-
PDF
- Split View
-
Views
-
Cite
Cite
R. Monte, R. Rabuñal, E. Casariego, H. López-Agreda, A. Mateos, S. Pértega, Analysis of the Factors Determining Survival of Alcoholic Withdrawal Syndrome Patients in a General Hospital, Alcohol and Alcoholism, Volume 45, Issue 2, March-April 2010, Pages 151–158, https://doi.org/10.1093/alcalc/agp087
Close - Share Icon Share
Abstract
Aim: To investigate the clinical variables associated with the risk of dying and the causes of death during the course of alcoholic withdrawal syndrome (AWS) in a general hospital. Methods: Cohort study of AWS patients admitted to Xeral Hospital in Lugo, Spain between 1987 and 2003. The characteristics of patients who died were contrasted with those who survived. The different clinical, epidemiological and biochemical variables reflective of alcohol consumption habits, basal health status and presentation features of the syndrome and its complications were all recorded. Results: There were 539 episodes of hospitalization for AWS in 436 patients (mean age 45.0, SD 12.0, 91.3% males), 71.1% of whom presented with delirium tremens. A total of 29 patients died, yielding a 6.6% mortality rate (95% confidence interval, CI: 4.2–9.1%). Eighteen patients (62%) died after being admitted to the intensive care unit (ICU). The following independent variables were associated with the risk of dying in a multivariate logistic regression model: cirrhosis [odds ratio (OR) 4.8 (95% CI 1.5–14.6), P = 0.006]; presenting with delirium tremens at diagnosis [OR 3.5 (95% CI 1.3–8.9), P = 0.008]; the existence of an underlying chronic pathology other than liver disease [OR 2.5 (95% CI 1–6.1), P = 0.01]; and the need for orotracheal intubation [OR 2.9 (95% CI 1.1–7.9), P = 0.03], especially if pneumonia requiring ICU is added [OR 8 (95% CI 3–21.3), P < 0.001]. Receiver operating characteristic analysis revealed an area under the curve of 0.818 (95% CI 0.742–0.894). Conclusions: The factors determining survival after admission to a general hospital for alcoholic withdrawal syndrome depend on the intensity of clinical manifestations (delirium tremens, ICU, orotracheal intubation) and the presence of associated comorbidity.
A specific analysis of fatality and its causes in alcoholic withdrawal syndrome (AWS) has rarely been made. On many occasions, different studies make no reference to causes of death (Ferguson et al., 1996; Foy et al., 1997; Lukan et al., 2002) and there are few available data from autopsies (Tavel et al., 1961; Salum, 1972). It is not easy to evaluate the role of alcohol deprivation per se in causing death. Frequently, death occurs when alcohol withdrawal symptoms have abated and patients present multiple medical complications, such as pneumonia, cirrhosis, gastrointestinal bleeding, etc., on top of those commonly attributed to withdrawal (Cushman, 1987; Foy et al., 1997; Horstmann et al., 1989). Nevertheless, classical studies on alcohol withdrawal and deprivation do describe cases of death during the withdrawal period in the apparent absence of other problems besides the withdrawal itself (Salum, 1972; Victor and Adams, 1953).
The mortality rate attributed to AWS has decreased from 37% at the beginning of the last century (Boston, 1908) to 2.4% in a recently published study (Puerta Louro et al., 2006). This improvement in survival rates has occurred parallel to the advances achieved in the treatment of AWS, especially in critical patient care. Studies that combine cases of major and minor withdrawal yield lower mortality rates, between 1 and 2.4% (Ferguson et al., 1996; Puerta Louro et al., 2006), probably due to the fact that the number of cases presenting delirium tremens (DT) is ‘diluted’ in the total. Survival rates also vary in function of where the study originates. Studies carried out in alcohol detoxification units, generally a part of psychiatric services, do not usually detail the causes of death when the treatment is neither administered in outpatient clinics (Whitfield et al., 1978) nor on the hospital floor (Brower et al., 1994; Shaw et al., 1998; Wetterling et al., 1994). The results of studies carried out on general hospital floors are heterogeneous and difficult to compare with each other because cases present very different basal health profiles regarding percentage of cases with DT, intensive care unit (ICU) transfers, percentage of patients undergoing surgery, etc. None of these studies have >100 patients with delirium tremens, and only two have been published in the last 10 years (Lukan et al., 2002; Puerta Louro et al., 2006).
