Published online Dec 7, 2012. doi: 10.3748/wjg.v18.i45.6614
Revised: June 19, 2012
Accepted: August 4, 2012
Published online: December 7, 2012
AIM: To compare the site, age and gender of cases of colorectal cancer (CRC) and polyps in a single referral center in Rome, Italy, during two periods.
METHODS: CRC data were collected from surgery/pathology registers, and polyp data from colonoscopy reports. Patients who met the criteria for familial adenomatous polyposis, hereditary non-polyposis colorectal cancer syndrome or inflammatory bowel disease were excluded from the study. Overlap of patients between the two groups (cancers and polyps) was carefully avoided. The χ2 statistical test and a regression analysis were performed.
RESULTS: Data from a total of 768 patients (352 and 416 patients, respectively, in periods A and B) who underwent surgery for cancer were collected. During the same time periods, a total of 1693 polyps were analyzed from 978 patients with complete colonoscopies (428 polyps from 273 patients during period A and 1265 polyps from 705 patients during period B). A proximal shift in cancer occurred during the latter years for both sexes, but particularly in males. Proximal cancer increased > 3-fold in period B compared to period A in males [odds ratio (OR) 3.31, 95%CI: 2.00-5.47; P < 0.0001). A similar proximal shift was observed for polyps, particularly in males (OR 1.87, 95%CI: 1.23-2.87; P < 0.0038), but also in females (OR 1.62, 95%CI: 0.96-2.73; P < 0.07).
CONCLUSION: The prevalence of proximal proliferative colonic lesions seems to have increased over the last decade, particularly in males.
- Citation: Corleto VD, Pagnini C, Cattaruzza MS, Zykaj E, Di Giulio E, Margagnoni G, Pilozzi E, D’Ambra G, Lamazza A, Fiori E, Ferri M, Masoni L, Ziparo V, Annibale B, Delle Fave G. Is proliferative colonic disease presentation changing? World J Gastroenterol 2012; 18(45): 6614-6619
- URL: https://www.wjgnet.com/1007-9327/full/v18/i45/6614.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i45.6614
Colorectal cancer (CRC) is one of the most common malignancies worldwide, the etiology of CRC involves environmental and genetic factors. In most cases, the cancer develops according to the classic adenoma-carcinoma sequence[1], as supported by epidemiological, clinical-pathological and molecular genetic studies. Thus, early detection and removal of adenomatous polyps are essential for cancer prevention. In fact, the risk of developing CRC within six years of a polypectomy is reduced by 75%-90%. However, not all CRC cases are preceded by adenomatous polyps, and some cancers have been shown to develop directly from aberrant crypts or flat lesions[2]. These alternative carcinogenetic pathways are relatively more frequent on the right side of the colon, making the efficacy of preventative strategies very challenging. In this regard, one of the topics of particular interest in CRC is the possible change in site distribution observed in recent decades. In fact, recent data from different studies report a change in the site distribution of CRC (proximal shift) related to gender, race[3,4] and older age[5]. Many factors are potentially involved in this phenomenon, and most of them are not easily evaluated. However, the crucial issue is to establish if and how much of these changes are due to a real biological event or related to multiple diagnostic biases. Indeed, the question is not theoretical, but rather implies important decisions related to strategies for screening, surveying and treating millions of people worldwide, with health and economic implications. In fact, proximal colon cancer represents a great challenge for physicians, both due to the technical limitations of screening strategies in the detection of right-sided colon lesions and to the peculiar behavior of these tumors[6]. Data on CRC location have been reported from different sources, such as cancer registries, colonoscopy reports, retrospective clinical analyses or autoptic data[5,7-9]. All of these sources have biases that could potentially under- or over-estimate the specific issue of the cancer location. However, data concerning a possible increase over time of right-sided colon cancers have been reported recently in large population studies[10,11]. The possible changes in the location of polyps over time have been less investigated, but a possible proximal shift in these lesions has been described by some studies[12-14]. Few studies have addressed the possible “right shift” of CRC in the Italian population[15,16], and only two studies have analyzed the changing distribution of both CRC and polyps over tim[17,18], thus data are scarce and not conclusive.
