Abstract
In this research, we explored the thermophysical characteristics of 3-ethyl-3-pentanol in combination with a selection of short-chain alcohols, specifically from C3 to C6 (1-propanol to 1-hexanol), over a range of temperatures from 293.15 K to 323.15 K. The exploration primarily focuses on examining both the excess molar volumes and viscosity deviations, revealing a consistent increase in negative excess molar volumes with the elongation of the alkyl chain. At the same time, an examination of viscosity presents deviations from expected ideals, displaying a positive pattern that lessens as the alkyl chain lengthens. This points to notable molecular interactions taking place among 3-ethyl-3-pentanol and the alcohols under study. Moreover, the study employed Free Volume Theory (FVT) to establish connections between the viscosities of both the individual substances and their combined mixtures. Notably, FVT closely matches our experimental data, with the largest difference seen being a 1.94% deviation in the mixture of 3-ethyl-3-pentanol and 1-pentanol. These results highlight the accuracy and relevance of FVT in providing insight into the viscosity of these mixtures, thereby deepening our understanding of their complex molecular dynamics.
Similar content being viewed by others
1 Introduction
In the realm of chemical research, understanding the behavior of alcohol mixtures is crucial for both theoretical and practical applications. These mixtures play pivotal roles in various industries, ranging from pharmaceuticals, where they serve as solvents for the formulation of drugs, to the cosmetics sector, acting as carriers for fragrances and active ingredients. In the energy sector, alcohol mixtures are fundamental in creating biofuels, enhancing combustion efficiency and reducing greenhouse gas emissions. Furthermore, in the field of material science, they are utilized in the production of polymers and resins, offering versatility and improved performance characteristics [1].
3-Ethyl-3-pentanol, with its unique properties, finds versatile applications across a multitude of industries, highlighting its significance beyond basic chemical research. In the pharmaceutical industry, it is prized for its efficacy as a solvent, aiding in the creation of complex drug formulations and enhancing the delivery of active ingredients. Its application in the production of biofuels also merits mention, as it contributes to the development of sustainable energy solutions by improving fuel efficiency and reducing emissions. This broad spectrum of applications underscores the versatility and industrial importance of 3-ethyl-3-pentanol, making it a substance of high interest for ongoing and future research endeavors [2].
Aliphatic alcohols, specifically 1-alkanols, are widely used across various industries due to their versatile chemical properties. These alcohols serve as important building blocks in the synthesis of various organic compounds, such as esters, ethers, and others, which find applications in the production of plastics, fragrances, and pharmaceuticals [3,4,5,6,7].
Prior research has highlighted a notable gap in the scientific literature, specifically the absence of data on the density and viscosity of binary mixtures composed of 3-ethyl-3-pentanol and 1-alkanols. In an effort to partially bridge this knowledge gap, this study investigates the density and viscosity of such mixtures. By analyzing these mixtures, the research aims to contribute insights into their thermophysical properties, thereby enriching the existing body of knowledge. This investigation not only provides a foundational understanding of the interactions within these mixtures but also offers potential implications for their application across various industrial sectors.
Subsequently, our research incorporates the application of Free Volume Theory (FVT) to elucidate the viscosity behaviors of 3-ethyl-3-pentanol and the 1-alkanol series, thus confirming the effectiveness of this method in linking fluid properties. Significantly, the viscosity measurements obtained through our study exhibit a strong concordance with the predictions made by the FVT, underscoring the reliability of our investigative approach.
2 Experimental Section
The chemicals used in the study, including 3-ethyl-3-pentanol (3E3P), 1-propanol (C3OH), 1-butanol (C4OH), 1-pentanol (C5OH), and 1-hexanol (C6OH), were obtained from suppliers such as Merck and Aldrich chemical. These chemicals were used as received without any further purification. The mass fraction purity of 3-ethyl-3-pentanol was 99%, and for 1-alkanols 99.5%, as shown in Table 1. The experimental density and viscosity data for pure 3-ethyl-3-pentanol were compared with previously published data [8,9,10] for validation. Table 2 presents this comparison. For the purpose of this research, previously published density and viscosity values of the pure 1-alkanol compounds were used [7], and the focus was on the analysis of 3-ethyl-3-pentanol and its mixtures with the selected 1-alkanols.
