Abstract
The S saturation and oxidation states of arc magmas are important factors in the formation of porphyry Cu–Au deposits. The Milin juvenile lower crustal cumulates (86.7–84.3 Ma) in the Gangdese provide insights into how sulfide saturation and oxidation states control porphyry mineralization. Zircons from the cumulates have low Ce4+/Ce3+ ratios (21–90) and reduced oxygen fugacities (ΔFMQ–1.8±0.5), which cannot be explained by fractional crystallization or crustal contamination, suggesting inheritance from a mantle source. Partial melting of the mantle under reduced conditions produced a sulfide-saturated primary arc magma with low chalcophile element contents owing to the residual sulfide in the mantle. The Milin lower crustal cumulates contain sulfides, indicating that the magma reached sulfide saturation in the early stages of magmatic differentiation. Based on our model, the primary arc magma before sulfide saturation contained 66.7 ppm Cu and 1.0 ppb Au. The residual magma after sulfide saturation in the lower crust contained 33–66 ppm Cu, 0.13–0.93 ppb Au; i.e., lower contents than those in arc basalts worldwide. Both these factors hindered the formation of Late Cretaceous large porphyry Cu–Au deposits in the Gangdese belt. Remelting of the Milin sulfide-rich cumulates can generate a Cu-rich andesitic magma only under high temperature and high-fO2 conditions, and a melt with low Cu content under low temperature even high-fO2 conditions. Thus, the temperature plays a crucial role in the remelting of the lower crust whether provide enough metals to match the Gangdese Miocene post-collisional porphyry Cu deposit.
Similar content being viewed by others
Introduction
Porphyry Cu–Au deposits are one of the most important ore deposit types worldwide and provide 75% of the Cu and 20% of global Au production (Sillitoe 2010). Porphyry Cu–Au deposits are mainly found at the margins of converging plates, such as island and continental arcs (Richards 2003, 2015). The primary magmas of subduction-related porphyry Cu–Au deposits are mainly mafic magmas formed by partial melting of the mantle wedge induced by dehydration of the subducting plate (Cooke et al. 2005; Sillitoe 2010; Richards 2015; Chen and Wu 2020). However, only a few subduction-related porphyries form giant Cu–Au deposits. The formation of porphyry Cu–Au deposits is affected by many factors, including: (1) the enrichment of metallic elements in the deep primary magma (Bell et al. 2009); (2) the magma oxidation state (Richards 2003); (3) the timing of sulfide saturation in the magma (Park et al. 2015, 2019; Hao et al. 2017); (4) the magma water concentration (Halter et al. 2005); (5) the ascending pathways of the ore-bearing magmas (Richards 2003); (6) the abundance of S and Cl in the magma (Grondahl and Zajacz 2022); and (7) the formation and migration of ore-bearing hydrothermal fluids (Mungall 2002; Wilkinson 2013). Copper and Au are highly compatible in sulfide melts and are thus strongly affected by sulfide saturation. The solubility of S in magmas is sensitive to the magma oxidation state (Jugo 2009). This study investigated how the magma oxidation state and sulfide saturation history of arc magmas control the metal fertility of arc magmas and mineralization potential.
The Gangdese belt has experienced a complex and protracted geological evolution, involving northward subduction in the Mesozoic Neo-Tethys Ocean and the Cenozoic collision between India and Eurasia (Yin and Harrison 2000; Zhang et al. 2021). This evolution has generated a 1500-km-long porphyry mineralization belt in the Tibetan Plateau with Cu resources of >56 million tons (Zheng et al. 2021). Neo-Tethyan oceanic subduction resulted in two periods of magmatism, which produced a large amount of Cretaceous and lesser amounts of Jurassic arc-related igneous rocks. The Jurassic rocks are associated with the giant Xiongcun porphyry Cu–Au deposit (~240 Mt with 0.4 wt% Cu; 173–161 Ma; Tang et al. 2010), whereas no large porphyry deposits associated with Cretaceous arc magmatism have been identified. The reason for this remains unknown.
Numerous Cenozoic porphyry Cu–Au deposits formed in a continental post-collisional setting have been discovered in the Gangdese belt (Hou et al. 2009, 2015; Yang et al. 2014, 2015; Richards 2015). Based on studies of Miocene continental post-collisional porphyry Cu–Au deposits, it has been concluded that these are different from typical arc porphyry deposits in terms of the deep geodynamic processes and magma sources (e.g., Hou et al. 2009, 2015). The magma and metal sources for porphyry Cu–Au deposits in a post-collisional setting are thought to result from the remelting of juvenile mafic lower crust that contains sulfides (Hou et al. 2015; Wang et al. 2019). However, recent studies have suggested that the contribution from the lower crust has been overestimated (Zhang et al. 2022a). It has also been proposed that the magmas associated with post-collisional porphyry Cu–Au deposits were formed by fractionation crystallization of lithospheric mantle-derived K-rich magmas (Chang and Audétat 2022).
Late Cretaceous mafic–ultramafic igneous rocks containing variable amounts of sulfides are found in the Milin area of the Gangdese belt and are thought to be the typical juvenile lower crust formed during subduction in the Neo-Tethys Ocean. We investigated the zircon chemistry and whole-rock platinum-group element (PGE), Au, and S contents of the Milin rocks. We used these data to constrain the magmatic oxygen fugacity (fO2) state and S saturation history during partial melting of the mantle and magmatic evolution, and how this affected mineralization. This provides new insights into the potential of the Late Cretaceous Gangdese arc magmatism to form porphyry Cu–Au deposits and whether lower crustal remelting can provide sufficient metallic elements for the formation of porphyry Cu–Au deposits.
Geological setting and sample descriptions
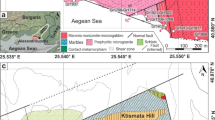
The Tibetan Plateau in southwest China is the largest and highest plateau in the world (Fig. 1a). It is divided into five blocks, including the Kunlun, Songpan–Ganzi, Qiangtang, Lhasa, and Himalayan blocks, from north to south (Fig. 1b). These blocks are separated by the Kunlun, Jinsha, Bangong Lake–Nujiang, and Yarlung–Zangbo suture zones, respectively, which record the closure of the Paleo-Asian and Paleo-, Meso-, and Neo-Tethys oceans, respectively (Yin and Harrison 2000; Zhang et al. 2021).
The Lhasa Block is a microcontinental block that separated from the Gondwana supercontinent and comprises mainly Precambrian basement, Paleozoic–Mesozoic sedimentary, and Mesozoic–Cenozoic igneous rocks (Zhu et al. 2011, 2013). It has experienced the continental collision between India and Eurasia in the early Cenozoic (65–55 Ma; e.g., Mo et al. 2003; Zhu et al. 2015), collision between the Lhasa and Qiangtang blocks in the Late Jurassic to Early Cretaceous (e.g., Pan et al. 2006; Zhu et al. 2013), and subduction in the Bangong Lake–Nujiang Tethys Ocean (to the south) and Yarlung–Zangbo Tethys Ocean (to the north) during the Mesozoic (Zhu et al. 2013). The Lhasa Block is a vast tectonomagmatic belt that has experienced multiple stages of magmatism. The Lhasa Block extends for 2500 km from east to west and is divided into northern, central, and southern sub-terranes by the Shiquan River–NamTso Mélange Zone (SNMZ) and the Luobadui–Milashan Fault (LMF) (Fig. 1b; Zhu et al. 2013). The northern Lhasa Block consists mainly of juvenile crust (Hou et al. 2015), Triassic–Cretaceous strata (sandstone, limestone, slate, and chert), and abundant volcanic rocks (Pan et al. 2006). The central Lhasa Block comprises Neoproterozoic metamorphic crystalline basement, which is covered by widely distributed Carboniferous–Permian clastic metasedimentary rocks (Hu et al. 2005). In addition, there are large areas of Jurassic–Cretaceous volcanic–sedimentary rocks, and a small amount of Ordovician, Silurian, and Devonian strata (Zhu et al. 2011, 2013). The southern Lhasa Block comprises sparse Precambrian crystalline basement, Upper Triassic–Lower Jurassic strata of the Yeba Formation (clastic sedimentary and volcanic rocks), and the Upper Jurassic–Cretaceous Sangri Formation (sandstone, slate, mudstone, and limestone) (Zhu et al. 2013).
The Gangdese belt is located at the southern margin of the central and southern Lhasa blocks and extends for 1500 km from east to west, with a width of 100–200 km from north to south (Fig. 1c). It records northward subduction of the Neo-Tethyan oceanic lithosphere and the collision between India and Eurasia (e.g., Yin and Harrison 2000; Ding and Lai 2003, Ding et al. 2014; Chu et al. 2006; Zhang et al. 2021). As a result, Jurassic volcanic rocks and abundant Cretaceous igneous rocks were generated during subduction, and large-scale magmatism (including K-rich magmatism) occurred during the collisional and post-collisional stages. There is a giant porphyry ore deposit associated with the Jurassic igneous rocks, but no large porphyry deposits associated with the Cretaceous magmatism have been identified. Unlike typical porphyry Cu–Au deposits worldwide, most porphyry deposits formed during the post-collisional stage in the Gangdese belt.
The eastern Gangdese arc consists mainly of three formations (i.e., the Bala, Gongga, and Rouqiecun formations) and four batholiths and complexes (i.e., the Linzhi, Kanniang, Wolong, and Lilong batholiths/complexes) (Fig. 2). The Bala Formation consists of bistagite, amphibolite, and biotite amphibole schist. The Gongga and Rouqiecun formations consist mainly of low-grade (greenschist-facies) metamorphic rocks represented by quartz and two-mica schists. The Linzhi Complex consists of Neoproterozoic to early Cenozoic garnet-bearing gneiss, amphibolite, and quartzite (Dong et al. 2011; Guo et al. 2011). The Kanniang Complex consists mainly of garnet-bearing gabbro. The Wolong batholith comprises Late Cretaceous granodiorite and granite (Sun 2021). The Lilong batholith is an ultramafic–intermediate intrusion and consists of hornblendite, gabbro, diorite, and granodiorite. These rocks have typical cumulate textures and are thought to be the typical juvenile lower crustal cumulates (Zhang et al. 2010; Sun 2021). The studied samples were collected from the Lilong batholith in western Milin County (Figs. 1c and 2).