In the present study, we analyze the clinical variables associated with the risk of dying, as well as the causes of death, during the course of AWS in a general hospital.
Materials and Methods
Study design
We have presented a retrospective cohort study on the clinical outcome and mortality rate of patients diagnosed with AWS. In a consecutive series of patients admitted to a general hospital with AWS, characteristics of cases with a fatal outcome are compared with those of survivors.
Study population
The study was carried out at the Xeral-Calde Hospital in Lugo (Spain) between January 1987 and December 2003. This center is a provincial university hospital with a 735-bed capacity, covering an area with a population of 221,441 inhabitants under the Spanish National Health Care System. All patients diagnosed with AWS and admitted either to medical or surgical services during this period were studied. Patients are not electively admitted for detoxification in our hospital. However, people who have developed DT in the area are managed in our hospital.
In cases of consecutive admissions of the same patient, these were considered as independent admissions if they were separated by at least 3 months and considered to be unrelated.
Data collection
Our hospital’s ethics committee approved the study. Cases were identified by code searching in the computerized database of the hospital. For each of the cases identified as AWS or DT in the database, the diagnoses of alcohol withdrawal syndrome and alcohol withdrawal delirium (DT) were reviewed by the investigators in accordance with the criteria established by the Diagnostic and Statistical Manual of Mental Disorders, 4th ed (DSM-IV)-TR manual (American Psychiatric Association, 2000). Data regarding demographic variables, relevant medical history, prior hospitalization for AWS, reason for current admission, alcohol withdrawal time prior to diagnosis in hours and alcohol consumption habits were extracted from clinical histories. The amount of daily alcohol consumption was calculated according to the formula (grams alcohol × milliliters × 0.8/100).
Clinical findings relevant to withdrawal symptoms (tremor, sweats, hallucinations, concomitant seizures and psychomotor agitation), vital signs (arterial blood pressure, heart rate) at diagnosis and highest axillary temperature recorded during the first 24 h post-diagnosis were collected. A diagnosis of delirium tremens was made only when clinical history included the presence of severe alterations in perception and attention, specified by medical staff in charge of patient’s care. Data on complications suffered during hospital admission, including transfers to ICUs and the need for orotracheal intubation, was collected in each case.
For patients with a previous diagnosis of cirrhosis, the existence of underlying liver disease was noted, in addition to its severity evaluated according to Child’s classification, as well as by Mendenhall’s classification and the Maddrey index when the latter two could be calculated. The diagnosis of cirrhosis or steatosis was stated by the research team according to clinical, biochemical and radiological data recorded in the clinical notes. Three patients had a liver biopsy available. Biochemical data collected at the time of AWS diagnosis were extracted from clinical histories, and when this was not possible, the most recent prior to diagnosis were used, provided they were taken during the episode under study.
All deaths were analyzed, those occurring on the general hospital floors and those in ICU, as well as the date of death, by the research team. Survival time was calculated as the difference between the date of diagnosis and that of discharge, expressed in days. The following data were used to determine the cause of death: evaluation of the patient’s attending physician in his or her clinical notes, autopsy results when available and all other clinical information and complementary tests performed during admission and hospital stay.
All patients received pharmacological treatment based on administration of clomethiazole at a dosage fixed between 2 and 3 g daily, orally, with increases and adjustments depending on intensity of symptoms and clinical course, according to the criteria of the attending physician. In cases of DT, 0.8% clomethiazole was used in intravenous infusion at an initial dosage of 100–150 ml/h, with adjustments depending on the clinical course, with a maintenance dose of 10–80 ml/h. Neuroleptics and benzodiazepines were used as adjunct treatment in cases of extreme agitation. All patients received parenteral thiamine at the time of admission. The criteria definition for AWS and DT, the treatment protocol and the standard of care remained constant over the period of study.