The density and viscosity measurements were performed using an Anton Paar SVM 3000 Stabinger viscometer, which employs a modified Couette principle. This apparatus consists of a rapidly rotating outer cylinder and a slower-moving inner cylinder. The SVM 3000 Stabinger viscometer is equipped with a thermoelectric thermometer to maintain an accurate temperature, as temperature variations can impact density and viscosity measurements. Prior to each measurement series, the instrument was calibrated using degassed and distilled water, as well as dry, moderate-pressure air. The chemicals utilized in the study were degassed, and the samples were stored in light-blocking containers to mitigate the influence of external factors. Ten distinct mixture formulations were prepared and analyzed for their properties. A high-precision Mettler AE 163 analytical balance from Switzerland, with an uncertainty of ± 0.14 mg, was employed for weighing the samples. Each sample was subjected to multiple measurement cycles, with three to five measurements taken in total. The mole fraction was determined with an uncertainty of 0.001, density measurements had a standard uncertainty of 0.002 g·cm−3, and viscosity measurements had a relative uncertainty of 0.1, ensuring the reliability of the collected data.
3 Results and Discussion
3.1 Volumetric behavior
Table 3 shows the recorded measurements of density and viscosity for mixtures containing 3-ethyl-3-pentanol and various alcohols, from 1-propanol to 1-hexanol, across temperatures ranging from 293.15 K to 323.15 K. These measurements enable the computation of excess molar volume of these solutions.
In this study, the volume taken up by individual pure substances was quantified as \(\mathop V\nolimits_{i}^{ * }\). Within the mixture, the overall volume and the mole fraction are denoted as V and xi, respectively. Data on excess molar volumes are available in Table S1, presented in SI units. To analyze the non-ideal characteristics of the mixture, the Redlich–Kister equation was employed [11]
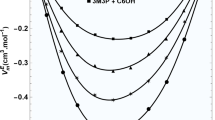
In this investigation, adjustable coefficients were utilized to correlate the experimental findings with the theoretical equation, details of which are available in Table S2 within the supplementary information document. The combination of 3-ethyl-3-pentanol with 1-alkanol led to the observation of negative excess molar volumes, indicating a reduction in total volume when mixed. This phenomenon hints at strong molecular forces at play within these mixtures. Notably, as the length of the 1-alkanols' alkyl chains extends, the negativity of the excess molar volumes diminishes. This pattern suggests that the molecular interactions within the mixtures weaken as the molecules experience increased steric hindrance, influencing their spatial arrangement and the strength of their mutual forces.
3-Ethyl-3-pentanol and 1-alkanols both possess the ability to form hydrogen bonds due to their -OH groups. Mixing 3-ethyl-3-pentanol with shorter-chain 1-alkanols, such as 1-propanol, facilitates a high efficiency in hydrogen bonding between the molecules, attributed to their comparable sizes and geometries. This similarity allows them to align closely, optimizing the formation of hydrogen bonds. This tight alignment leads to a reduction in overall volume, observed as negative excess molar volumes. However, as the length of the 1-alkanols' alkyl chains increases, the molecules become larger and more cumbersome, leading to steric hindrance that disrupts efficient molecular packing. As a result, the capacity for effective hydrogen bonding diminishes, weakening the intermolecular forces and resulting in less pronounced negative excess molar volumes. The gradual increase in alkyl chain length correlates with a decrease in the strength of molecular interactions, illustrating how the compactness and interaction potential diminish with longer-chain alcohols. Figure 1 visually depicts how changes in excess molar volume correlate with both the mole fraction of 3-ethyl-3-pentanol and the chain length of the alcohols, based on measurements taken at 293.15 K, showing the impact of molecular structure on mixture volumes.
The observed behavior of increasing excess molar volume with temperature in mixtures of 3-ethyl-3-pentanol and 1-alkanols can be attributed to the interplay between the weakening of molecular interactions and the thermal expansion properties of liquids. As temperature rises, the kinetic energy of the molecules increases, reducing the effectiveness of hydrogen bonds and dipole–dipole interactions that contribute to negative excess molar volumes at lower temperatures. This weakening of interactions allows molecules to move more freely and apart from each other, leading to an overall increase in the mixture's volume.
3.2 Dynamic Viscosities
Table 3 displays viscosity data for the pure substances and their respective mixtures. To analyze how mixing these compounds affects viscosity, we applied the following formula to calculate the change in viscosity, denoted as Δη.
The above question is instrumental in quantifying how the viscosity of the mixture diverges from what would be expected under ideal conditions. Notably, all binary mixtures exhibited positive deviations in viscosity, a finding detailed in Table S1 within the SI file.