The studied samples are mainly olivine–pyroxene hornblendite, pyroxene hornblendite, hornblendite, and hornblende gabbro (Fig. 3a–c). The rocks all have cumulate textures and contain disseminated sulfides such as chalcopyrite, pentlandite, pyrrhotite, and pyrite. Sulfides occur mainly at the intervals of silicate mineral grains. Rounded sulfide droplets of pyrrhotite, chalcopyrite, pentlandite, and pyrite are widely distributed in hornblende in the various lithologies (Fig. 3d–h). This indicates the sulfides are magmatic in origin. The olivine–pyroxene hornblendite consists of olivine (25–30 vol.%), pyroxene (10–15 vol.%), and hornblende (50–55 vol.%) (Fig. 3a). The pyroxene hornblendite consists of pyroxene (25–30 vol.%) and hornblende (65–70 vol.%). The pyroxenes have two main forms, with one being subhedral and the other being inclusions in hornblende. Hornblendite is the main sulfide-rich cumulate rock and consists mainly of hornblende (~95 vol.%) (Fig. 3b). The hornblende gabbro consists mainly of pyroxene (30–35 vol.%), plagioclase (45–50 vol.%), and hornblende (~15 vol.%) with minor biotite (Fig. 3c).
Representative photomicrographs of the Milin mafic–ultramafic cumulates showing (a) olivine–pyroxene hornblendite, (b) hornblendite, (c) hornblende gabbro, and (d–h) disseminated sulfide in the silicate minerals. Ol = olivine; Hbl = hornblende; Cpx = clinopyroxene; Pl = plagioclase; Ccp = chalcopyrite; Pn = pentlandite; Po = pyrrhotite; Py = pyrite
Analytical methods
Whole-rock major elements were analyzed using X-ray fluorescence (XRF) spectrometry at the ALS Laboratory Group, Guangzhou, China. The powdered samples (0.7 g) and fluxing agent (7 g; Li2B4O7) were fused and cooled into glass discs for analysis. The precision is <±5%. Trace elements were measured using a Perkin–Elmer ELAN-DRC-e inductively coupled plasma mass spectrometer (ICP–MS) at the State Key Laboratory of Ore Deposit Geochemistry (SKLODG), Institute of Geochemistry, Chinese Academy of Sciences, Guiyang, China. The powdered samples (50 mg) were dissolved in HF + HNO3 in high-pressure Teflon bombs for 8 h at ~190°C. The solutions were then evaporated to dryness and dissolved in 2% HNO3 for ICP–MS analysis. The international standard AGV-2 was used to monitor the data accuracy, and the precision is <±10% for all elements.
Two hornblende gabbro samples from the Milin area were selected for zircon separation at the Langfang Regional Geological Survey, Hebei Province, China. We observed the morphology of the zircons in cathodoluminescence (CL) images and in transmitted and reflected light and selected zircon grains for U–Pb dating and trace element analysis. This process was conducted by laser ablation ICP–MS (GeoLas Pro 193 nm ArF excimer laser and Agilent 7500x ICP–MS) at the SKLODG. The laser beam diameter was 32 μm, with an 8 Hz repetition rate and energy density of 10 J/cm2. Helium was used as the carrier gas. Data for each zircon were obtained for 60 s after 20 s of background data acquisition. The zircon standard 91500 was used as an external standard for the age corrections. The standard materials NIST 610, BHVO-2G, BCR-2G, and BIR-1G were used to correct the zircon trace element data. The zircon U–Pb isotope ratios and trace element contents were obtained using ICPMSDataCal 10.8 software (Liu et al. 2009). Concordia plots were obtained using Isoplot 3.0 (Ludwig 2003). The common Pb correction followed the method of Andersen (2002).
In situ zircon Lu–Hf isotopic compositions were obtained using a Neptune Plus III multi-collector ICP–MS coupled to a New Wave 213 nm laser ablation system at SKLODG. Analyses employed a laser spot size of 44 μm and He as a carrier gas, with a repetition rate of 10 Hz and energy density of ~5.3 J/cm2. During the analyses, the Penglai standard zircon yielded a weighted-mean 176Hf/177Hf ratio of 0.282904 ± 0.000012, which is in good agreement with the recommended Hf isotope ratio for this standard (176Hf/177Hf = 0.282906 ± 0.000010; Li et al. 2010).
Gold and S were analyzed at the ALS Laboratory Group, Guangzhou, China. The samples (25–30 g) were weighed and then digested in aqua regia in a high-density polyethylene bottle. Gold was analyzed using an Agilent 7900 ICP–MS. The detection limit for Au was 0.1 ppb. The samples were placed in an induction furnace at 1350°C, and S released in the form of SO2 was introduced into an infrared detection system. The S contents were measured according to the change in the energy received by the C–S analyzer (CS844 LECO). The detection limit for S was 0.001 wt%.
The PGEs were measured using an improved digestion technique and isotope dilution (ID)–ICP–MS at SKLODG (Qi et al. 2011). The powdered samples (~5 g) were added to ~5 mL of water in a polytetrafluoroethylene (PTFE) beaker. Subsequently, 25 mL of HF was added and the beakers were placed on a hotplate to remove silicates. After the solutions had evaporated, 5 mL of HF and 15 mL of HNO3 were added and the samples were placed in an oven for 48 h at 190°C. The samples were then evaporated to dryness on a hotplate, and 5 mL of HCl was added to remove the residual HF + HNO3 in the sample. After drying, the residues were dissolved in 40 mL of HCl. The solutions were centrifuged at 2800 rev/min for 5 min. The upper part of the solutions was used to collect the PGEs by Te co-precipitation. The precipitates were dissolved in aqua regia and diluted to 10 mL, then the PGEs were extracted on a mixed ion exchange column. Finally, the filtered solutions were evaporated down to ~3 ml for ICP–MS analysis. Platinum, Pd, Ru, and Ir were measured using isotope dilution, and the Rh contents were calculated using 194Pt as an internal standard. The international standard WGB-1 and TDB were used to monitor the data accuracy. The detection limits are 0.004 ppb for Ir, 0.008 ppb for Ru, 0.006 ppb for Rh, 0.014 for Pt, and 0.012 for Pd.
Results
Zircon U–Pb age, Hf isotope, and trace element data
The in-situ zircon U–Pb and Hf isotope and trace element data are listed in Tables S1–3. The zircon grains from the two hornblende gabbro samples exhibit weak oscillatory zoning and have crystal lengths of 50–200 μm, with length/width ratios of 1 to 2. Twenty-nine zircon grains from sample 20ML-26 yielded a weighted-mean 206Pb/238U age of 84.3 ± 1.4 Ma (MSWD = 1.11; Fig. 4a). These zircons have initial 176Hf/177Hf ratios of 0.282959–0.283164, with corresponding εHf(t) values of 8.37–15.81 (Fig. 5). Eighteen zircon analyses of sample 20ML-32 yielded a weighted-mean 206Pb/238U age of 86.7 ± 1.9 Ma (MSWD = 6.4; Fig. 4b), which represents the crystallization age. The zircons have initial 176Hf/177Hf ratios of 0.283058–0.283149, with corresponding εHf(t) values of 11.94–15.18 (Fig. 5).
The remaining zircons have high heavy rare earth element (REE) and low light REE contents, with high total REE contents (ƩREE = 47–1004 ppm), and marked negative to small positive Eu anomalies (EuN/EuN* = 0.12–1.38) (Fig. 4c–d). The REE patterns and high Th/U ratios (0.13–0.67) suggest these are magmatic zircons (Hoskin and Schaltegger 2003). Previous studies have shown that zircon Ce4+/Ce3+ ratios are closely related to the magmatic fO2 (Ballard et al. 2002; Loucks et al. 2020). The zircon Ce4+/Ce3+ ratios are calculated from the method of Ballard et al. (2002) based on Ce contents in zircon and melt. Some mineral inclusions (e.g., rutile and apatite) occur in the zircon grains, and the inclusions can strongly affect the zircon trace element contents. Therefore, some anomalous results (e.g., La > 0.1 ppm and Ti > 20 ppm) were excluded in this study (Zhu et al. 2022). The zircons from the 20ML-26 and 20ML-32 have low Ce4+/Ce3+ ratios of 21–90 and 33–76, with low estimated mean fO2 values of ΔFMQ–3.5±0.5, and ΔFMQ–1.4±0.2 based on the method of Loucks et al. (2020), and these samples have a total mean fO2 values of ΔFMQ–1.8±0.5.
Major and trace element geochemistry
The whole-rock geochemical compositions of the mafic–ultramafic cumulates are listed in Table S4. The loss-on-ignition (LOI) values of the samples are generally <3 wt% (1.22–3.00 wt%). The samples have low SiO2 contents of 41–50 wt%. The variations between major elements and MgO are shown in Fig. 6. SiO2 and Na2O + K2O increase initially with decreasing MgO until 17 and 15 wt%, respectively, and then decrease (Fig. 6a and b). Fe2O3T decreases initially with decreasing MgO until 17 wt% and then increases (Fig. 6c). Al2O3 increases with decreasing MgO (Fig. 6d). Ni and Co decrease with decreasing MgO (Fig. 6e and f). The major element variations reflect the accumulation of silicate minerals (e.g., olivine, pyroxene, and hornblende) during early magmatic evolution.
On primitive mantle-normalized trace element (Fig. 7a) and chondrite-normalized REE diagrams (Fig. 7b), all the samples have similar patterns, with enrichments in large-ion lithophile elements (LILEs; e.g., Rb, Ba, and K) and depletions in high-field-strength elements (HFSEs; e.g., Nb, Ta, Zr, and Hf). The REE patterns are flat ([La/Yb]N = 0.86–2.79), and the samples have no obvious Eu anomalies (Eu/Eu* = 0.8–1.07; mean = 0.96).