Statistical analysis
For the statistical analysis, the chi-squared test was used to evaluate differences between two qualitative variables and the Kolmogorov–Smirnov test for distribution comparison. After previous assessment of homoscedasticity, Student’s T test was used to compare quantitative variables between two groups. To ascertain the univariate risk of progressing from minor to major alcohol withdrawal, relative risk with 95% confident intervals (CI) was used. A logistic regression model was utilized for multivariate analysis. In the univariate analysis, a 5% significance was required for inclusion of variables, although, in the maximum model, we decided to include non-significant but clinically relevant variables that might influence results. The variables included in the maximum model were: sex, seizures, amount of daily alcohol consumption, cirrhosis, admission for alcohol withdrawal syndrome, the presence of DT at diagnosis, hemoglobin value, albumin value, comorbidity, ICU admission, mechanical ventilation and intra-ICU pneumonia. The collinearity of the maximum model was assessed. A forward procedure was used as a modeling strategy; the log-likelihood ratio test was used for model comparison and goodness-of-fit assessment. Interaction factors were analyzed but were not included in the final model as they were not found to be significant. Adjusted odds risk ratios (OR) for DT and 95% CI for each independent covariate were calculated from the estimated beta coefficients derived from the logistic regression model. To assess discrimination capacity of results, a receiver operating characteristic (ROC) curve was constructed by standard procedure using its graphic representation and the area under the curve. SPSS 15.0 package was used for analysis. Values of P <0.05 were considered significant.
Results
We studied 539 episodes of hospitalization for AWS in 436 patients. The mean age was 45.0 (SD 12.0), with a range between 20 and 75; 492 were men (91.3%). Alcohol withdrawal was the cause of admission in 336 cases (62.3%), while 203 (37.6%) were admitted for other causes and subsequently developed AWS during their hospital stay. One hundred forty-nine (149) of these episodes presented in medical wards and 54 in surgical services. In 221 cases (41%), the syndrome coursed with concomitant seizures; 97% of the ‘gran mal’ variety. At diagnosis, 303 cases were considered to be presenting with AWS while 236 were considered to be presenting with alcohol withdrawal delirium (DT). One hundred forty-seven cases (48.5%) progressed from AWS to DT. Of these, 91 were admitted for AWS and 51 for other reasons. It meant that, by the end of their hospital stay, 156 cases were counted as AWS (28.9%) and 383 (71.1%) as cases of DT. No differences were found in the proportion of patients with minor AWS that developed DT during the period of study when we considered the number of cases over 4-year intervals. Seventy-two patients were admitted to hospital on more than one occasion, accounting for a total of 175 admissions in this group: 51 were admitted twice, 15 on three occasions and six on four or more.
The average time since alcohol consumption had significantly declined or ceased until the diagnosis of alcohol withdrawal syndrome in patients admitted for AWS was 55.4 h (SD 31.1) compared to 41.1 h (SD 23.9) in patients admitted for a medical or surgical condition (P < 0.001).
The ICU transfer rate was 37.8% (95% CI 33.1–37.8). Of these, 188 presented alcohol withdrawal delirium, which means that the percentage of ICU admissions within the group suffering DT constitutes 49%. Focusing on the reason for hospitalization, 31.5% of the patients transferred to the ICU were admitted to hospital for AWS and 41.9% for other causes. One hundred fifty cases (78.5%) were transferred to intensive care due to uncontrollable agitation, 15 (7.8%) due to iatrogenic respiratory depression, six (3.1%) because of a disease unrelated to delirium and 19 (10.1%) for other reasons. One hundred thirty-three patients (69.6% of the cases) admitted to the ICU required orotracheal intubation (OTI) and mechanical ventilation, which was prolonged for 9.6 days (SD 9.5).
A total of 29 patients died, yielding a 6.6% mortality rate (95% CI 4.2–9.1%). With regard to the total number of AWS episodes, the overall death rate was 5.4%, and 7.5% in the group of patients had alcohol withdrawal delirium. Twenty-six cases (89.6%) were men and the mean age was 45.4 years (SD 12.5). Eighteen patients (62%) died after being admitted to the ICU and the rest on general hospital floors. Thirteen cases (44.8%) were admitted for alcohol withdrawal and the rest had been admitted for different reasons, subsequently presenting AWS. Table 1 shows the most significant characteristics of all deceased patients as well as the triggers and causes of fatal outcomes.
