaginal fornix, (ii) age and vaginal width at the pelvic flexure and (iii) height and vaginal width at the pelvic flexure. A nonstatistically significant trend was noted between the overall length of the vagina and weight (P-value = 0.07).
Factors that affect the baseline dimensions of the human vagina
| Factors . | Variables . | Correlation . | P-value . |
|---|---|---|---|
| Fornix (transverse) | |||
| Age | Negative | 0.26 | |
| Weight | Negative | 0.28 | |
| Height | Positive | 0.4 | |
| Parity | Positive | 0.36 | |
| Fornix (longitudinal) | |||
| Age | Negative | 0.13 | |
| Weight | Positive | 0.13 | |
| Height | Positive | 0.15 | |
| Parity | Positive | 0.02a | |
| Cervical os | |||
| Age | Positive | 0.93 | |
| Weight | Negative | 0.94 | |
| Height | Negative | 0.28 | |
| Parity | Negative | 0.63 | |
| Flexion width | |||
| Age | Positive | 0.03a | |
| Weight | Positive | 0.74 | |
| Height | Negative | 0.04a | |
| Parity | Negative | 0.29 | |
| Mid-lower vagina | |||
| Age | Positive | 0.3 | |
| Weight | Negative | 0.9 | |
| Height | Negative | 0.29 | |
| Parity | Negative | 0.3 | |
| Introitus width | |||
| Age | Negative | 0.55 | |
| Weight | Positive | 0.15 | |
| Height | Positive | 0.59 | |
| Parity | Positive | 0.86 | |
| Surface contact | |||
| Age | Positive | 0.80 | |
| Weight | Positive | 0.24 | |
| Height | Negative | 0.5 | |
| Parity | Positive | 0.89 | |
| Length | |||
| Age | Positive | 0.76 | |
| Weight | Positive | 0.07b | |
| Height | Negative | 0.33 | |
| Parity | Negative | 0.56 |
| Factors . | Variables . | Correlation . | P-value . |
|---|---|---|---|
| Fornix (transverse) | |||
| Age | Negative | 0.26 | |
| Weight | Negative | 0.28 | |
| Height | Positive | 0.4 | |
| Parity | Positive | 0.36 | |
| Fornix (longitudinal) | |||
| Age | Negative | 0.13 | |
| Weight | Positive | 0.13 | |
| Height | Positive | 0.15 | |
| Parity | Positive | 0.02a | |
| Cervical os | |||
| Age | Positive | 0.93 | |
| Weight | Negative | 0.94 | |
| Height | Negative | 0.28 | |
| Parity | Negative | 0.63 | |
| Flexion width | |||
| Age | Positive | 0.03a | |
| Weight | Positive | 0.74 | |
| Height | Negative | 0.04a | |
| Parity | Negative | 0.29 | |
| Mid-lower vagina | |||
| Age | Positive | 0.3 | |
| Weight | Negative | 0.9 | |
| Height | Negative | 0.29 | |
| Parity | Negative | 0.3 | |
| Introitus width | |||
| Age | Negative | 0.55 | |
| Weight | Positive | 0.15 | |
| Height | Positive | 0.59 | |
| Parity | Positive | 0.86 | |
| Surface contact | |||
| Age | Positive | 0.80 | |
| Weight | Positive | 0.24 | |
| Height | Negative | 0.5 | |
| Parity | Positive | 0.89 | |
| Length | |||
| Age | Positive | 0.76 | |
| Weight | Positive | 0.07b | |
| Height | Negative | 0.33 | |
| Parity | Negative | 0.56 |
Statistically significant P ≤ 0.05.
Trend P-value 0.05–0.1.
Fornix: transverse measurement in the posterior vaginal fornix; cervical os: transverse measurement at the level of 1 cm below cervix; flexion width: transverse measurement as the vagina passes through the pelvic diaphragm; mid-lower vagina: transverse measurement 3 cm above the level of the introitus; introitus width: transverse measurement 1 cm above the level of the introitus; surface contact: sum of transverse measurements at the four demarcated sites in the upper and lower vagina and of longitudinal measurement in the vaginal fornices; linear spread of gel: total linear length along the long axis of the vagina.