Chondrite-normalized REE patterns and primitive mantle-normalized trace element diagrams for the Milin mafic–ultramafic cumulates. Normalization values were taken from Sun and McDonough (1989)
Chalcophile element and sulfur contents
The PGE, Cu, Au, and S contents of the analyzed rocks are listed in Table 1. The Milin mafic–ultramafic cumulates contain 0.16–22.43 ppb Pd (mean = 5.7 ppb), 0.13–15.53 ppb Pt (mean = 3.94 ppb), 0.01–0.42 ppb Rh (mean = 0.12 ppb), 0.01–0.16 ppb Ru (mean = 0.06 ppb), and 0.005–0.33 ppb Ir (mean = 0.1 ppb). The total PGE contents are highly variable (0.34–29.91 ppb). The Pt and Pd contents increase initially with decreasing MgO until ~17 wt% and then decrease (Fig. S1a–b). The Ir and Ru contents decrease with decreasing MgO (Fig. S1c–d). The Cu and Au contents of the samples are 12.6–432 ppm (mean = 151 ppm) and 0.6–10.2 ppb (mean = 3.62 ppb), respectively. The Cu contents exhibit no clear trend with MgO (Fig. S1e). The Au contents increase initially with decreasing MgO until ~17 wt% and then decrease (Fig. S1f). The Cu/Pd ratios of the samples are 5.43 × 103 to 1.12 × 106 and are higher than those of the mantle (103 to 104; Barnes and Maier 1999). These ratios are negatively correlated with Pd (Fig. 8b). The S contents vary from 150 to 7800 ppm (mean = 1360 ppm). The correlation between PGEs, Cu, and Au with S is weak (Fig. S2), which is a common phenomenon in sulfide-bearing rocks (Maier et al. 2004; Lightfoot et al. 2012; Keays and Tegner 2015). This weak correlation can be attributed to the different partition coefficients and R-factors (mass ratio of silicate to sulfide liquid; Campbell and Naldrett 1979) between sulfide melt and silicate magma during sulfide saturation.
(a) Primitive mantle-normalized PGE patterns and (b) plot of Pd versus Cu/Pd for the Milin mafic–ultramafic cumulates. Primitive mantle values are from Barnes and Maier (1999)
Discussion
Reduced arc magmas during the Cretaceous
The mantle wedge oxygen fugacity exerts a key control on arc magma evolution and mineralization in subduction zones. Owing to metasomatism by subduction-derived melts or fluids, mantle wedges are generally considered to be more oxidized than the sources of depleted mantle-derived magmas, such as mid-ocean ridge basalts (i.e., MORBs; Ballhaus 1993; Brounce et al. 2014; Richards 2015). For example, Ballhaus (1993) showed that the fO2 of a mantle wedge ranges from FMQ+1 to FMQ+3 using the olivine–orthopyroxene–spinel equilibrium approach. Brounce et al. (2014) showed that the fO2 of island arc primary magmas varied between FMQ+1 and FMQ+1.6 using the Fe3+/ΣFe ratio of olivine-hosted melt inclusions.
Recent studies have shown that the fO2 was high in arc magmas generated in the early subduction stage (e.g., Jurassic) of the Neo-Tethys Ocean, which is consistent with typical arc magmas worldwide. For example, Chen et al. (2019) reported Ce4+/Ce3+ ratios of 126–3444 for zircons from the Bima Formation arc volcanic rocks (195–165 Ma), and Zou et al. (2015) and Xie et al. (2018) reported that zircons from the Xiongcun porphyry deposit (179–164 Ma) have Ce4+/Ce3+ ratios of 197–3737 (Table S5). These high zircon Ce4+/Ce3+ ratios are indicative of a high fO2 in these arc magmas (Ballard et al. 2002; Trail and Bruce Watson 2012). We estimated the fO2 (Fig. 9) of these Jurassic arc magmas using published zircon data (Wei 2014; Zou et al. 2015; Ma et al. 2017; Xie et al. 2018; Chen et al. 2019), based on the method of Loucks et al. (2020). The Jurassic Bima and Yeba formation volcanic rocks yielded a mean fO2 of ΔFMQ+1.3 and ΔFMQ+1.0, respectively. The Xiongcun porphyry deposit yielded a mean fO2 of ΔFMQ+1.3. These results are consistent with the high fO2 of the Jurassic arc magmas. In contrast, zircons from the Milin mafic–ultramafic cumulates have low Ce4+/Ce3+ ratios (21–90), implying the Late Cretaceous arc magmas had a low fO2. The estimated fO2 of the Milin cumulates ranges from ΔFMQ–3.5 to ΔFMQ–1.0, with a mean of ΔFMQ–1.8±0.5 (Fig. 9), which is much lower than for the Jurassic arc magmas. Therefore, the fO2 of the arc magmas in the Gangdese belt decreased significantly from the Jurassic to Late Cretaceous.
The decrease in the fO2 may have been due to the mantle source or magmatic evolution. During magmatic evolution, variations in fO2 are caused by fractional crystallization and crustal contamination. Carmichael (1991) proposed that during fractional crystallization, the fO2 variations depend on the Fe3+/ΣFe ratio of the residual magma. If the separated minerals cause the Fe3+/ΣFe ratio of the residual magma to increase, then the residual magma becomes more oxidized. However, most studies have shown that fractional crystallization does not lead to significant changes in fO2 (de Hoog et al. 2004; Crabtree and Lange 2012; Grocke et al. 2016). For example, when MgO decreases from 10 to 7, and to 5 wt% in MORBs, the Fe3+/ΣFe ratio only increases by 0.015 and 0.03, respectively (Cottrell and Kelley 2011; Shorttle et al. 2015). Previous studies have shown that garnet and hornblende crystallized during fractionation of the parental magmas to the Milin mafic–ultramafic cumulates (Sun 2021; Zhang et al. 2021). The effects of garnet and hornblende fractionation on magma fO2 are controversial (King et al. 2000; Tang et al. 2018; Lee and Tang 2020; Zhang et al. 2022b; Holycross and Cottrell 2023). Some studies have suggested that garnet and hornblende crystallization will increase the Fe3+/ΣFe ratio and fO2 in the residual magma (Tang et al. 2018; Lee and Tang 2020; Zhang et al. 2022b). Consequently, the low fO2 of the Milin cumulates cannot have been caused by garnet and hornblende fractionation. The crystallization of magnetite will reduce the fO2 of magma. The Xiongcun porphyry deposit and Jurassic volcanic rocks (e.g., Bima and Yeba formation) in the Gangdese experience magnetite differentiation (Zou et al. 2015; Xie et al. 2018; Chen et al. 2019), but their fO2 are still higher than those of the Milin samples. This means that the differentiation of magnetite cannot be the cause of the low fO2 in Milin area. Therefore, the low fO2 of the Late Cretaceous arc magmas was not caused by fractional crystallization.
The addition of oxidizing or reducing crustal material can obviously change the fO2 of magma. For example, the magma of the Duke complex is considered to be reduced by the addition of C-bearing country rocks (Thakurta et al. 2008). However, the Milin mafic–ultramafic cumulates have obvious depleted Hf isotopic compositions (8.37–15.81), similar to those (10.2–17.7; Fig. 5) of the Jurassic arc magmas, indicating that crustal contamination was negligible because crustal material usually has enriched Hf isotope compositions. As such, crustal contamination did not cause the decrease in fO2 from the Jurassic to Late Cretaceous. In fact, the low fO2 may be inherited the mantle source. For instance, Hu et al. (2023) reported the Troodos mantle ophiolite have reduced fO2 values. Furthermore, the chalcophile element depletion in the primary magmas indicates that the primary magmas had a low fO2, implying that the decrease in fO2 may have been inherited from the mantle source. Recent studies have shown that several global oceanic anoxic events (OAEs) occurred worldwide during 120–86 Ma (i.e., in the Cretaceous; Huber et al. 2002; Chen et al. 2011). During OAEs, organic matter-rich black shales were widely deposited in marine and terrestrial environments. Therefore, the low fO2 of the Late Cretaceous mantle source may be caused by the addition of reducing fluid by organic matter-rich sediments, but this still needs further study.
First stage of chalcophile element depletion—residual sulfide in the mantle during partial melting
The fO2 of a magmatic system has a prominent role on S solubility (Jugo 2009), which in turn controls the behavior of chalcophile and siderophile elements (Lee et al. 2012). The S content of the depleted mantle is generally <200 ppm (Nielsen et al. 2014; Ding and Dasgupta 2017). However, owing to metasomatism during subduction, S-containing fluid or melt will enter the mantle wedge and increase the S content of the mantle wedge (300–500 ppm; de Hoog et al. 2001; Wallace and Edmonds 2011; Richards 2015; Li et al. 2022). As a variable-valence element, the valence state (S2− and S6+) of S is markedly affected by the fO2. Sulfide (S2−) and sulfate (S6+) are mainly present under low-fO2 (e.g., ≤FMQ) and high-fO2 (e.g., >FMQ+2; Jugo 2009) conditions. The solubility of S can increase by 10 times with increasing fO2 (Jugo 2009).
Typical arc magmas are produced by 8%–20% partial melting of the mantle wedge (Kelley et al. 2006). At a high fO2, only a small percentage of partial melting is needed to dissolve all the sulfide in the mantle. The chalcophile elements, such as PGEs, Au, and Cu, which are hosted in the sulfide, will thus enter the basaltic magma during partial melting. This process results in the formation of a primary arc magma with metal fertility. Within these primary arc magmas, Copper, Pd and Au can reach up to 200–300 ppm, 20–30 ppb and 8 ppb, respectively (Sun et al. 2004; Dale et al. 2012; Park et al. 2013; Chiaradia 2014; Richards 2015). These concentrations are expected to have Cu/Pd ratios similar to those found in primitive mantle (103 to 104). For example, Pt and Pd in Tongan primary arc magmas (MgO > 9 wt%) are up to 21.0 and 21.2 ppb, respectively, and the Cu/Pd ratios are 4.6 × 103 to 1 × 104 (Dale et al. 2012; Park et al. 2013). Sulfide saturated from this magma would be enriched in PGEs and have low Cu/Pd ratios. For example, the sulfide saturated from magmas of the Tolbachik volcano in the Kamchatka arc contain up to 299 ppm Pd and 115 ppm Pt and have low Cu/Pd ratios (9 × 102 to 4.3 × 104; Zelenski et al. 2017).