Characteristics of patients who died post-admission for AWS
| . | Sex . | Age . | Reason for admission . | ICU . | OTIc . | Trigger . | Cause of death . | Necropsy . |
|---|---|---|---|---|---|---|---|---|
| 1 | Male | 46 | Traumatism | No | No | Sudden death | No | |
| 2 | Male | 24 | Withdrawal | No | No | Sudden death | No | |
| 3 | Male | 40 | Withdrawal | Yes | Yes | Pneumonia | Cardiogenic shock | No |
| 4 | Male | 30 | Withdrawal | Yes | Yes | Pneumonia | Multi-organ failure | No |
| 5 | Male | 42 | Withdrawal | No | No | Respiratory arrest due to sedation | No | |
| 6 | Male | 40 | Withdrawal | Yes | Yes | Cirrhosis | Liver failure | No |
| 7 | Male | 55 | Pneumonia | No | No | UDBd | Sudden death | No |
| 8 | Male | 32 | Diabetic ketoacidosis | Yes | Yes | Pneumonia | Respiratory distress | No |
| 9 | Male | 28 | Withdrawal | No | No | Respiratory arrest due to sedation | No | |
| 10 | Male | 41 | UDBd | Yes | Yes | Cirrhosis | Liver failure | No |
| 11 | Male | 44 | Tuberculosis | No | No | Respiratory failure | No | |
| 12 | Male | 44 | Withdrawal | Yes | Yes | Acute pulmonary edema | Cardiogenic shock | No |
| 13 | Male | 53 | Withdrawal | Yes | Yes | Pneumonia | Multi-organ failure | No |
| 14 | Male | 30 | Withdrawal | Yes | Yes | Pneumonia | Multi-organ failure | No |
| 15 | Male | 36 | Withdrawal | Yes | Yes | Pneumonia | Multi-organ failure | No |
| 16 | Male | 43 | Withdrawal | No | No | Massive pulmonary embolism | Yes | |
| 17 | Male | 55 | Withdrawal | Yes | Yes | Auriculo-ventricular (AV) blockage | Cardiogenic shock | No |
| 18a | Female | 41 | Withdrawal | Yes | Yes | Cirrhosis | Liver failure | No |
| 19 | Male | 63 | Gastric cancer | Yes | Yes | Gastric cancer | No | |
| 20 | Male | 52 | Sigma volvulus | No | No | Volvulus relapse | Hyperpotassemia | No |
| 21 | Male | 77 | AMIe | Yes | Yes | Cardiogenic shock | No | |
| 22 | Female | 50 | UDBa | No | No | Cirrhosis | Liver failure | No |
| 23 | Male | 40 | Pancreatitis | Yes | Yes | Pneumonia | Multi-organ failure | No |
| 24 | Female | 50 | Withdrawal | Yes | Yes | Pneumonia | Respiratory distress | No |
| 25 | Male | 62 | Traumatism | Yes | Yes | Pneumonia | Multi-organ failure | No |
| 26b | Male | 33 | Pneumonia | Yes | Yes | Cirrhosis | Respiratory distress | No |
| 27 | Male | 70 | Sepsis | No | No | Cirrhosis | Septic shock | No |
| 28 | Male | 63 | UDBd | Yes | Yes | Hypovolemic shock | Multi-organ failure | No |
| 29 | Male | 50 | Auricular fibrillation | No | No | Sudden death | No |
| . | Sex . | Age . | Reason for admission . | ICU . | OTIc . | Trigger . | Cause of death . | Necropsy . |
|---|---|---|---|---|---|---|---|---|
| 1 | Male | 46 | Traumatism | No | No | Sudden death | No | |
| 2 | Male | 24 | Withdrawal | No | No | Sudden death | No | |
| 3 | Male | 40 | Withdrawal | Yes | Yes | Pneumonia | Cardiogenic shock | No |
| 4 | Male | 30 | Withdrawal | Yes | Yes | Pneumonia | Multi-organ failure | No |
| 5 | Male | 42 | Withdrawal | No | No | Respiratory arrest due to sedation | No | |
| 6 | Male | 40 | Withdrawal | Yes | Yes | Cirrhosis | Liver failure | No |
| 7 | Male | 55 | Pneumonia | No | No | UDBd | Sudden death | No |
| 8 | Male | 32 | Diabetic ketoacidosis | Yes | Yes | Pneumonia | Respiratory distress | No |
| 9 | Male | 28 | Withdrawal | No | No | Respiratory arrest due to sedation | No | |