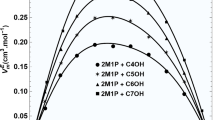
The occurrence of positive deviations in viscosity suggests that the intermolecular forces intensify when these alcohols blend, exceeding the forces observed in the individual substances. This enhancement is largely due to hydrogen bonding among the hydroxyl groups of 3-ethyl-3-pentanol and the 1-alkanols. In these mixtures, the presence of hydroxyl groups serves as focal points for hydrogen bonding, culminating in a viscosity increase. This increase in viscosity stems from the more structured and orderly arrangement of molecules and the heightened resistance to flow, a direct consequence of the strengthened intermolecular forces.
As the alkyl chain length of the 1-alkanols extends, there is a decrease in the viscosity deviations observed in the mixtures. This phenomenon can be understood by examining the impact of extended alkyl chains on the mixture's molecular dynamics. The presence of longer alkyl chains brings about steric hindrance, which acts as a physical barrier to the close packing of molecules, thereby hampering the formation of efficient hydrogen bonds. Steric hindrance essentially refers to the physical limitations imposed on molecular proximity due to the increased bulk of the chains, which weakens intermolecular forces as the chains become longer. As a result, although hydrogen bonding continues to play a role in determining the viscosity of the mixture, its effect becomes less pronounced with the elongation of the alkyl chain, leading to a reduced positive deviation in viscosity.
The noted reduction in viscosity deviation as temperature rises in mixtures of 3-ethyl-3-pentanol and 1-alkanols is due to the dynamic relationship between molecular movement and the diminishing strength of certain intermolecular forces, including hydrogen bonding. As temperature rises, the kinetic energy of the molecules within these mixtures increases, facilitating their ability to overcome the attractive forces holding them together. This increased molecular motion disrupts the structured arrangements formed by hydrogen bonding, which is more pronounced at lower temperatures, leading to a reduction in viscosity deviation. Figure 2 visually presents how the viscosity deviations change with the mixture composition and the length of the alkyl chain in 3-ethyl-3-pentanol and 1-alkanol mixtures at 293.15 K.
4 Free Volume Theory
A wide array of viscosity models has been developed by researchers, yet attaining exact forecasts is still a considerable challenge. While empirical models offer user convenience and fairly accurate predictions of viscosity, theoretically based models excel in predicting the viscosity of dilute gases but often fall short when applied to dense fluids. Semi-empirical models, blending in molecular characteristics of fluids, are increasingly preferred for their enhanced accuracy in viscosity estimation and their extended applicability across different fluid types. The Free Volume Theory (FVT) has established itself as an effective approach for predicting the viscosity of both gases and liquids. At its core, FVT relies on the concept of the free space that a molecule can move into when subjected to shear stress. The seminal contributions to FVT were made by Allal et al. [12,13,14], who formulated a model that describes the viscosity of dense fluids in relation to temperature and pressure.
The essential principle behind FVT is the necessity of "free" volume (Vf) for the rearrangement of molecules within a liquid. This Vf diminishes as the liquid is cooled and its volume contracts, making its precise definition difficult. Doolittle [10] addressed this by viewing Vf as the volume arising from the liquid's total thermal expansion without undergoing a phase transition. He quantified Vf by deducting the molecular volume, which is determined by extrapolating the liquid's volume to absolute zero temperature, suggesting that Vf only approaches zero as the temperature nears absolute zero. FVT emerged from integrating Doolittle's free-volume model, which includes a formula for the free-volume fraction as developed by Allal et al., with the correlation between viscosity and microstructure. This correlation is identified as the multiplication of the fluid's shear modulus by the average relaxation time of a molecule [15]. In the FVT framework, viscosities are divided into the dilute gas viscosity η0 and the residual viscosity ηf using the following equation
η0 can be calculated from the following equation
In the given equation, MW represents the molecular weight, while νc is the critical volume, and Ω* is the reduced collision integral. To account for the effects of hydrogen bonding, the acentric factor ω, and the dipole moment μr on the dilute gas viscosity η0, researchers have introduced a hydrogen bonding correction factor, denoted as χ. By incorporating these parameters, the equation can more accurately estimate η0 by considering the specific molecular properties and interactions that influence viscosity [12,13,14].
The residual viscosity ηf is a component of liquid viscosity that is dependent on pressure and plays a crucial role in determining the overall viscosity of a liquid. The exact definition of residual viscosity can vary across different viscosity models. Nonetheless, it is commonly associated with the free volume (fV) via a specific relationship that connects these two properties.