The present study aims to address this issue retrospectively by analyzing records from a large set of patients, either operated on for CRC or diagnosed with colon polyps by colonoscopy, during two distinct periods of time at an Italian single referral center.
We performed a retrospective, observational study of CRC and polyps at a single referral center (“Sapienza” University Hospital - Rome, Italy) for two periods of time: from 1989 to 1993 (period A) and from 2003 to 2007 (period B). The aim of the study was to compare the location of CRC and polyps and to study the differences in the age and gender distributions between the two periods.
The age and gender of the patients and the location, histology, morphology and dimensions of their lesions were recorded. For discrimination between the proximal and distal colon, the boundary was situated at the juncture of the splenic flexure, as was performed in previous studies[16,19].
Overlap of patients in the two groups (CRC and polyps) was carefully avoided. The study was approved by the institutional University review board; because this study was a retrospective analysis of an existing data set, written informed consent was not obtained from the participating subjects.
During the two periods, endoscopic examinations were performed using Olympus videocolonscopes (CF100I in period A, CFQ145I in period B).
CRC data were obtained from surgery registries, and the diagnoses were all confirmed by histological examination of surgical resections. Overall, 768 consecutive patients diagnosed with cancer who underwent surgery were analyzed. Of these, 352 were operated on from 1989-1993 (period A) and 416 from 2003-2007 (period B).
Polyp data were obtained from colonoscopies. Only complete colonoscopy examinations with adequate bowel preparation were considered. Subjects with uncompleted examinations or unsatisfactory cleansing were excluded, unless a second complete colonoscopy was performed within three months. Only patients with sporadic polyps were included, and patients who met the criteria for familial adenomatous polyposis, hereditary non-polyposis colorectal cancer syndrome or other polyposis syndromes, or who had been diagnosed with or suspected to have inflammatory bowel disease (ulcerative colitis or Crohn’s disease), were excluded from the study.
Four senior gastroenterologists, each with more than 10 years of endoscopic experience, performed 4176 colonoscopies (1030 and 3146 for periods A and B, respectively). Polyps were detected in 27% and 23% of colonoscopies in periods A and B, respectively.
A total of 978 patients were analyzed, and 1693 polyps were found.
The data obtained from each polyp were included in the descriptive analysis. For patients with more than one polyp, the most advanced lesion, either in the proximal or in the distal segment of the colon, was taken into consideration in the multivariate analysis.
Proportions were calculated for the categorical data, and means and standard deviations were calculated for the quantitative data. χ2 and t tests were used to assess the differences between periods A and B. Multivariate logistic regression was used to estimate the relative risk of finding a proximal CRC and polyp, adjusting for age, sex and the diagnosis period (A vs B) as independent variables. The limit of statistical significance for all tests was set at 0.05.
As shown in Table 1, a higher percentage of cancers was recorded in men than in women, and there was no statistically significant difference between the periods. Patients were older in period B than in period A. In particular, there were fewer patients with CRCs in period B than in period A in all age groups less than 70 years.
| Period A (1989-1993) | Period B (2003-2007) | P value | |
| 352 patients | 416 patients | ||
| Male | 202 (57.4) | 253 (60.8) | 0.335 |
| Female | 150 (42.6) | 163 (39.2) | |
| Age (yr) | |||
| < 50 | 49 (13.9) | 27 (6.5) | < 0.0001 |
| 50-59 | 70 (19.9) | 55 (13.2) | |
| 60-69 | 121 (34.4) | 95 (22.8) | |
| 70-79 | 90 (25.6) | 180 (43.3) | |
| ≥ 80 | 22 (6.2) | 59 (14.2) |
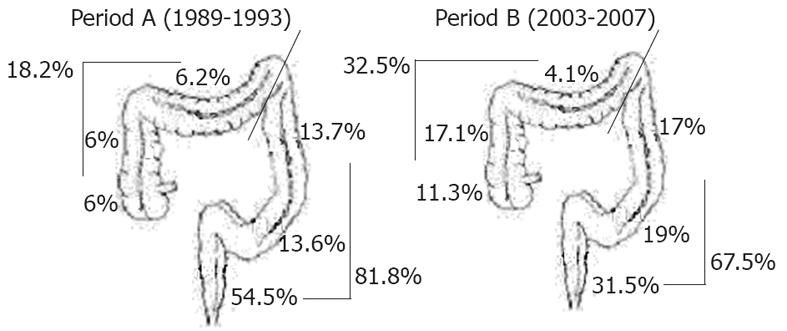
From period A to B, proximal CRC incidence increased by an absolute 14.3% (from 18.2% to 32.5%, P < 0.0001). In particular, the increase was observed in the cecum and in the ascending colon (12.0% vs 28.4% in periods A and B, respectively), whereas in distal CRC cases, a consistent reduction was noted in the rectum, with a decrease from 54.5% to 31.5% in periods A and B, respectively (P < 0.0001) (Figure 1).