The studied samples are mafic–ultramafic cumulates and were early-formed cumulates generated from arc magmas. These rocks contain a variable amount of sulfides (Fig. 3) but are characterized by PGE depletions and high Cu/Pd ratios (5.43 × 103 to 1.12 × 106; Fig. 8). Due to the higher sulfide/silicate partition coefficients for PGEs than Cu, such as PGEs (103 to 105; Bezmen et al. 1994; Zhang and Li 2021; 105 to 106; Mungall and Brenan 2014) and Cu (102 to 103; Crocket et al. 1997), the Cu/Pd ratios of sulfide saturated from a magma would be lower than the initial magma ratios. Therefore, the primary magma of the Milin lower crust would have had higher Cu/Pd ratios than the sulfide-bearing mafic–ultramafic rocks and much higher values than those of the mantle. The high Cu/Pd ratios of Milin sulfide-bearing cumulates imply that the primary magmas were characterized by depletions in PGEs and other chalcophile elements (e.g., Cu and Au). This, together with the low total PGE contents, are inconsistent with sulfide saturated from a chalcophile element-enriched magma generated by partial melting of mantle with a high fO2. In fact, the fO2 (ΔFMQ–1.8±0.5) of the Milin primary magma is more reduced than typical arc magmas. Under such low-fO2 conditions, a high degree of partial melting is required to dissolve all the sulfide in the mantle. At a reasonable degree of partial melting, S-saturated arc magma would be generated and residual sulfide would remain in the mantle. Therefore, the arc magma would have low PGEs contents and high Cu/Pd ratios. This is the case for the Milin cumulates. The estimated chalcophile element contents of the primary arc magma generated by partial melting of the mantle wedge under reduced conditions are shown in Fig. 10. The results show that partial melting of 15% would yield a primary magma with 63 ppm Cu, 0.29 ppb Au, 0.029 ppb Pd, and 0.036 ppb Pt at FMQ–1 (Table S6). Therefore, the Milin primary magmas are characterized by moderate Cu depletion and large Au and PGE depletions.
Mantle melting models. The high temperature during partial melting of the mantle leads to a decrease in the Cu and Au partition coefficients, and thus we assumed that the partition coefficients for Cu, Au, Pd, and Pt between sulfide liquid and silicate melt are 8 × 102, 7 × 103, 2.85 × 105, and 4 × 105, respectively (Mungall and Brenan 2014; Li and Audétat 2015). The initial S, Cu, Au, Pd, and Pt contents in the primitive mantle were 300 ppm, 30 ppm, 1 ppb, 4 ppb, and 7 ppb, respectively (Barnes and Maier 1999; Zhao et al. 2022). The S solubility was 1400 ppm under ΔFMQ–1 condition (Zhao et al. 2022)
The variation of Cu and Pd in these rocks, as shown in Fig. 11, can be modeled using a two-stage sulfide saturation approach with different R-factors. We assume that the arc magmas contained 10 ppb Pd, 5 ppb Au, and 100 ppm Cu if all the Cu-rich Fe sulfide liquid in the mantle had been dissolved in the magma. This assumption is based on the average contents of chalcophile elements in chalcophile elements-undepleted arc magma worldwide (Sun et al. 2007; Dale et al. 2012; Park et al. 2013). The partition coefficients between sulfide liquid and silicate for Au, Cu, and Pd are 8 × 103, 1 × 103, and 2.85 × 105, respectively (Mungall and Brenan 2014; Li and Audétat 2015). The modeling results indicate that during the first stage sulfide saturation, which corresponds to the sulfide retained in the mantle during partial melting, the primary magmas become depleted in chalcophile elements, containing only 0.07 ppb Pd, 1.0 ppb Au, and 66.7 ppm Cu under an R-factor of 2000. The variation of Pd and Cu in the Milin cumulates was formed during the second stage sulfide saturation from the chalcophile elements-depleted primary magma, with R-factors ranging roughly from 103 to 105 (Fig. 11).
Model calculation of the variation of Pd with Cu in the Milin mafic–ultramafic cumulates. Cyan solid points present the sulfide liquids segregated from PGE-rich basaltic magma with 10 ppb Pd and 100 ppm Cu under variable R-factor values. Red solid points show the sulfide liquids segregated from the PGE-depleted magma with 0.07 ppb Pd, and 66.7 ppm Cu under variable R-factor values. The sulfide/silicate partition coefficients for Cu and Pd are 1 × 103 and 2.85 × 105, respectively (Mungall and Brenan 2014; Li and Audétat 2015). Percentages of sulfide in the rocks are labeled as 0.1%, 1% and 10%
In summary, the low fO2 in the mantle source resulted in the presence of residual sulfides in the mantle during partial melting, and thus the Milin primary magmas were depleted in chalcophile elements.
Second stage of chalcophile element depletion caused by early sulfur saturation
The S solubility in the Milin primary magma was low due to the low fO2, and thus the primary magma was saturated in sulfide before it left the mantle. In general, the S solubility increases as pressure decreases (Wendlandt 1982; Mavrogenes and O’Neill 1999). Therefore, as pressure decreases during magma ascent into the lower crust, the magma will become sulfide-undersaturated. As shown in Fig. 3, the early crystallized minerals contain sulfide droplets, suggesting the magma reached sulfide saturation in the early stages of magmatic differentiation. The S solubility is mainly affected by pressure, temperature, magma composition, and oxygen fugacity. The fO2 of the magma was too low to decrease the S solubility. The magma Fe content has an important effect on the S solubility, and thus magnetite crystallization typically leads to sulfide saturation. However, the Milin cumulates are the products of early crystallization of magma, and magnetite crystallization was negligible.
For a typical primary arc basaltic magma, the S solubility is about 1400 ppm at 2 GPa under reduced condition and, when the pressure drops to 1 GPa, the S solubility increases to 1700 ppm (Fortin et al. 2015). Owing to S being an incompatible element in olivine, clinopyroxene, and other early liquidus minerals, S contents will increase with fractional crystallization in the residual magma. The Milin primary magma was S-saturated, and thus the S content in the primary magma was equal to its solubility (i.e., the Milin primary magma contained 1400 ppm S). Only 20% fractional crystallization is required to increase the S content in the magma to 1700 ppm, which then results in S saturation. In addition, the S solubility decreases by about 400 ppm for every decrease of 100°C at 1 GPa (Holzheid and Grove 2002), which suggests that a temperature decrease could also have resulted in sulfide saturation. Therefore, we propose that as fractional crystallization proceeded and temperature decreased during early magmatic evolution, S became saturated in the Milin basaltic magmas. As such, metallic elements were sequestered by the saturated sulfide, which further reduced the metal contents of the residual magma. We estimate that the residual basaltic magma after S saturation in the early stage of magmatic differentiation contained 33-66 ppm Cu, 0.13–0.93 ppb Au, and 0–0.02 ppb Pd. These contents are much lower than those of typical arc basaltic magmas (Cu = 93–135 ppm; Au = 3.84–6.06 ppb; Pd = 7.61–13.7 ppb; Pt = 2.80–7.61 ppb; Sun et al. 2007; Park et al. 2013).
Mineralization implications for the Gangdese belt
Late Cretaceous arc magmatism
The Gangdese belt has experienced multiple stages of magmatism and porphyry Cu–Au mineralization. The large Xiongcun porphyry Cu–Au deposit was associated with Jurassic arc magmatism (Tang et al. 2010). There are abundant Late Cretaceous arc igneous rocks in the Gangdese belt, but no large porphyry deposits associated with these have been found. There are many factors involved in the formation of porphyry Cu–Au deposits, but it is important that there are enough metallic elements in the magma. Our results show that residual sulfide in the mantle during partial melting was due to the low fO2, which resulted in the primary magma being moderated depleted in Cu and highly depleted in Au and PGEs. Furthermore, the primary magma reached sulfide saturation in the lower crust during the early stages of magmatic differentiation. Abundant chalcophile elements were sequestered in the saturated sulfide in the lower crust. The residual magma after S saturation contained 33–66 ppm Cu, 0.13–0.93 ppb Au and 0–0.02 ppb Pd, much lower than typical arc basaltic magmas.
It has been found that some large Cu porphyries are formed in association with magma with lower content of Cu. Recent studies have shown that the redissolution of sulfide can form magmatic–hydrothermal fluids containing large amounts of metallic elements and thus provide sufficient materials for the formation of porphyry Cu–Au deposits (Wilkinson 2013; Mungall et al. 2015; Bai et al. 2020). However, the exsolution of hydrothermal fluids from magma is physical release and it more likely to occurs in the shallow crust. Although saturated sulfides can be transported by ascending andesitic arc magmas (Heinrich and Connolly 2022), the magma from the lower crust must pass through multi-level magma chambers to reach the shallow crust (Fig. 12; Annen et al. 2006), which will reduce the magma flow rate and lead to sulfide being precipitated in the multi-level magma chambers. As a result, the Milin lower crustal sulfides were difficult to transfer to the shallow crust and could not be dissolved in aqueous fluids.
Schematic diagram of the evolution of the Milin arc magmas in the Gangdese arc. Under the low fO2 conditions, partial melting of the mantle wedge with metasomatism by subduction-related fluid or melt produced a sulfide-saturated primary arc magma, with depletions in chalcophile elements due to the residual sulfide in the mantle. The primary arc magmas reached sulfide saturation owing to fractional crystallization proceeded and the temperature decreased during the early stages of magmatic differentiation. In this case, the chalcophile elements were sequestered into the early formed sulfide, which further reduced the chalcophile element contents in the residual basaltic magma. Both these factors hindered the formation of large Late Cretaceous porphyry Cu–Au deposits in the Gangdese belt
Based on our results, we propose that sulfide saturation in the early stages of magmatic differentiation and residual sulfide in the mantle during partial melting hindered the formation of large porphyry Cu–Au deposits associated with the Late Cretaceous arc magmatism in the Gangdese belt (Fig. 12).