The formula serves to encapsulate the complex relationship between molecular interactions and the available free volume, which in turn significantly impacts the residual viscosity. This mathematical relationship is articulated as follows in reference [12].
Within this framework, the density (ρ) is quantified in moles per liter (mol·L−1), reflecting the concentration of molecules in the liquid. The friction coefficient (ζ0), which plays a role in the diffusion process and molecular mobility, is measured in kilograms per second (kg·s−1). Furthermore, the average quadratic length (L2), which provides insight into the molecular size, is expressed in square angstroms (Å2). The parameter B is linked to the extent of free-volume overlap among molecules, indicating how molecular free volumes interconnect and affect the system's overall behavior. The friction coefficient (ζ0) can be calculated using the following equation:
In the above equation, bf represents a specified length associated with energy dissipation, measured in angstroms (Å), while E stands for the energy involved in the dissipation process. The formulation of this equation is crucial for elucidating the connection between the friction coefficient and the energy dissipation process, shedding light on how energy is absorbed and dispersed as molecules move and interact within the system.
Within this framework, the energy term E is conceptualized as comprising two distinct components: one that reflects the characteristics of an ideal gas, and another that is influenced by the system's density. The first component is linked to a variable (α), which is employed to characterize the connection between the system's density and this specific component of energy. This bifurcation in the energy term facilitates a more streamlined analysis, allowing for a clearer understanding of the system's behavior by integrating considerations of both ideal gas properties and factors that are dependent on density. This approach aids in dissecting the complex interactions and phenomena observed within the system, as outlined in references [12, 13].
Through the integration of the previously mentioned equations, a detailed expression for the residual component can be formulated. This ultimate equation encapsulates the critical elements of the system's dynamics, incorporating the diverse factors and components that have been examined earlier. It provides a holistic view of how these elements interact to influence the system's residual behavior, offering a deeper insight into the underlying mechanisms governing the system's properties and responses.
With
Equation 10 includes three tunable parameters, each reflecting a specific aspect of the system's behavior and characteristics. The length parameter Lv is linked to the molecular structure and the characteristic relaxation time, shedding light on the molecules' spatial arrangement and their relaxation dynamics. The α parameter elucidates the connection between the energy barrier and the system's density, measuring the variation of the energy barrier with changes in density. The B parameter is related to the extent of free-volume overlap among molecules, demonstrating how individual molecules' free volumes interconnect. By carefully adjusting these parameters through an objective function, it becomes possible to accurately simulate and forecast the viscosity of the pure system across various conditions and arrangements, as discussed in reference [15].
Employing Free Volume Theory (FVT) to calculate the viscosity of binary mixtures involves implementing specific mixing guidelines that integrate the characteristics of the individual substances with interaction coefficients. These guidelines are crucial for accurately predicting the viscosity of a mixture, as they consider the roles and interactions of each constituent.
This study utilized three distinct parameters to accurately replicate the viscosity measurements of 1-alkanols and 3-methyl-3-pentanol obtained through experimentation. By employing an optimization process, these parameters were finely adjusted to suit the specific compound under examination, based on the viscosity information collected from tests on the pure substances. The values for these parameters, fine-tuned to align with the Free Volume Theory (FVT) framework, were determined and are detailed in Table 4. The accuracy of the model in forecasting the viscosity of pure liquids is notably high, with discrepancies kept under 1.67%.
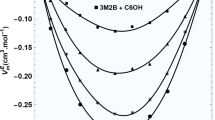
After determining the essential parameters for each pure substance, the study proceeded to evaluate the viscosity of binary mixtures using established mixing rules. The mixtures, which combined 3-ethyl-3-pentanol with 1-propanol, 1-butanol, 1-pentanol, and 1-hexanol, demonstrated impressively low average absolute deviations (AAD) of 1.79%, 1.86%, 1.94%, and 1.37%, respectively. These AAD figures represent the average percentage difference between the viscosities measured experimentally and those estimated by the FVT model, underlining the model's precision in calculating the viscosity of these binary mixtures. Figure 3 provides a graphical representation of the slight variances between the experimentally observed viscosities and the predictions made by the model for mixtures of 3-ethyl-3-pentanol and 1-alkanol at 293.15 K. This visualization clearly demonstrates the model's accuracy and its capability to closely predict the viscosities of binary mixtures, thereby confirming its applicability for assessing viscosity behaviors in such mixtures.