| 10 | Male | 41 | UDBd | Yes | Yes | Cirrhosis | Liver failure | No |
| 11 | Male | 44 | Tuberculosis | No | No | Respiratory failure | No | |
| 12 | Male | 44 | Withdrawal | Yes | Yes | Acute pulmonary edema | Cardiogenic shock | No |
| 13 | Male | 53 | Withdrawal | Yes | Yes | Pneumonia | Multi-organ failure | No |
| 14 | Male | 30 | Withdrawal | Yes | Yes | Pneumonia | Multi-organ failure | No |
| 15 | Male | 36 | Withdrawal | Yes | Yes | Pneumonia | Multi-organ failure | No |
| 16 | Male | 43 | Withdrawal | No | No | Massive pulmonary embolism | Yes | |
| 17 | Male | 55 | Withdrawal | Yes | Yes | Auriculo-ventricular (AV) blockage | Cardiogenic shock | No |
| 18a | Female | 41 | Withdrawal | Yes | Yes | Cirrhosis | Liver failure | No |
| 19 | Male | 63 | Gastric cancer | Yes | Yes | Gastric cancer | No | |
| 20 | Male | 52 | Sigma volvulus | No | No | Volvulus relapse | Hyperpotassemia | No |
| 21 | Male | 77 | AMIe | Yes | Yes | Cardiogenic shock | No | |
| 22 | Female | 50 | UDBa | No | No | Cirrhosis | Liver failure | No |
| 23 | Male | 40 | Pancreatitis | Yes | Yes | Pneumonia | Multi-organ failure | No |
| 24 | Female | 50 | Withdrawal | Yes | Yes | Pneumonia | Respiratory distress | No |
| 25 | Male | 62 | Traumatism | Yes | Yes | Pneumonia | Multi-organ failure | No |
| 26b | Male | 33 | Pneumonia | Yes | Yes | Cirrhosis | Respiratory distress | No |
| 27 | Male | 70 | Sepsis | No | No | Cirrhosis | Septic shock | No |
| 28 | Male | 63 | UDBd | Yes | Yes | Hypovolemic shock | Multi-organ failure | No |
| 29 | Male | 50 | Auricular fibrillation | No | No | Sudden death | No |
Died on hospital floor after stay in ICU.
Only case of minor AWS.
Orotracheal intubation.
Upper digestive bleeding.
Acute myocardial infarction.
Characteristics of patients who died post-admission for AWS
| . | Sex . | Age . | Reason for admission . | ICU . | OTIc . | Trigger . | Cause of death . | Necropsy . |
|---|---|---|---|---|---|---|---|---|
| 1 | Male | 46 | Traumatism | No | No | Sudden death | No | |
| 2 | Male | 24 | Withdrawal | No | No | Sudden death | No | |
| 3 | Male | 40 | Withdrawal | Yes | Yes | Pneumonia | Cardiogenic shock | No |
| 4 | Male | 30 | Withdrawal | Yes | Yes | Pneumonia | Multi-organ failure | No |
| 5 | Male | 42 | Withdrawal | No | No | Respiratory arrest due to sedation | No | |
| 6 | Male | 40 | Withdrawal | Yes | Yes | Cirrhosis | Liver failure | No |
| 7 | Male | 55 | Pneumonia | No | No | UDBd | Sudden death | No |
| 8 | Male | 32 | Diabetic ketoacidosis | Yes | Yes | Pneumonia | Respiratory distress | No |
| 9 | Male | 28 | Withdrawal | No | No | Respiratory arrest due to sedation | No | |
| 10 | Male | 41 | UDBd | Yes | Yes | Cirrhosis | Liver failure | No |
| 11 | Male | 44 | Tuberculosis | No | No | Respiratory failure | No | |
| 12 | Male | 44 | Withdrawal | Yes | Yes | Acute pulmonary edema | Cardiogenic shock | No |
| 13 | Male | 53 | Withdrawal | Yes | Yes | Pneumonia | Multi-organ failure | No |
| 14 | Male | 30 | Withdrawal | Yes | Yes | Pneumonia | Multi-organ failure | No |
| 15 | Male | 36 | Withdrawal | Yes | Yes | Pneumonia | Multi-organ failure | No |
| 16 | Male | 43 | Withdrawal | No | No | Massive pulmonary embolism | Yes | |
| 17 | Male | 55 | Withdrawal | Yes | Yes | Auriculo-ventricular (AV) blockage | Cardiogenic shock | No |
| 18a | Female | 41 | Withdrawal | Yes | Yes | Cirrhosis | Liver failure | No |