Implications for the metal sources of the post-collisional porphyry Cu–Au deposits
Because of the early saturation and accumulation of sulfide during magmatic differentiation, the Milin lower crustal cumulates contain 1361 ppm S and 151 ppm Cu; i.e., much higher than the contents of their mantle source. In the Gangdese belt, numerous Cenozoic porphyry Cu deposits formed in a post-collisional setting. It has long been proposed that the ore-bearing magmas formed by remelting of thickened, sulfide-rich, juvenile lower crust (Richards 2009; Hou et al. 2015; Wang et al. 2019). However, recent studies have shown that the contribution from the lower crust to the porphyry Cu deposits was overestimated (Zhang et al. 2022a). To assess the importance of lower crust in the formation process of the post-collisional porphyry Cu deposit, we conducted remelting simulations of the Milin lower crustal mafic cumulates. Remelting of mafic–ultramafic cumulates generally produces andesitic magmas. We assumed the temperature and pressure were 800°C–1000°C and 1 GPa, respectively. Under these conditions, sulfide exists mainly as monosulfide solid solution (MSS; Li and Audétat 2015). Given the partition coefficients of Cu between silicate minerals (olivine, pyroxene, and hornblende) and melt range of 0.05 to 0.2 (Fellows and Canil 2012; Liu et al. 2014), the bulk D was set to 0.1 for silicate assemblages. The partition coefficients of Cu between MSS and melt are estimated according to Li and Audétat (2015). The S solubility of the andesitic magma are calculated from the equations of Jugo et al. (2010), Fortin et al. (2015), and Zajacz and Tsay (2019). More detailed parameters of the model are listed in the caption of Fig. 13. According to the results of partial melting experiments (e.g., Beard and Lofgren 1991; Qian and Hermann 2013; Gao et al. 2016), a water-rich mafic crustal material can reach a maximum of 30%–50% partial melting at 800 °C. The Milin mafic–ultramafic rocks are rich in hornblendes and represents a water-rich lower crust. Moreover, Lu et al. (2015) reported that additional water was added during the partial melting process of the Gangdese Miocene lower crust. Therefore, a high degree (50%) of melting is assumed in our model. The estimated contents of metals in the magma generated by remelting of the Milin lower crustal cumulates are listed in Table S7 and shown in Fig. 13.
Remelting models for Cu in the Milin mafic–ultramafic cumulate. The average contents of the Milin mafic–ultramafic cumulate are 1361 ppm S and 151 ppm Cu. We assumed the temperature and pressure were 800°C–1000°C and 1 GPa. Under these conditions, the sulfide exists mainly as monosulfide solid solution (MSS; Li and Audétat 2015; Sun 2021). DCu = 2100, 1900, 1700 and 830, 740, 660 at 1 GPa, 800°C, 1000°C and FMQ, FMQ+1, FMQ+2 conditions, respectively. The S solubility of the andesitic magma are 230, 410, 600 and 560, 1000, 2400 ppm under 800°C, 1000°C and FMQ, FMQ+1, FMQ+2 conditions, respectively (Jugo et al. 2010; Fortin et al. 2015; Zajacz and Tsay 2019)
The results show that the Cu content in the melt produced by 800°C and <50% partial melting of the lower crust is 19–26 ppm (Fig. 13a and b). Because of the lower magma S solubility compared to the S content in the lower crust, the melt generated is always S-saturated at 800°C. Thus, the Cu content in the melt do not increase markedly with an increasing degree of partial melting, even under high fO2 condition (Fig. 13a and b). On the contrary, there are higher Cu (48–112 ppm) content in the initial melt under 1000°C condition, which are higher than those (Cu ~40 ppm) of typical andesitic arc magmas (~3 wt% MgO; Sun et al. 2007; Park et al. 2015; Richards 2015). This is due to higher S solubility of magma and low partition coefficient of Cu between MSS and magma at 1000°C (Holzheid and Grove 2002; Li and Audétat 2015). The increased S solubility enables the melt to dissolve more sulfide. This, together with the low partition coefficient, results in a higher metal content in the melt compared to the content generated at low temperatures.
Our modeling show that the remelting of the lower crust can generate a magma with fertile metals under conditions of high temperature and high fO2, and magma with barren metals under low temperatures even with high fO2. Indeed, there are Miocene ultrapotassic magmas with high fO2 in the Gangdese belt (Li et al. 2020), and their underplating and addition may contribute to the high fO2 of the magma generated by the lower crustal remelting (Hou et al. 2004; Wang et al. 2014). Therefore, the high temperature is crucial to whether the lower crust remelting can provide abundant metal to match the Gangdese Miocene post-collisional porphyry Cu deposit. Previous studies have shown that the formation temperature of ore-bearing porphyry in the post-collision stage of the Gangdese belt is relatively low. For instance, Lu et al. (2015) reported that zircon saturation temperatures of the ore-bearing porphyries ranging from 680 to 780°C. Wu (2016) calculated that the crystallization temperature of hornblende in different porphyry deposits was between 700 and 800°C. However, it is unclear whether the lower crust source was remelted at high temperatures, which is depend on the heat budgets allow by the ultrapotassic magmas. If the temperature of the remelting is not high enough, additional metal may be contributed to the formation of the Gangdese Miocene post-collisional porphyry Cu deposits. Consequently, further investigation on temperature for source melting is probably needed.
Conclusions
The studied mafic–ultramafic cumulates (86.7–84.3 Ma) in the lower crust were the products of Late Cretaceous subduction in the Neo-Tethys Ocean. The cumulates have low Ce4+/Ce3+ ratios (21–90) and low fO2 values (mean ΔFMQ–1.8±0.5) that are lower than those of the Jurassic arc magmatism (126–3737; ΔFMQ+1.0 to ΔFMQ+1.3), suggesting the fO2 of the arc magmas in the Gangdese belt decreased from the Jurassic to Late Cretaceous. The reduced nature of the magmas cannot be explained by fractional crystallization or crustal contamination, suggesting inheritance from a mantle source.
Under low-fO2 conditions, residual sulfide remained in the mantle during partial melting, resulting in significant depletions in chalcophile elements in the Milin primary arc magmas. In the lower crust, sulfide saturation in the magma during the early stages of magmatic differentiation led to a further decrease in chalcophile element content in the residual magmas. Both these factors hindered the formation of large Late Cretaceous porphyry Cu–Au deposits in the Gangdese belt. Remelting of the Milin sulfide-rich cumulates can produce a Cu-rich andesitic magma only under high temperature and high-fO2 conditions, and a magma with low Cu content under low temperature even high fO2 conditions. Therefore, the temperature of the lower crust remelting is a crucial factor in determining whether it can supply sufficient metals to match the Gangdese Miocene post-collisional porphyry Cu deposit, and further investigations are necessary.
References
Andersen T (2002) Correction of common lead in U-Pb analyses that do not report 204Pb. Chem Geol 192:59–79. https://doi.org/10.1016/S0009-2541(02)00195-X
Annen C, Blundy JD, Sparks RSJ (2006) The genesis of intermediate and silicic magmas in deep crustal hot zones. J Petrol 47(3):505–539. https://doi.org/10.1093/petrology/egi084
Bai ZJ, Zhong H, Hu RZ, Zhu WG (2020) Early sulfide saturation in arc volcanic rocks of southeast China: Implications for the formation of co-magmatic porphyry–epithermal Cu–Au deposits. Geochim Cosmochim Acta 280:66–84. https://doi.org/10.1016/j.gca.2020.04.014
Ballard JR, Palin JM, Campbell IH (2002) Relative oxidation states of magmas inferred from Ce“IV”/Ce“III” in zircon: Application to porphyry copper deposits of northern Chile. Contrib Mineral Petrol 144:347–364. https://doi.org/10.1007/s00410-002-0402-5
Ballhaus C (1993) Redox states of lithospheric and asthenospheric upper mantle. Contrib to Mineral Petrol 114:331–348. https://doi.org/10.1007/BF01046536
Barnes SJ, Maier WD (1999) The fractionation of Ni, Cu and the noble metals in silicate and sulfide liquids. Geol Soci Can Mine Soci Cana 13:69–106
Beard JS, Lofgren GE (1991) Dehydration melting and water-saturated melting of basaltic and andesitic greenstones and amphibolites at 1, 3, and 6.9 kbar. J Petrol 32:365–401. https://doi.org/10.1093/petrology/32.2.365
Bell AS, Simon A, Guillong M (2009) Experimental constraints on Pt, Pd and Au partitioning and fractionation in silicate melt-sulfide-oxide-aqueous fluid systems at 800 °C, 150 MPa and variable sulfur fugacity. Geochim Cosmochim Acta 73:5778–5792. https://doi.org/10.1016/j.gca.2009.06.037
Bezmen NI, Asif M, Brugmann GE, Romanenko IM, Naldrett AJ (1994) Distribution of Pd, Rh, Ru, Ir, Os, and Au between sulfide and silicate melts. Geochim Cosmochim Acta 58:1251–1260. https://doi.org/10.1016/0016-7037(94)90379-4
Brounce MN, Kelley KA, Cottrell E (2014) Variations in Fe3+/∑Fe of Mariana Arc Basalts and Mantle Wedge fO2. J Petrol 55:2513–2536. https://doi.org/10.1093/petrology/egu065