5 Conclusion
This study explores the examination of both thermodynamic and transport characteristics of binary mixtures formed by combining 3-ethyl-3-pentanol with a range of 1-alkanols, from 1-propanol to 1-hexanol. The observation of negative excess molar volumes alongside positive deviations in viscosity highlights the occurrence of enhanced molecular interactions within these mixtures, exceeding those observed in their singular forms. These interactions are largely attributed to the formation of hydrogen bonds among the hydroxyl groups of 3-ethyl-3-pentanol and the 1-alkanols. Nevertheless, the introduction of alkyl chains brings about steric hindrance, which limits the molecules' ability to come into close proximity and organize effectively. The study underscores the influence of steric hindrance, a consequence of the lengthening alkyl chains in alcohols, on reducing the efficiency of molecular arrangements and weakening the intermolecular forces. By applying the Free Volume Theory (FVT) model, a precise approximation of the viscosity measurements for these binary mixtures was achieved, showing a maximum deviation of 1.94% from the observed data.
Data Availability
All data generated or analyzed during this study are included in this article and its Supplementary Information files.
References
M. Almasi, exploring thermophysical properties of butyl lactate with short-chain 1-alkanols using experimental and theoretical perspectives. Int. J. Thermophys. 45, 16 (2024)
D. González-Salgado, J. Troncoso, F. Plantier, J.L. Daridon, D. Bessieres, Study of the volumetric properties of weakly associated alcohols by means of high-pressure speed of sound measurements. J. Chem. Thermodyn. 38, 893–899 (2006)
M. Almasi, M. Mohebbifar, Modeling and measurement of density and viscosity of ethyl Lactate + 1-alkanol mixtures. J. Mol. Liq. 400, 124459 (2024)
M. Almasi, Thermodynamic insights into 3-Methyl-2-Butanol and C3–C6 1-alkanol: Experimental study and CPA modeling. Int. J. Thermophys. 45, 40 (2024)
M. Almasi, Analyzing intermolecular interactions in 2-Methyl-1-pentanol and C4–C7 1-alkanol mixtures: thermodynamic and transport investigations. Int. J. Thermophys. 45, 54 (2024)
M. Almasi, Thermodynamic study of interactions between 1-alkanol and butanone. Chem. Phys. 527, 110474 (2019)
M. Almasi, R. Hernandez, Theoretical and experimental study of triethanolamine and 1-alkanol mixtures. Fluid Phase Equilib. 571, 113810 (2023)
M. Zabransky, V. Ruzicka, V. Mayer, E.S. Domalski, J. Phys. Chem. Ref. Data Monograph 6 (1996).
E.C. Bingham, L.W. Spooner, The fluidity method for the determination of association. J. Rheol. 3, 221–224 (1932)
G. Edgar, G. Calingaert, R.E. Marker, The preparation and properties of the isomeric heptanespart. i. Preparation. J. Am. Chem. Soc. 51, 1483–1491 (1929)
O.J. Redlich, A.T. Kister, Thermodynamics of nonelectrolyte solutions: algebraic representation of thermodynamic properties and the classification of solutions. Ind. Eng. Chem. 40, 345–348 (1948)
A.K. Doolittle, Studies in Newtonian flow. II. The dependence of the viscosity of liquids on free space. J. Appl. Phys. 22, 1471–1475 (1951)
A. Allal, M. Moha-Ouchane, C. Boned, A new free volume model for dynamic viscosity and density of dense fluids versus pressure and temperature. Phys. Chem. Liquids 39, 1–30 (2001)
A. Allal, C. Boned, A. Baylaucq, Free-volume viscosity model for fluids in the dense and gaseous states. Phys. Rev. E 64, 011203 (2001)
N. Gao, Y. Yang, Z. Wang, X. Guo, S. Jiang, J. Li, Y. Hu, Z. Liu, C. Xu, Viscosity of ionic liquids: Theories and models. Chem. Rev. 124, 27–123 (2024)
Acknowledgements
M. Almasi acknowledges the economic support given by the Malayer University.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All parts by the corresponding author.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Almasi, M. Investigating the Density and Viscosity of 3-Ethyl-3-Pentanol and Short-Chain 1-Alkanol: A Free Volume Theory Approach. Int J Thermophys 45, 74 (2024). https://doi.org/10.1007/s10765-024-03369-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-024-03369-5