In the multivariate analysis, the risk of finding a proximal CRC, after adjusting for age and sex, showed a statistically significant interaction term between period B and gender. Thus, two regression equations were run. For men, the risk of developing a proximal cancer in period B was more than 3 times greater than that in period A, adjusting for age [odds ratio (OR) 3.31, 95%CI: 2.00-5.47; P = 0.0001], whereas for females, there was an increased risk, but this increase was not statistically significant (OR 1.21, 95%CI: 0.72-2.04; P = 0.4637). There was no significant evidence of an effect of age in males (OR 1.34, 95%CI: 0.86-2.11; P = 0.1999) or in females (OR 1.23, 95%CI: 0.74-2.07; P = 0.4272).
As shown in Table 2, polyps were more frequently found in males than in females, with no statistically significant difference between the periods (P = 0.0892). We evaluated 428 polyps from 273 patients in period A and 1265 polyps from 705 patients in period B. The mean number of polyps per patient increased from 1.6 in period A to 1.8 in period B (P = 0.01).
| Period A (1989-1993)273 patients | Period B (2003-2007)705 patients | P value | |
| Male | 178 (65.2) | 418 (59.3) | 0.0892 |
| Female | 95 (34.8) | 287 (40.7) | |
| Age (yr) | |||
| < 50 | 50 (19.2) | 91 (12.9) | < 0.0015 |
| 50-59 | 44 (16.9) | 154 (21.9) | |
| 60-69 | 103 (39.5) | 226 (32.1) | |
| 70-79 | 57 (21.8) | 185 (26.3) | |
| ≥ 80 | 7 (2.7) | 48 (6.8) | |
| Total No. of polyps | 428 | 1265 | |
| No. of polyps, mean ± SD | 1.6 ± 1 | 1.8 ± 1.3 | 0.0102 |
| No. of polyps, median | 1 | 1 | |
| Range | 1-6 | 1-14 | |
| Dimensions | |||
| < 5 mm | 114 (26.6) | 530 (41.9) | < 0.00005 |
| 5-9 mm | 228 (53.3) | 454 (35.9) | |
| 10-19 mm | 44 (10.3) | 191 (15.1) | |
| 20-29 mm | 23 (5.4) | 52 (4.1) | |
| 30-39 mm | 9 (2.1) | 25 (2) | |
| 40+ mm | 10 (2.3) | 13 (1) | |
| Histopathological pattern | |||
| Hyperplastic | 91 (33.6) | 438 (38.7) | < 0.00005 |
| Mild/moderate dispalsia1 | 151 (55.7) | 597 (52.7) | |
| Severe dysplasia2 | 27 (10) | 87 (7.7) | |
| Others3 | 2 (0.7) | 10 (0.9) | |
No univocal trend in age distribution between the two periods was observed. With regard to the percentage of patients with polyps in period A to period B, a decrease was observed for age groups < 50 years and 60-69 years, whereas an increase was observed for the age groups 50-59, 70-79 and ≥ 80 years (P < 0.0015). A similar trend was observed in both males and females (data not shown).