| 19 | Male | 63 | Gastric cancer | Yes | Yes | Gastric cancer | No | |
| 20 | Male | 52 | Sigma volvulus | No | No | Volvulus relapse | Hyperpotassemia | No |
| 21 | Male | 77 | AMIe | Yes | Yes | Cardiogenic shock | No | |
| 22 | Female | 50 | UDBa | No | No | Cirrhosis | Liver failure | No |
| 23 | Male | 40 | Pancreatitis | Yes | Yes | Pneumonia | Multi-organ failure | No |
| 24 | Female | 50 | Withdrawal | Yes | Yes | Pneumonia | Respiratory distress | No |
| 25 | Male | 62 | Traumatism | Yes | Yes | Pneumonia | Multi-organ failure | No |
| 26b | Male | 33 | Pneumonia | Yes | Yes | Cirrhosis | Respiratory distress | No |
| 27 | Male | 70 | Sepsis | No | No | Cirrhosis | Septic shock | No |
| 28 | Male | 63 | UDBd | Yes | Yes | Hypovolemic shock | Multi-organ failure | No |
| 29 | Male | 50 | Auricular fibrillation | No | No | Sudden death | No |
| . | Sex . | Age . | Reason for admission . | ICU . | OTIc . | Trigger . | Cause of death . | Necropsy . |
|---|---|---|---|---|---|---|---|---|
| 1 | Male | 46 | Traumatism | No | No | Sudden death | No | |
| 2 | Male | 24 | Withdrawal | No | No | Sudden death | No | |
| 3 | Male | 40 | Withdrawal | Yes | Yes | Pneumonia | Cardiogenic shock | No |
| 4 | Male | 30 | Withdrawal | Yes | Yes | Pneumonia | Multi-organ failure | No |
| 5 | Male | 42 | Withdrawal | No | No | Respiratory arrest due to sedation | No | |
| 6 | Male | 40 | Withdrawal | Yes | Yes | Cirrhosis | Liver failure | No |
| 7 | Male | 55 | Pneumonia | No | No | UDBd | Sudden death | No |
| 8 | Male | 32 | Diabetic ketoacidosis | Yes | Yes | Pneumonia | Respiratory distress | No |
| 9 | Male | 28 | Withdrawal | No | No | Respiratory arrest due to sedation | No | |
| 10 | Male | 41 | UDBd | Yes | Yes | Cirrhosis | Liver failure | No |
| 11 | Male | 44 | Tuberculosis | No | No | Respiratory failure | No | |
| 12 | Male | 44 | Withdrawal | Yes | Yes | Acute pulmonary edema | Cardiogenic shock | No |
| 13 | Male | 53 | Withdrawal | Yes | Yes | Pneumonia | Multi-organ failure | No |
| 14 | Male | 30 | Withdrawal | Yes | Yes | Pneumonia | Multi-organ failure | No |
| 15 | Male | 36 | Withdrawal | Yes | Yes | Pneumonia | Multi-organ failure | No |
| 16 | Male | 43 | Withdrawal | No | No | Massive pulmonary embolism | Yes | |
| 17 | Male | 55 | Withdrawal | Yes | Yes | Auriculo-ventricular (AV) blockage | Cardiogenic shock | No |
| 18a | Female | 41 | Withdrawal | Yes | Yes | Cirrhosis | Liver failure | No |
| 19 | Male | 63 | Gastric cancer | Yes | Yes | Gastric cancer | No | |
| 20 | Male | 52 | Sigma volvulus | No | No | Volvulus relapse | Hyperpotassemia | No |
| 21 | Male | 77 | AMIe | Yes | Yes | Cardiogenic shock | No | |
| 22 | Female | 50 | UDBa | No | No | Cirrhosis | Liver failure | No |
| 23 | Male | 40 | Pancreatitis | Yes | Yes | Pneumonia | Multi-organ failure | No |
| 24 | Female | 50 | Withdrawal | Yes | Yes | Pneumonia | Respiratory distress | No |
| 25 | Male | 62 | Traumatism | Yes | Yes | Pneumonia | Multi-organ failure | No |
| 26b | Male | 33 | Pneumonia | Yes | Yes | Cirrhosis | Respiratory distress | No |
| 27 | Male | 70 | Sepsis | No | No | Cirrhosis | Septic shock | No |
| 28 | Male | 63 | UDBd | Yes | Yes | Hypovolemic shock | Multi-organ failure | No |
| 29 | Male | 50 | Auricular fibrillation | No | No | Sudden death | No |
Died on hospital floor after stay in ICU.