Factors that affect the baseline dimensions of the human vagina
| Factors . | Variables . | Correlation . | P-value . |
|---|---|---|---|
| Fornix (transverse) | |||
| Age | Negative | 0.26 | |
| Weight | Negative | 0.28 | |
| Height | Positive | 0.4 | |
| Parity | Positive | 0.36 | |
| Fornix (longitudinal) | |||
| Age | Negative | 0.13 | |
| Weight | Positive | 0.13 | |
| Height | Positive | 0.15 | |
| Parity | Positive | 0.02a | |
| Cervical os | |||
| Age | Positive | 0.93 | |
| Weight | Negative | 0.94 | |
| Height | Negative | 0.28 | |
| Parity | Negative | 0.63 | |
| Flexion width | |||
| Age | Positive | 0.03a | |
| Weight | Positive | 0.74 | |
| Height | Negative | 0.04a | |
| Parity | Negative | 0.29 | |
| Mid-lower vagina | |||
| Age | Positive | 0.3 | |
| Weight | Negative | 0.9 | |
| Height | Negative | 0.29 | |
| Parity | Negative | 0.3 | |
| Introitus width | |||
| Age | Negative | 0.55 | |
| Weight | Positive | 0.15 | |
| Height | Positive | 0.59 | |
| Parity | Positive | 0.86 | |
| Surface contact | |||
| Age | Positive | 0.80 | |
| Weight | Positive | 0.24 | |
| Height | Negative | 0.5 | |
| Parity | Positive | 0.89 | |
| Length | |||
| Age | Positive | 0.76 | |
| Weight | Positive | 0.07b | |
| Height | Negative | 0.33 | |
| Parity | Negative | 0.56 |
| Factors . | Variables . | Correlation . | P-value . |
|---|---|---|---|
| Fornix (transverse) | |||
| Age | Negative | 0.26 | |
| Weight | Negative | 0.28 | |
| Height | Positive | 0.4 | |
| Parity | Positive | 0.36 | |
| Fornix (longitudinal) | |||
| Age | Negative | 0.13 | |
| Weight | Positive | 0.13 | |
| Height | Positive | 0.15 | |
| Parity | Positive | 0.02a | |
| Cervical os | |||
| Age | Positive | 0.93 | |
| Weight | Negative | 0.94 | |
| Height | Negative | 0.28 | |
| Parity | Negative | 0.63 | |
| Flexion width | |||
| Age | Positive | 0.03a | |
| Weight | Positive | 0.74 | |
| Height | Negative | 0.04a | |
| Parity | Negative | 0.29 | |
| Mid-lower vagina | |||
| Age | Positive | 0.3 | |
| Weight | Negative | 0.9 | |
| Height | Negative | 0.29 | |
| Parity | Negative | 0.3 | |
| Introitus width | |||
| Age | Negative | 0.55 | |
| Weight | Positive | 0.15 | |
| Height | Positive | 0.59 | |
| Parity | Positive | 0.86 | |
| Surface contact | |||
| Age | Positive | 0.80 | |
| Weight | Positive | 0.24 | |
| Height | Negative | 0.5 | |
| Parity | Positive | 0.89 | |
| Length | |||
| Age | Positive | 0.76 | |
| Weight | Positive | 0.07b | |
| Height | Negative | 0.33 | |
| Parity | Negative | 0.56 |
Statistically significant P ≤ 0.05.
Trend P-value 0.05–0.1.
Fornix: transverse measurement in the posterior vaginal fornix; cervical os: transverse measurement at the level of 1 cm below cervix; flexion width: transverse measurement as the vagina passes through the pelvic diaphragm; mid-lower vagina: transverse measurement 3 cm above the level of the introitus; introitus width: transverse measurement 1 cm above the level of the introitus; surface contact: sum of transverse measurements at the four demarcated sites in the upper and lower vagina and of longitudinal measurement in the vaginal fornices; linear spread of gel: total linear length along the long axis of the vagina.
Discussion
Neither a single shape nor one summary measurement can characterize the dimensions of the resting vagina in women of reproductive age. Using a noninvasive imaging modality we were able to gain some insight into the anatomy. We confirmed that the axis and dimensions of the upper and lower vagina are different (data not shown). The axis of the lower vagina (from the introitus to the pelvic diaphragm), in relation to a standing woman, is vertical and posterior. The upper vagina changes its axis at the level of the pelvic diaphragm (from the pelvic diaphragm to the cervix), and it becomes more horizontal. We have previously noted that the transverse shape of the upper vagina at the level of the cervix is not always an ‘H’ and instead is often a ‘W’ (Barnhart et al., 2004). We also note that measurements of the transverse diameter of the vagina vary along its length. The width of the vagina is narrowest at the level of the introitus with minimal change in width noted at the level of the pelvic diaphragm. Above the pelvic diaphragm, the transverse width of the vagina is greater, around and behind the cervix (the transverse width of the fornix).
The differences in dimensions of the vagina along its length most likely result from the constriction by the surrounding pelvic tissues and the intrinsic compliance of the vaginal walls, resulting in the greater width and compliance of the upper vagina. The differences in the axis and the width of the vagina may not be readily appreciated by the clinician as an inserted speculum straightens the axis of the vagina. Once the speculum is opened, it is sometimes difficult to appreciate the differences in width along the length of the vaginal canal.