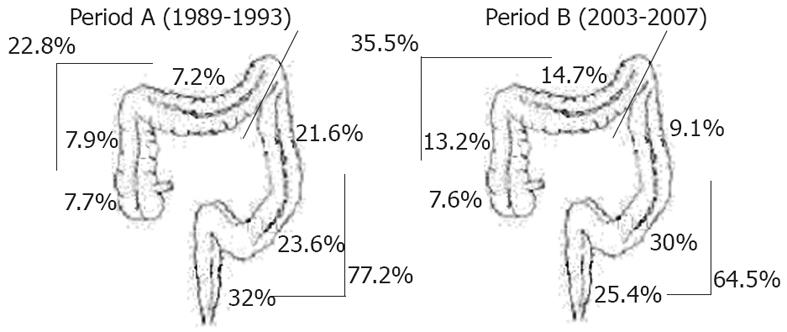
From period A to B, the incidence of proximal polyps increased by an absolute 12.7% (from 22.8% to 35.5%, P < 0.00005). In analyzing the anatomical segments separately, an increase in incidence of proximal polyps was observed in the ascending colon (from 7.9% to 13.2%) and in the transverse colon (from 7.2% to 14.7%). In the distal colon, a reduction in polyps was observed in the descending colon (from 21.6% to 9.1%) and in the rectum (from 32% to 25.4%), whereas an increase was noted in the sigmoid colon (from 23.6% to 30%) (P < 0.00005) (Figure 2).
In the multivariate logistic regression analysis, after adjusting for age, a male’s risk of developing a proximal polyp in period B was almost 90% greater than his risk in period A (OR 1.87, 95%CI: 1.23-2.87; P = 0.004), whereas for females, there was an increase of more than 60% in the risk, which was close to statistical significance (OR 1.62, 95%CI: 0.96-2.73; P = 0.07). When considering age groups stratified by greater or less than 70 years, no differences in proximal polyp detection was demonstrated for either gender.
The size and histopathological pattern of the polyps were also analyzed.
There was a statistically significant increase in the percentages of micropolyps (< 5 mm) from 26.6% to 41.9% and from 10.3% to 15.1% for polyps of 10-19 mm in size, whereas the percentage of large polyps (40 mm) diminished from 2.3% to 1.0% in period B vs A (P < 0.00005).
Histopathology data were available for 63.3% and 89.5% of polyps in period A and B, respectively. No statistically significant differences in the overall number of hyperplastic polyps and adenomas with mild/moderate and severe dysplasia were observed between periods A and B. Nonetheless, when the histopathological pattern was analyzed according to polyp location, from period A to B, adenomas with mild/moderate dysplasia in the proximal colon increased significantly from 21.8% to 41.2% (P < 0.001), whereas adenomas with severe dysplasia decreased from 37% to 23%, which was not statistically significant.
In recent decades, screening strategies for early diagnosis and/or prevention of CRC have been consistently implemented. Nonetheless, colon malignancies still remain the third most common cancer and an important cause of death in Western countries[20]. Thus, many efforts have been made in order to improve the efficacy of screening strategies, which often differ even regionally in the same country. Colonoscopy is considered the “gold standard” for the diagnosis and removal of pre-malignant colon lesions, even though a careful risk stratification strategy is required in order to optimize resources for screening purposes. In this setting, the presumed right-side increase in pre-malignant lesions and CRC may represent a further stimulus to perform high-quality endoscopic examination of the right side of the colon, which is often difficult to explore carefully (especially the cecum)[6]. Moreover, even though in the last year colonoscopies have increased in number and quality, it has been demonstrated that a relatively high proportion of cases of CRC may develop without macroscopic evidence of pre-malignant lesions, introducing further challenges to prevention strategies. Despite the consistent number of studies that analyze differences in the location of colon CRC and polyps, data are still not univocal and indeed remain difficult to interpret. As already mentioned, results are often difficult to compare due to the different sources from which the data are collected. Another important reason concerns the length of the observation, which varies from a few years to decades according to different studies.
This study retrospectively evaluated the differences in the site distribution of CRC and polyps between two 5-year periods over a period of 10 years, analyzing data from surgical registries and from endoscopic reports in a single referral center. The relatively short interval time between the two periods (10 years) could have, at least in part, influenced the observed differences, which may be more striking with a wider interval time.