Campbell IH, Naldrett AJ (1979) The influence of silicate: Sulfide ratios on the geochemistry of magmatic sulfides. Econ Geol 74:1503–1506. https://doi.org/10.2113/gsecongeo.74.6.1503
Carmichael ISE (1991) The redox states of basic and silicic magmas: a reflection of their source regions? Contrib Mineral Petrol 106:129–141. https://doi.org/10.1007/BF00306429
Chang J, Audétat A (2022) Post-subduction porphyry Cu magmas in the Sanjiang region of southwestern China formed by fractionation of lithospheric mantle–derived mafic magmas. Geology 51(1):64–68. https://doi.org/10.1130/G50502.1
Chen H, Wu C (2020) Metallogenesis and major challenges of porphyry copper systems above subduction zones. Sci China Earth Sci 63:899–918. https://doi.org/10.1007/s11430-019-9595-8
Chen X, Richards JP, Liang H, Zou Y, Zhang J, Huang W, Ren L, Wang F (2019) Contrasting arc magma fertilities in the Gangdese belt, Southern Tibet: Evidence from geochemical variations of Jurassic volcanic rocks. Lithos 324-325:789–802. https://doi.org/10.1016/j.lithos.2018.12.008
Chen X, Wang CS, Huang YJ (2011) Progress in the study of Cretaceous rapid climate change evidence of glaciation in a greenhouse world. Geo Science 25(3):409–418 (In Chinese with English abstract)
Chiaradia M (2014) Copper enrichment in arc magmas controlled by overriding plate thickness. Nat Geosci 7(1):43–46. https://doi.org/10.1038/ngeo2028
Chu MF, Chung SL, O’Reilly SY, Pearson NJ, Wu FY, Li XH, Liu D, Ji J, Chu CH, Lee HY (2011) India’s hidden inputs to Tibetan orogeny revealed by Hf isotopes of Transhimalayan zircons and host rocks. Earth Planet Sci Lett 307(3-4):479–486. https://doi.org/10.1016/j.epsl.2011.05.020
Chu MF, Chung SL, Song B, Liu D, O’Reilly SY, Pearson NJ, Ji J, Wen DJ (2006) Zircon U-Pb and Hf isotope constraints on the Mesozoic tectonics and crustal evolution of southern Tibet. Geology 34(9):745–748. https://doi.org/10.1130/G22725.1
Cooke DR, Hollings P, Walshe JL (2005) Giant porphyry deposits: Characteristics, distribution, and tectonic controls. Econ Geol 100:801–818. https://doi.org/10.2113/gsecongeo.100.5.801
Cottrell E, Kelley KA (2011) The oxidation state of Fe in MORB glasses and the oxygen fugacity of the upper mantle. Earth Planet Sci Lett 305:270–282. https://doi.org/10.1016/j.epsl.2011.03.014
Crabtree SM, Lange RA (2012) An evaluation of the effect of degassing on the oxidation state of hydrous andesite and dacite magmas: A comparison of pre- and post-eruptive Fe2+ concentrations. Contrib Mineral Petrol 163:209–224. https://doi.org/10.1007/s00410-011-0667-7
Crocket JH, Fleet ME, Stone WE (1997) Implications of composition for experimental partitioning of platinum-group elements and gold between sulfide liquid and basalt melt: The significance of nickel content. Geochim Cosmochim Acta 61:4139–4149. https://doi.org/10.1016/S0016-7037(97)00234-2
Dale CW, Macpherson CG, Pearson DG, Hammond SJ, Arculus RJ (2012) Inter-element fractionation of highly siderophile elements in the Tonga Arc due to flux melting of a depleted source. Geochim Cosmochim Acta 89:202–225. https://doi.org/10.1016/j.gca.2012.03.025
de Hoog JCM, Hattori KH, Hoblitt RP (2004) Oxidized sulfur-rich mafic magma at Mount Pinatubo, Philippines. Contrib to Mineral Petrol 146:750–761. https://doi.org/10.1007/s00410-003-0532-4
de Hoog JCM, Mason PRD, Van Bergen MJ (2001) Sulfur and chalcophile elements in subduction zones: Constraints from a laser ablation ICP-MS study of melt inclusions from Galunggung volcano, Indonesia. Geochim Cosmochim Acta 68(18):3147–3164. https://doi.org/10.1016/S0016-7037(01)00634-2
Ding L, Lai Q (2003) New geological evidence of crustal thickening in the Gangdese block prior to the Indo-Asian collision. Chinese Sci Bull 48:1604–1610. https://doi.org/10.1007/bf03183969
Ding L, Xu Q, Yue Y, Wang H, Cai F, Li S (2014) The Andean-type Gangdese Mountains: Paleoelevation record from the Paleocene-Eocene Linzhou Basin. Earth Planet Sci Lett 392:250–264. https://doi.org/10.1016/j.epsl.2014.01.045
Ding S, Dasgupta R (2017) The fate of sulfide during decompression melting of peridotite–implications for sulfur inventory of the MORB-source depleted upper mantle. Earth Planet Sci Lett 459:183–195. https://doi.org/10.1016/j.epsl.2016.11.020
Dong X, Zhang Z, Santosh M, Wang W, Yu F, Liu F (2011) Late neoproterozoic thermal events in the northern lhasa terrane, south tibet: Zircon chronology and tectonic implications. J Geodyn 52(5):389–405. https://doi.org/10.1016/j.jog.2011.05.002
Fellows SA, Canil D (2012) Experimental study of the partitioning of Cu during partial melting of Earth’s mantle. Earth Planet Sci Lett 337:133–143. https://doi.org/10.1016/j.epsl.2012.05.039
Fortin MA, Riddle J, Desjardins-Langlais Y, Baker DR (2015) The effect of water on the sulfur concentration at sulfide saturation (SCSS) in natural melts. Geochim Cosmochim Acta 160:100–116. https://doi.org/10.1016/j.gca.2015.03.022
Gao P, Zheng YF, Zhao ZF (2016) Experimental melts from crustal rocks: A lithochemical constraint on granite petrogenesis. Lithos 266–267:133–157. https://doi.org/10.1016/j.lithos.2016.10.005
Grocke SB, Cottrell E, de Silva S, Kelley KA (2016) The role of crustal and eruptive processes versus source variations in controlling the oxidation state of iron in Central Andean magmas. Earth Planet Sci Lett 440:92–104. https://doi.org/10.1016/j.epsl.2016.01.026
Grondahl C, Zajacz Z (2022) Sulfur and chlorine budgets control the ore fertility of arc magmas. Nat Commun 13:4218. https://doi.org/10.1038/s41467-022-31894-0
Guo L, Zhang HF, Harris N, Pan FB, Xu WC (2011) Origin and evolution of multi-stage felsic melts in eastern Gangdese belt: Constraints from U-Pb zircon dating and Hf isotopic composition. Lithos 127(1):54–67. https://doi.org/10.1016/j.lithos.2011.08.005
Halter WE, Heinrich CA, Pettke T (2005) Magma evolution and the formation of porphyry Cu-Au ore fluids: Evidence from silicate and sulfide melt inclusions. Miner Depos 39:845–863. https://doi.org/10.1007/s00126-004-0457-5
Hao H, Campbell IH, Park JW, Cooke DR (2017) Platinum-group element geochemistry used to determine Cu and Au fertility in the Northparkes igneous suites, New South Wales, Australia. Geochim Cosmochim Acta 216:372–392. https://doi.org/10.1016/j.gca.2017.05.009
Heinrich CA, Connolly JAD (2022) Physical transport of magmatic sulfides promotes copper enrichment in hydrothermal ore fluids. Geology 50(10):1101–1105. https://doi.org/10.1130/G50138.1
Holycross M, Cottrell E (2023) Garnet crystallization does not drive oxidation at arcs. Science 380(6644):506–509. https://doi.org/10.1126/science.ade3418
Holzheid A, Grove TL (2002) Sulfur saturation limits in silicate melts and their implications for core formation scenarios for terrestrial planets. Am Mineral 87(2–3):227–237. https://doi.org/10.2138/am-2002-2-304
Hoskin PWO, Schaltegger U (2003) The composition of zircon and igneous and metamorphic petrogenesis. Rev Mineral Geochem 53:27–62. https://doi.org/10.2113/0530027
Hou Z, Yang Z, Qu X, Meng X, Li Z, Beaudoin G, Rui Z, Gao Y, Zaw K (2009) The Miocene Gangdese porphyry copper belt generated during post-collisional extension in the Tibetan Orogen. Ore Geol Rev 36:25–51. https://doi.org/10.1016/j.oregeorev.2008.09.006
Hou Z, Yang ZM, Lu Y, Kemp A, Zheng Y, Li Q, Tang J, Yang ZS, Duan L (2015) A genetic linkage between subduction- and collision-related porphyry Cu deposits in continental collision zones. Geology 43(3):247–250. https://doi.org/10.1130/G36362.1
Hou ZQ, Gao YF, Qu XM, Meng X, Li Z, Beaudoin G, Rui Z, Gao Y, Zaw K (2004) Origin of adakitic intrusives generated during mid-Miocene east-west extension in southern Tibet. Earth Planet Sci Lett 220:139–155. https://doi.org/10.1016/S0012-821X(04)00007-X
Hu D, Wu Z, Jiang W, Shi Y, Ye P, Liu Q (2005) SHRIMP zircon U-Pb age and Nd isotopic study on the nyainqêntanglha group in tibet. Sci China Ser D Earth Sci 48(9):1377–1386. https://doi.org/10.1360/04yd0183
Hu WJ, Zhou MF, Julia MR, John M, Wu YD, B ZJ (2023) The Redox State of Incipient Oceanic Subduction Zones: An Example from the Troodos Ophiolite (Cyprus). J Geophys Res Solid Earth 128(4):e2022JB025008. https://doi.org/10.1029/2022JB025008
Huber BT, Norris RD, MacLeod KG (2002) Deep-sea paleotemperature record of extreme warmth during the Cretaceous. Geology 30:123–126. https://doi.org/10.1130/0091-7613(2002)030<0123:DSPROE>2.0.CO;2
Ji WQ, Wu FY, Chung SL, Li JX, Liu CZ (2009) Zircon U-Pb geochronology and Hf isotopic constraints on petrogenesis of the Gangdese batholith, southern Tibet. Chem Geol 262:229–245. https://doi.org/10.1016/j.chemgeo.2009.01.020
Jugo PJ (2009) Sulfur content at sulfide saturation in oxidized magmas. Geology 37:415–418. https://doi.org/10.1130/G25527A.1
Jugo PJ, Wilke M, Botcharnikov RE (2010) Sulfur K-edge XANES analysis of natural and synthetic basaltic glasses: Implications for S speciation and S content as function of oxygen fugacity. Geochim Cosmochim Acta 74:5926–5938. https://doi.org/10.1016/j.gca.2010.07.022
Keays RR, Tegner C (2015) Magma chamber processes in the formation of the low-sulphide magmatic Au-PGE mineralization of the platinova reef in the skaergaard intrusion, East Greenland. J Petrol 56(12):2319–2340. https://doi.org/10.1093/petrology/egv075