There are differences in vaginal dimensions among women. The length of the vagina (from external cervical os to introitus) ranged from approximately 4.1–9.5 cm, a greater than 100% difference from the shortest to the longest length. There was also a large range in the width of the vagina at all demarcated sites measured. The width and the range of the width tended to increase from the introitus to the fornix. The largest range in the width of the vagina was noted in the width of the posterior fornix, which in the undistended vagina is the portion of the vagina behind (posterior) to the barrel of the cervix. The cervix extends from the upper wall of the vagina into the canal with the external os pointing in the general direction of the introitus. The area of the vagina cephalad to the external os, thus posterior to the barrel of the cervix, is the fornix. Using MRI we were able to measure the posterior fornix in both the transverse plane (right to left) and in the longitudinal plane (in the sagittal plane). Thus, the ‘true length’ of the vagina is the length from the external os to the introitus plus the longitudinal length of the posterior fornix. These two aspects in length, however, are not in the same ‘linear plane’, and the connection between these two lengths will also often include some change in linear direction.
Interestingly, while there were differences in vaginal dimensions among women, there were only small differences in the dimensions when the same woman was imaged multiple times. Over the course of these trials, some women had repeated measurements as much as six months apart, and there was very little variation in these measurements, suggesting that the anatomy does not change substantially over short periods, and measurements using MRI have low intra-person variability.
Notably, few statistically significant associations could be drawn between the potentially influencing factors and baseline dimensions of the vagina. Although it has been suggested that there are racial differences in vaginal dimensions, our data did not demonstrate any such clear-cut differences. We noted that age of a woman is associated with an increase in the transverse diameter at the pelvic flexure. This is consistent with the clinical finding of increasing laxity of the vaginal walls in women of advanced age. Interestingly, by contrast, height of the subject was negatively associated with width of the vagina at the level of the flexion. Weight of a woman tended to be positively associated with overall length of the vagina and presented as a nonstatistically significant trend (P-value 0.05–0.10). Given the number of statistical comparisons performed in this study, it is possible that some of these findings may be due to chance.
Surprisingly, parity had little association with the overall surface contact and length of the vagina. Parity is associated with a significant increase in the length of the vaginal fornix. The potential effect of parity may be via stretching and elongation of the birth canal at the time of vaginal childbirth. This effect is especially significant in the upper part of the vagina where the dilation, thinning (effacement) and taking up of the cervix is an active process, as opposed to the lower vagina where passive stretching takes place during parturition.
Our summary measurement of surface contact is not a true measure of surface area. It was devised to objectively compare the spread of a vagina gel under experimental protocol to assess the effect of a variable such as time since insertion or gel volume. It is notable that our summary measurements of 137.58 ± 18.37 mm with a range of 103.9–165 mm appear to be larger than estimates of surface area reported in other studies. Prior studies that used casts to measure vaginal dimensions reported a surface area of 87.46 mm2, SD 7.8 mm2 and range 65.73–107.07 mm2. A direct comparison of these measurements cannot be performed due to differences in methodology and because the casts showed either extrusion of cast material by a small vagina or improper filling in a roomy vagina, leading to understated measurements (Pendergrass et al., 2003). Our measurements of the width of the vagina are similar to those reported, which range from 23.9 to 64.5 mm (Pendergrass et al., 1996, 2003). However, we are unable to characterize the shape of the vagina as a ‘heart, slug, pumpkin seed or parallel sides’ as suggested by other studies (Pendergrass et al., 1996, 2000, 2003).
The dimensions and shape of the vagina are of great importance in medicine and surgery; however, there appears to be no single way to characterize the size and shape of the human vagina. Although differences exist between women, there are few covariates associated with these differences. There does not appear to be large variation in the dimensions of the vagina within the same woman. Given the large range in the dimensions noted, it is most likely that one size for a vaginal device will not fit all women (Mauck et al., 2004). Prior research has shown that using a single size for fitting two cervical caps leads to the correct fit in only 33% of women. Moreover, it is possible that one volume of a gel intended to cover the vaginal epithelium may not be appropriate for all women. This information can be used to better design various devices used in the vagina. We have previously proved that deployment of a potential microbicide gel in the upper and lower vagina is affected by factors such as ambulation, time since insertion, volume and product (Barnhart et al., 2005). Baseline vaginal dimension may also be an important factor.
Acknowledgements
Support for this subproject [CSA-03-333] was provided by the Global Microbicide Project [GMP], a program of CONRAD, Eastern Virginia Medical School. The views expressed by the authors do not necessarily reflect the views of CONRAD or GMP.
References
Author notes
1Penn Fertility Care, Department of Obstetrics and Gynecology and Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania, Philadelphia, PA, 2Department of Internal Medicine, University of California, San Francisco, CA, 3Department of Radiology, University of Pennsylvania and 4Children’s Hospital of Philadelphia, Philadelphia, PA, USA