Bearing in mind the aforementioned limitations for data interpretation, many recent large studies have reported a trend for “proximalization” of CRC in different geographic areas[10,15,21,22]. Conversely, other studies have questioned the possible “right shift” in CRC location[23,24] or have observed the phenomenon only in specific subgroups[3,25,26]. Moreover, some other authors have explained that the putative increase in proximal CRCs is mainly consequent to the decrease in rectal cancer cases[8,16]. In this study, we confirmed the proximal shift in CRC over time and observed a 3-fold increase in the risk of finding proximal cancer in males in period B vs period A. In line with previous findings in Italian populations[16], the single anatomical segments analysis emphasized that the relative increase in proximal CRC cases over time was partly due to a reduction in the number of rectal cancer cases (54.5% vs 31.5% in periods A and B, respectively). In fact, excluding rectal cancers, the trend for a proximal shift in CRCs over time, although maintained, showed less of a difference (data not shown). This relatively small, but homogeneous study from a single referral center, confirmed the trend of a proximal shift in CRC location during recent years, and is thus further confirmation of the phenomenon previously described in large cohorts of patients from different areas.
Published data on polyp prevalence are scarce and less consistent than are data for CRC, and the related studies mainly concern advanced adenomas[27]. However, some studies have suggested a proximal shift in those lesions over time[12-14,17,18]. In this study, we observed a proximal shift in polyps between periods A and B, albeit less consistent than that observed for CRC. As already observed for CRC, the proximalization of lesions was more evident in males (90% increase in period B vs A). The increase in total polyps, and in particular in proximal lesions, refers mainly to micropolyps and low-grade dysplastic polyps, that could be partially explained by the increase in colonoscopies for cancer prevention in period B vs period A, and to the “see and sampling” strategy that has become more popular in recent years. Notably, the present data on polyp dimensions and histopathological patterns need to be interpreted with caution, both due to the high rate of missing histological data [157 (36.7%) and 133 (10.5%) polyps with missing histological reports in periods A and B, respectively] and due to the fact that the two variables (size and histology) are not independent.
Besides possible biological explanations of increased proliferative right-sided colon lesions over time, many confounding factors related to the global technical and behavioral medical changes throughout the years could have partially contributed to this location shift. With regard to the latter, the most important consideration concerns the impact of increased sensibilization for CRC prevention in the last decade that could have potentially influenced either CRC or polyp presentation in our population during period B. In fact, the older age of CRC patients in that period could be at least partially due to the preventive effect of the screening approach, and the same “proximal shift” could be an effect of better prevention of distal lesions (which are more easily detected by screening methods such as sigmoidoscopy). Considering polyps, the modifications of colonoscopy indications, particularly due to an increased trend to cancer prevention, may have influenced the different findings in the two periods, even though only a slight decrease in the proportion of colonoscopies with polyp detection was found between the two periods (23% in period B vs 27% in period A). Regarding technical progress, the right-sided CRC increase could be a result of the recent different surgical treatment options for right-sided CRC, in particular the laparoscopic approach, that in many centers has made surgery much more possible in elderly patients compared to previous years. This detail is particularly true considering that for CRC evaluation, we included exclusively surgical registry data without considering the surgical approach. Moreover, procedural improvements (i.e., standardization of retraction time) and the amelioration of bowel cleansing could have potentially influenced the observed difference in polyp detection between the two time periods. Nonetheless, no substantial improvements in technical equipment occurred between the two periods, since high definition endoscopes were not available in both periods.
Nonetheless, even if the precise amount and specific causes of the right shift in pre-malignant and malignant colon lesions remain to be established, the present retrospective analysis appears to confirm, albeit with some limitations and possible confounding factors, a trend of an increase in such lesions over time. As a consequence, endoscopists and clinicians in daily clinical practice, as well as future strategies for screening campaigns, should take into account the possible increase in proximal colonic proliferative disorders. In this regard, the whole colon should be considered as a potential target for neoplastic changes, and partial colon examinations should be avoided or limited to particular conditions. Novel endoscopic instruments with higher resolution power could result in an improvement per se in the detection of colonic lesions. However, besides the technical devices, better bowel preparation (cecum cleaning), the constant improvement of endoscopists’ skills, and a standardized technical endoscopic approach[28] are all fundamental basic tools that can improve the endoscopic examination quality in order to obtain a more accurate observation of the whole colon.