Kelley KA, Plank T, Grove TL, Stolper EM, Newman S, Hauri E (2006) Mantle melting as a function of water content beneath back-arc basins. J Geophys Res Solid Earth 111(89):B08209. https://doi.org/10.1029/2005JB003732
King PL, Hervig RL, Holloway JR, Delaney JS, Dyar MD (2000) Partitioning of Fe3+/Fetotal between amphibole and basanitic melt as a function of oxygen fugacity. Earth Planet Sci Lett 178(1–2):97–112. https://doi.org/10.1016/S0012-821X(00)00071-6
Lee CTA, Tang M (2020) How to make porphyry copper deposits. Earth Planet Sci Lett 529:115868. https://doi.org/10.1016/j.epsl.2019.115868
Lee CTA, Luffi P, Chin EJ, Bouchet R, Dasgupta R, Morton DM, Le Roux V, Yin QZ, Jin D (2012) Copper systematics in arc magmas and implications for crust-mantle differentiation. Science 336(6077):64–68. https://doi.org/10.1126/science.1217313
Li JL, Gao J, Huang GF, Ma ZP, Wang XY (2022) Geochemical behavior and recycling of sulfur in subduction zones. Acta Petr Sin 38(5):1345–1359. https://doi.org/10.18654/1000-0569/2022.05.05 (in Chinese with English abstract)
Li W, Yang Z, Chiaradia M, Lai Y, Yu C, Zhang J (2020) Redox state of southern Tibetan upper mantle and ultrapotassic magmas. Geology 48(7):733–736. https://doi.org/10.1130/G47411.1
Li XH, Long WG, Li QL, Liu Y, Zheng YF, Yang YH, Chamberlain KR, Wan DF, Guo CH, Wang XC, Tao H (2010) Penglai Zircon Megacrysts: A Potential New Working Reference Material for Microbeam Determination of Hf-O Isotopes and U-Pb Age. Geostand Geoanalytical Res 34:117–134. https://doi.org/10.1111/j.1751-908X.2010.00036.x
Li Y, Audétat A (2015) Effects of temperature, silicate melt composition, and oxygen fugacity on the partitioning of V, Mn Co, Ni, Cu, Zn, As, Mo, Ag, Sn, Sb, W, Au, Pb, and Bi between sulfide phases and silicate melt. Geochim Cosmochim Acta 162:25–45. https://doi.org/10.1016/j.gca.2015.04.036
Lightfoot PC, Keays RR, Evans-Lamswood D, Wheeler R (2012) S saturation history of Nain Plutonic Suite mafic intrusions: Origin of the Voisey’s Bay Ni-Cu-Co sulfide deposit, Labrador, Canada. Miner Depos 47(1):23–50. https://doi.org/10.1007/s00126-011-0347-6
Liu X, Xiong X, Audétat A, Li Y, Song M, Li L, Sun W, Ding X (2014) Partitioning of copper between olivine, orthopyroxene, clinopyroxene, spinel, garnet and silicate melts at upper mantle conditions. Geochim Cosmochim Acta 125:1–22. https://doi.org/10.1016/j.gca.2013.09.039
Liu Y, Gao S, Hu Z, Gao C, Zong K, Wang D (2009) Continental and oceanic crust recycling-induced melt-peridotite interactions in the Trans-North China Orogen: U-Pb dating, Hf isotopes and trace elements in zircons from mantle xenoliths. J Petrol 51:37–571. https://doi.org/10.1093/petrology/egp082
Loucks RR, Fiorentini ML, Henriquez GJ (2020) New magmatic oxybarometer using trace elements in zircon. J Petrol 61(3):egaa034. https://doi.org/10.1093/petrology/egaa034
Lu YJ, Loucks RR, Fiorentini ML, Yang ZM, Hou ZQ (2015) Fluid flux melting generated postcollisional high Sr/Y copper ore-forming water-rich magmas in Tibet. Geology 43(7):583–586. https://doi.org/10.1130/G36734.1
Ludwig KR (2003) User’s manual for Isoplot 3.00, a geochronological toolkit for Microsoft Excel, vol 4. Berkeley Geochronology Center special publication, pp 1–70
Ma X, Xu Z, Chen X, Meert JG, He Z, Liang F, Meng Y, Ma S (2017) The origin and tectonic significance of the volcanic rocks of the Yeba Formation in the Gangdese magmatic belt, South Tibet. J Earth Sci 28:265–282. https://doi.org/10.1007/s12583-016-0925-8
Maier WD, Gomwe T, Barnes SJ, Li C, Theart H (2004) Platinum group elements in the Uitkomst Complex, South Africa. Econ Geol 99(3):0499–0516. https://doi.org/10.2113/gsecongeo.99.3.0499
Mavrogenes JA, O’Neill HSC (1999) The relative effects of pressure, temperature, and oxygen fugacity on the solubility of sulfide in mafic magmas. Geochim Cosmochim Acta 63(7–8):1173–1180. https://doi.org/10.1016/S0016-7037(98)00289-0
Meisel T, Moser J (2004) Reference materials for geochemical PGE analysis: new analytical data for Ru, Rh, Pd, Os, Ir, Pt and Re by isotope dilution ICP-MS in 11 geological reference materials. Chem Geol 208(1–4):319–338. https://doi.org/10.1016/j.chemgeo.2004.04.019
Mo XX, Zhao ZD, Deng JF, Dong GC, Zhou S, Guo TY, Zhang SQ, Wang LL (2003) Response of volcanism to the India-Asia collision. Earth Sci Front 10(3):135–148. https://doi.org/10.3321/j.issn:1005-2321.2003.03.013 (in Chinese with English abstract)
Mungall J, Brenan J (2014) Partitioning of platinum-group elements and Au between sulfide liquid and basalt and the origins of mantle-crust fractionation of the chalcophile elements. Geochim Cosmochim Acta 125:265–289. https://doi.org/10.1016/j.gca.2013.10.002
Mungall JE (2002) Roasting the mantle: Slab melting and the genesis of major Au and Au-rich Cu deposits. Geology 30(10):915–918. https://doi.org/10.1130/0091-7613(2002)030<0915:RTMSMA>2.0.CO;2
Mungall JE, Brenan JM, Godel B, Barnes SJ, Gaillard F (2015) Transport of metals and sulphur in magmas by flotation of sulphide melt on vapour bubbles. Nat Geosci 8:216–219. https://doi.org/10.1038/ngeo2373
Nielsen SG, Shimizu N, Lee CTA, Behn MD (2014) Chalcophile behavior of thallium during MORB melting and implications for the sulfur content of the mantle. Geochem Geophys Geosyst 15(12):4905–4919. https://doi.org/10.1002/2014GC005536
Pan GT, Mo XX, Hou ZQ, Zhu DC, Wang LQ, Li GM, Zhao ZD, Geng QR, Liao LZ (2006) Spatial-temporal framework of the Gangdese Orogenic belt and its evolution. Acta Petr Sin 22(3):521–533. https://doi.org/10.3321/j.issn:1000-0569.2006.03.001 (in Chinese with English abstract)
Park JW, Campbell IH, Arculus RJ (2013) Platinum-alloy and sulfur saturation in an arc-related basalt to rhyolite suite: Evidence from the Pual Ridge lavas, the Eastern Manus Basin. Geochim Cosmochim Acta 101:76–95. https://doi.org/10.1016/j.gca.2012.10.001
Park JW, Campbell IH, Kim J, Moon JW (2015) The Role of Late Sulfide Saturation in the Formation of a Cu- and Au-rich Magma: Insights from the Platinum Group Element Geochemistry of Niuatahi–Motutahi Lavas, Tonga Rear Arc. J Petrol 56:59–81. https://doi.org/10.1093/petrology/egu071
Park JW, Campbell IH, Malaviarachchi SPK, Cocker H, Hao H, Kay SM (2019) Chalcophile element fertility and the formation of porphyry Cu ± Au deposits. Miner Depos 54(5):657–670. https://doi.org/10.1007/s00126-018-0834-0
Qi L, Gao J, Huang X, Hu J, Zhou MF, Zhong H (2011) An improved digestion technique for determination of platinum group elements in geological samples. J Anal At Spectrom 26:1900–1904. https://doi.org/10.1039/c1ja10114e
Qian Q, Hermann J (2013) Partial melting of lower crust at 10-15 kbar: Constraints on adakite and TTG formation. Contrib Mineral Petrol 165:1195–1224. https://doi.org/10.1007/s00410-013-0854-9
Richards JP (2003) Tectono-magmatic precursors for porphyry Cu-(Mo-Au) deposit formation. Econ Geol 98:1515–1533. https://doi.org/10.2113/gsecongeo.98.8.1515
Richards JP (2009) Post-subduction porphyry Cu-Au and epithermal Au deposits: Products of remelting of subduction-modified lithosphere. Geology 37:247–250. https://doi.org/10.1130/G25451A.1
Richards JP (2015) The oxidation state, and sulfur and Cu contents of arc magmas: Implications for metallogeny. Lithos 233:27–45. https://doi.org/10.1016/j.lithos.2014.12.011
Shorttle O, Moussallam Y, Hartley ME, Maclennan J, Edmonds M, Murton BJ (2015) Fe-XANES analyses of Reykjanes Ridge basalts: Implications for oceanic crust’s role in the solid Earth oxygen cycle. Earth Planet Sci Lett 427:272–285. https://doi.org/10.1016/j.epsl.2015.07.017
Sillitoe RH (2010) Porphyry copper systems. Econ Geol 105:3–41. https://doi.org/10.2113/gsecongeo.105.1.3
Sun QS (2021) Enrichment mechanism of sulfide in cumulates in the lower crust of Gangdese belt, Tibet. Master's thesis, China University of Geosciences (in Chinese with English abstract)
Sun SS, McDonough WF (1989) Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. Geol Soc Spec Publ 42:313–345. https://doi.org/10.1144/GSL.SP.1989.042.01.19
Sun W, Arculus RJ, Kamenetsky VS, Binns RA (2004) Release of gold-bearing fluids in convergent margin magmas prompted by magnetite crystallization. Nature 431:975–977. https://doi.org/10.1002/abio.370040210
Sun WD, Binns RA, Fan AC, Kamenetsky VS, Wysoczanski R, Wei GJ, Hu YH, Arculus RJ (2007) Chlorine in submarine volcanic glasses from the eastern manus basin. Geochim Cosmochim Acta 71:1542–1552. https://doi.org/10.1016/j.gca.2006.12.003
Tang JX, Li FJ, Li ZJ, Zhang L, Tang XQ, Deng Q, Lang XH, Huang Y, Yao XF, Wang Y (2010) Time Limit for Formation of Main Geological Bodies in Xiongcun Copper Gold Deposit, Xietongmen County, Tibet: Evidence from Zircon U⁃Pb Ages and Re⁃Os Age of Molybdenite. Miner Depos 29(3):461–475 (in Chinese with English abstract)
Tang M, Erdman M, Eldridge G, Lee CTA (2018) The redox “filter” beneath magmatic orogens and the formation of continental crust. Sci Adv 4(5):eaar444. https://doi.org/10.1126/sciadv.aar4444
Thakurta J, Ripley EM, Li C (2008) Geochemical constraints on the origin of sulfide mineralization in the Duke Island Complex, southeastern Alaska. Geochem Geophys Geosyst 9:Q07003. https://doi.org/10.1029/2008GC001982