Proximalization of colorectal cancer during the last decades has been variously reported. Data for the right shift in polyps are scant and controversial.
Data from large cohorts of patients followed for decades and relatively short studies suggest a change in colonic proliferative disease during recent years, with an increase in right-sided lesions.
The data show a proximalization of proliferative colonic lesions (cancers and polyps), in a single referral center, in two different periods of time with a ten-year interval between these periods.
These results should be interpreted with caution, due to many possible unavoidable biases that may interfere when undertaking this type of study, either for different endoscopical/surgical approaches or for biological factors. However, the authors suggest that this phenomenon that may have important implications either for improvement of endoscopic accuracy or for screening programs.
The study involves a single institution but covers 2 separate 5 year periods 10 years apart. The authors compared data regarding the anatomic location of both colorectal cancers and colonic polyps found by colonoscopy during each period. The data suggest that proximal proliferative lesions have increased in prevalence, particularly in males. That proximal colonic premalignant and malignant conditions have increased in prevalence over the last 2 decades has also been shown in other studies. In order to be sure that comparisons between 2 separate periods are valid, even within a single institution, the procedures in question should be as identical as possible, allowing for changes in technology, operator experience, etc.
Peer reviewers: John Beynon, BSc, MB BS, MS, FRCS (ENG.), Consultant Colorectal Surgeon, Singleton Hospital, Sketty Lane, Swansea SA2 8QA, United Kingdom; Roderick M Quiros, MD, FACS, Surgical Oncologist, Cancer Care Associates, 801 Ostrum Street, Bethlehem, PA 18015, United States
S- Editor Shi ZF L- Editor Webster JR E- Editor Li JY
| 1. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8087] [Cited by in F6Publishing: 7708] [Article Influence: 226.7] [Reference Citation Analysis (1)] |
| 2. | Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977-1981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3107] [Cited by in F6Publishing: 3009] [Article Influence: 97.1] [Reference Citation Analysis (1)] |
| 3. | Wu X, Cokkinides V, Chen VW, Nadel M, Ren Y, Martin J, Ellison GL. Associations of subsite-specific colorectal cancer incidence rates and stage of disease at diagnosis with county-level poverty, by race and sex. Cancer. 2006;107:1121-1127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Okamoto M, Shiratori Y, Yamaji Y, Kato J, Ikenoue T, Togo G, Yoshida H, Kawabe T, Omata M. Relationship between age and site of colorectal cancer based on colonoscopy findings. Gastrointest Endosc. 2002;55:548-551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Strul H, Kariv R, Leshno M, Halak A, Jakubowicz M, Santo M, Umansky M, Shirin H, Degani Y, Revivo M. The prevalence rate and anatomic location of colorectal adenoma and cancer detected by colonoscopy in average-risk individuals aged 40-80 years. Am J Gastroenterol. 2006;101:255-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 145] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Hewett DG, Kahi CJ, Rex DK. Does colonoscopy work? J Natl Compr Canc Netw. 2010;8:67-76; quiz 77. [PubMed] [Cited in This Article: ] |
| 7. | Anderson WF, Umar A, Brawley OW. Colorectal carcinoma in black and white race. Cancer Metastasis Rev. 2003;22:67-82. [PubMed] [Cited in This Article: ] |
| 8. | Matanoski G, Tao XG, Almon L, Adade AA, Davies-Cole JO. Demographics and tumor characteristics of colorectal cancers in the United States, 1998-2001. Cancer. 2006;107:1112-1120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Jass JR, Young PJ, Robinson EM. Predictors of presence, multiplicity, size and dysplasia of colorectal adenomas. A necropsy study in New Zealand. Gut. 1992;33:1508-1514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 106] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Singh H, Demers AA, Xue L, Turner D, Bernstein CN. Time trends in colon cancer incidence and distribution and lower gastrointestinal endoscopy utilization in Manitoba. Am J Gastroenterol. 2008;103:1249-1256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Saltzstein SL, Behling CA. Age and time as factors in the left-to-right shift of the subsite of colorectal adenocarcinoma: a study of 213,383 cases from the California Cancer Registry. J Clin Gastroenterol. 2007;41:173-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Offerhaus GJ, Giardiello FM, Tersmette KW, Mulder JW, Tersmette AC, Moore GW, Hamilton SR. Ethnic differences in the anatomical location of colorectal adenomatous polyps. Int J Cancer. 1991;49:641-644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Park SY, Kim BC, Shin SJ, Lee SK, Kim TI, Kim WH. Proximal shift in the distribution of adenomatous polyps in Korea over the past ten years. Hepatogastroenterology. 2009;56:677-681. [PubMed] [Cited in This Article: ] |
| 14. | Levi F, Randimbison L, La Vecchia C. Trends in the subsite distribution of colorectal carcinomas and polyps: an update. Cancer. 1998;83:2040-2042. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 15. | Sarli L, Michiara M, Sgargi P, Iusco D, De Lisi V, Leonardi F, Bella MA, Sgobba G, Roncoroni L. The changing distribution and survival of colorectal carcinoma: an epidemiological study in an area of northern Italy. Eur J Gastroenterol Hepatol. 2005;17:567-572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Ponz de Leon M, Marino M, Benatti P, Rossi G, Menigatti M, Pedroni M, Di Gregorio C, Losi L, Borghi F, Scarselli A. Trend of incidence, subsite distribution and staging of colorectal neoplasms in the 15-year experience of a specialised cancer registry. Ann Oncol. 2004;15:940-946. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Fenoglio L, Cena P, Bracco C, Pomero F, Migliore E, Benedetti V, Morino M, Perin PC. Proximalisation of colorectal carcinoma: a 10-year study in Italy. Dig Dis Sci. 2008;53:736-740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Fenoglio L, Castagna E, Comino A, Luchino C, Senore C, Migliore E, Capucci F, Panzone S, Silvestri A, Ghezzo L. A shift from distal to proximal neoplasia in the colon: a decade of polyps and CRC in Italy. BMC Gastroenterol. 2010;10:139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Yamaji Y, Mitsushima T, Ikuma H, Watabe H, Okamoto M, Yoshida H, Kawabe T, Wada R, Omata M. Right-side shift of colorectal adenomas with aging. Gastrointest Endosc. 2006;63:453-458; quiz 464. [PubMed] [Cited in This Article: ] |
| 20. | Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4370] [Cited by in F6Publishing: 4241] [Article Influence: 235.6] [Reference Citation Analysis (0)] |
| 21. | Li M, Gu J. Changing patterns of colorectal cancer in China over a period of 20 years. World J Gastroenterol. 2005;11:4685-4688. [PubMed] [Cited in This Article: ] |
| 22. | Meza R, Jeon J, Renehan AG, Luebeck EG. Colorectal cancer incidence trends in the United States and United kingdom: evidence of right- to left-sided biological gradients with implications for screening. Cancer Res. 2010;70:5419-5429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 23. | Rabeneck L, Davila JA, El-Serag HB. Is there a true "shift" to the right colon in the incidence of colorectal cancer? Am J Gastroenterol. 2003;98:1400-1409. [PubMed] [Cited in This Article: ] |
| 24. | Gomez D, Dalal Z, Raw E, Roberts C, Lyndon PJ. Anatomical distribution of colorectal cancer over a 10 year period in a district general hospital: is there a true "rightward shift"? Postgrad Med J. 2004;80:667-669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Rim SH, Seeff L, Ahmed F, King JB, Coughlin SS. Colorectal cancer incidence in the United States, 1999-2004 : an updated analysis of data from the National Program of Cancer Registries and the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115:1967-1976. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 26. | Murphy G, Devesa SS, Cross AJ, Inskip PD, McGlynn KA, Cook MB. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer. 2011;128:1668-1675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 27. | Regula J, Rupinski M, Kraszewska E, Polkowski M, Pachlewski J, Orlowska J, Nowacki MP, Butruk E. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 2006;355:1863-1872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 529] [Cited by in F6Publishing: 522] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 28. | Rex DK. Maximizing detection of adenomas and cancers during colonoscopy. Am J Gastroenterol. 2006;101:2866-2877. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 195] [Article Influence: 10.8] [Reference Citation Analysis (0)] |