Trail D, Bruce Watson E, Tailby ND (2012) Ce and Eu anomalies in zircon as proxies for the oxidation state of magmas. Geochim Cosmochim Acta 97:70–87. https://doi.org/10.1016/j.gca.2012.08.032
Wallace PJ, Edmonds M (2011) The sulfur budget in magmas: Evidence from melt inclusions, submarine glasses, and volcanic gas emissions. Rev Mineral Geochem 73:215–246. https://doi.org/10.2138/rmg.2011.73.8
Wang R, Richards JP, Hou ZQ, Yang ZM, Gou ZB, DuFrane SA (2014) Increasing magmatic oxidation state from paleocene to miocene in the eastern gangdese belt, tibet: Implication for collision-related porphyry Cu-Mo ±Au mineralization. Econ Geol 109:1943–1965. https://doi.org/10.2113/econgeo.109.7.1943
Wang X, Zhang JF, Rushmer T, Adam J, Turner S, Xu WC (2019) Adakite-like potassic magmatism and crust-mantle interaction in a post-collisional setting: an experimental study of melting beneath the Tibetan Plateau. J Geophys Res Solid Earth 124:12782–12798. https://doi.org/10.1029/2019JB018392
Wei YQ (2014) The geochronology, geochemistry and petrogenesis of the volcanic rocks of Yeba Formation, southern Tibet. Master's Thesis. China University of Geosciences, Beijing (in Chinese with English abstract)
Wendlandt RF (1982) Sulfide saturation of basalt and andesite melts at high pressures and temperatures. Am Mineral 67(9–10):877–885
Wilkinson JJ (2013) Triggers for the formation of porphyry ore deposits in magmatic arcs. Nat Geosci 6:917–925. https://doi.org/10.1038/ngeo1940
Wu S (2016) The super-large Zhunuo porphyry Cu deposit in the Gangdese belt, southern Tibet: magmatism and mineralization. Ph.D. China University of Geosciences (Beijing) (in Chinese with English abstract)
Xie F, Tang J, Chen Y, Lang X (2018) Apatite and zircon geochemistry of Jurassic porphyries in the Xiongcun district, southern Gangdese porphyry copper belt: Implications for petrogenesis and mineralization. Ore Geol Rev 96:98–114. https://doi.org/10.1016/j.oregeorev.2018.04.013
Xie FW (2019) The Jurassic magmatism and its mineralization potentiality in the southern Lhasa subterrane. Ph.D. Chinese Academy of Geological Sciences (in Chinese with English abstract)
Yang ZM, Hou Z, Xu J, Bian X, Wang G, Yang ZS, Tian S, Liu Y, Wang Z (2014) Geology and origin of the post-collisional Narigongma porphyry Cu-Mo deposit, southern Qinghai, Tibet. Gondwana Res 26:536–556. https://doi.org/10.1016/j.gr.2013.07.012
Yang ZM, Hou ZQ, Chang ZS, Li QY, Liu YF, Qu HC, Sun MY, Xu B (2015) Cospatial Eocene and Miocene granitoids from the Jiru Cu deposit in Tibet: Petrogenesis and implications for the formation of collisional and postcollisional porphyry Cu systems in continental collision zones. Lithos 245:243–257. https://doi.org/10.1016/j.lithos.2015.04.002
Yin A, Harrison TM (2000) Geologic evolution of the Himalayan-Tibetan orogen. Annu Rev Earth Planet Sci 28:211–280. https://doi.org/10.1146/annurev.earth.28.1.211
Yuan C, Zhou MF, Sun M, Zhao Y, Wilde S, Long X, Yan D (2010) Triassic granitoids in the eastern Songpan Ganzi Fold Belt, SW China: Magmatic response to geodynamics of the deep lithosphere. Earth Planet Sci Lett 290(3–4):481–191. https://doi.org/10.1016/j.epsl.2010.01.005
Zajacz Z, Tsay A (2019) An accurate model to predict sulfur concentration at anhydrite saturation in silicate melts. Geochim Cosmochim Acta 261:288–304. https://doi.org/10.1016/j.gca.2019.07.007
Zelenski M, Kamenetsky VS, Mavrogenes JA, Danyushevsky LV, Matveev D, Gurenko AA (2017) Platinum-group elements and gold in sulfide melts from modern arc basalt (Tolbachik volcano, Kamchatka). Lithos 290-291:172–188. https://doi.org/10.1016/j.lithos.2017.08.012
Zhang JB, Chang J, Wang R, Audétat A (2022a) Can Post-Subduction Porphyry Cu Magmas Form by Partial Melting of Typical Lower Crustal Amphibole-Rich Cumulates? Petrographic and Experimental Constraints from Samples of the Kohistan and Gangdese Arc Roots. J Petrol 63:1–22. https://doi.org/10.1093/petrology/egac101
Zhang JB, Wang R, Hong J (2022b) Amphibole fractionation and its potential redox effect on arc crust: Evidence from the Kohistan arc cumulates. Am Mineral 107(9):1779–1788. https://doi.org/10.2138/am-2022-8141
Zhang M, Li Y (2021) Breaking of Henry’s law for sulfide liquid–basaltic melt partitioning of Pt and Pd. Nat Commun 12:5994. https://doi.org/10.1038/s41467-021-26311-x
Zhang ZM, Ding HX, Dong X, Tian ZL, Palin RM, Santosh M, Chen YF, Jiang YY, Qin AK, Kang DY, Li WT (2021) The Mesozoic magmatic, metamorphic, and tectonic evolution of the eastern Gangdese magmatic arc, southern Tibet. GSA Bull 134(7-8):1721–1740. https://doi.org/10.1130/B36134.1
Zhang ZM, Dong X, Geng GS, Wang W, Yu F, Liu F (2010) Precambrian Metamorphism of the Northern Lhasa Terrane, South Tibet and Its Tectonic Implications. Acta Geo Sin 84(4):9–456 (in Chinese with English abstract)
Zhao SY, Yang AY, Langmuir CH, Zhao TP (2022) Oxidized primary arc magmas: Constraints from Cu/Zr systematics in global arc volcanics. Sci Adv 8:eabk0718. https://doi.org/10.1126/sciadv.abk0718
Zheng YY, Wu S, Ci Q, Chen X, Gao SB, Liu XF, Jiang XW, Zheng SL, Li M, Jiang XJ (2021) Cu-Mo-Au Metallogenesis and Minerogenetic Series during Superimposed orogenesis process in Gangdese. Earth Sci 46(6):1909–1940. https://doi.org/10.3799/dqkx.2020.392 (in Chinese with English abstract)
Zhu DC, Wang Q, Zhao ZD, Chung SL, Cawood PA, Niu Y, Liu SA, Wu FY, Mo XX (2015) Magmatic record of India-Asia collision. Sci Rep 5:14289. https://doi.org/10.1038/srep14289
Zhu DC, Zhao ZD, Niu Y, Dilek Y, Hou ZQ, Mo XX (2013) The origin and pre-Cenozoic evolution of the Tibetan Plateau. Gondwana Res 23(4):1429–1454. https://doi.org/10.1016/j.gr.2012.02.002
Zhu DC, Zhao ZD, Niu Y, Mo XX, Chung SL, Hou ZQ, Wang LQ, Wu FY (2011) The Lhasa Terrane: Record of a microcontinent and its histories of drift and growth. Earth Planet Sci Lett 301(1-2):241–255. https://doi.org/10.1016/j.epsl.2010.11.005
Zhu JJ, Hu RZ, Bi XW, Hollings P, Zhong H, Gao JF, Pan LC, Huang ML, Wang DZ (2022) Porphyry Cu fertility of eastern Paleo-Tethyan arc magmas: Evidence from zircon and apatite compositions. Lithos 424-425:106775. https://doi.org/10.1016/j.lithos.2022.106775
Zou YQ, Huang WT, Liang HY, Wu J, Lin SP, Wang XZ (2015) Identification of porphyry genetically associated with mineralization and its zircon U-Pb and biotite Ar-Ar age of the Xiongcun Cu-Au deposit, southern Gangdese, Tibet. Acta Petr Sin 31(7):53–2062 (in Chinese with English abstract)
Acknowledgments
We thank Dapeng Wang and Yifan Yin for their help in the platinum-group elements analysis, and thank Yanwen Tang, and Junjie Han for their help in the zircon chemistry analysis. The helpful and constructive reviews by Zoltan Zajacz and an anonymous reviewer on this version are greatly appreciated and significantlyimproved the quality of the paper. Editor Eduardo Mansur is thanked for handling this paper.
Funding
This study was financially supported by the National Natural Science Foundation of China (42122024, 42121003), the Second Tibetan Plateau Scientific Expedition and Research (STEP) (2019QZKK0806-02), CAS “Light of West China” Program (xbzg-zdsys-202310), Guizhou Provincial High level Innovation Talent program (GCC[2023]057) and Guizhou Provincial 2021 Science and Technology Subsidies (No. GZ2021SIG).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Editorial handling: E. Mansur
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
Supplementary Figure 1: Plots of (a) Pt, (b) Pd, (c) Ir, (d) Ru, (e) Cu, and (f) Au versus MgO contents for the Milin mafic–ultramafic cumulates. Supplementary Figure 2: Plots of (a) Pt, (b) Pd, (c) Rh, (d) PGEs, (e) Cu, and (f) Au versus S contents for the Milin mafic–ultramafic cumulates. (PDF 418 kb)
ESM 2
Supplementary Table 1: LA-ICP-MS zircon U–Pb dating data for the Milin mafic–ultramafic cumulates (XLSX 23 kb)
ESM 3
Supplementary Table 2: Zircon Hf isotope results for the Milin mafic–ultramafic cumulates (XLSX 18 kb)
ESM 4
Supplementary Table 3: Zircon trace element compositions for the Milin mafic–ultramafic cumulates (XLSX 30 kb)
ESM 5
Supplementary Table 4: Whole-rock major (wt%) and trace element (ppm) compositions of the Milin mafic–ultramafic cumulates (XLSX 18 kb)
ESM 6
Supplementary Table 5: The fertile and barren porphyry composition characteristics of different ages in Gangdese belt (XLSX 12 kb)
ESM 7
Supplementary Table 6: The estimated chalcophile element contents of the primary arc magma generated by partial melting of the mantle wedge under reduced conditions (XLSX 11 kb)
ESM 8
Supplementary Table 7: The estimated contents of chalcophile elements in the melt produced by remelting of the Milin mafic–ultramafic cumulates (XLSX 11 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, JL., Bai, ZJ., Zhong, H. et al. Sulfide saturation in reduced magmas during generation of the Gangdese juvenile lower crust: Implications for porphyry Cu–Au mineralization in the Gangdese belt, Tibet. Miner Deposita (2024). https://doi.org/10.1007/s00126-024-01269-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00126-024-01269-0