Only case of minor AWS.
Orotracheal intubation.
Upper digestive bleeding.
Acute myocardial infarction.
The data on the 29 fatal cases are compared with those of the non-fatal AWS episodes (510), with the aim of determining which factors impacted survival. Table 2 provides a univariate analysis of the differences observed among the diverse clinical variables with regard to circumstances and AWS presentation characteristics between the two groups. The variables associated with a greater risk of dying were admission for a reason other than AWS and presenting with DT at the moment of AWS diagnosis. Patients with more than one admission did not show a trend to repeat the clinical presentation (DT or AWS) in subsequent admissions. Neither the presence of previous admissions for alcohol withdrawal nor the fact that patients were transferred to the ICU in previous alcohol withdrawal episodes influenced survival.
Univariate analysis of factors predictive of death: clinical variables
| . | Death . | RR . | 95% CI . | P . | |
|---|---|---|---|---|---|
| No (n = 510) . | Yes (n = 29) . | ||||
| Sex (male) | 466 (91.3%) | 26 (89.6%) | 1.2 | 0.3–3.8 | 0.7 |
| Agea | 45.4 (12) | 45.4 (12.5) | 0.9 | ||
| Reason for admission | |||||
| Withdrawal | 323 (63.3%) | 13 (44.8%) | 2 | 1.01–4.2 | 0.04 |
| Other reason | 185 (36.2%) | 16 (55.1%) | |||
| Withdrawal time (h)a | 55.6 (33.6) | 52.2 (33) | 0.6 | ||
| Grams of alcohol per daya | 39.3 (89.7) | 257.8 (93.9) | 0.3 | ||
| Smokerb | 6 (7.1%) | 12 (5.2%) | 0.7 | 0.3–1.9 | 0.5 |
| Seizures | 208 (40.7%) | 13 (44.8%) | 1.2 | 0.6–2.4 | 0.6 |
| Delirium on admission | 216 (42.3%) | 20 (68.9%) | 2.8 | 1.3–6.1 | 0.005 |
| Previous admission for AWS | 95 (18.6%) | 6 (20,6%) | 1.1 | 0.4–2.7 | 0.7 |
| ICU in previous AWS | 39 (41%) | 4 (66%) | 2.7 | 0.5–14 | 0.2 |
| BPsa,d | 137.9 (20.4) | 131.4 (27.3) | 0.1 | ||
| BPdc | 79.7 (13.8) | 78 (15.8) | 0.4 | ||
| HRe | 99.4 (18.1) | 104.1 (25.3) | 0.1 | ||
| BT 24 hf | 37.5 (0.7) | 37.6 (0.8) | 0.1 | ||
| . | Death . | RR . | 95% CI . | P . | |
|---|---|---|---|---|---|
| No (n = 510) . | Yes (n = 29) . | ||||
| Sex (male) | 466 (91.3%) | 26 (89.6%) | 1.2 | 0.3–3.8 | 0.7 |
| Agea | 45.4 (12) | 45.4 (12.5) | 0.9 | ||
| Reason for admission | |||||
| Withdrawal | 323 (63.3%) | 13 (44.8%) | 2 | 1.01–4.2 | 0.04 |
| Other reason | 185 (36.2%) | 16 (55.1%) | |||
| Withdrawal time (h)a | 55.6 (33.6) | 52.2 (33) | 0.6 | ||
| Grams of alcohol per daya | 39.3 (89.7) | 257.8 (93.9) | 0.3 | ||
| Smokerb | 6 (7.1%) | 12 (5.2%) | 0.7 | 0.3–1.9 | 0.5 |
| Seizures | 208 (40.7%) | 13 (44.8%) | 1.2 | 0.6–2.4 | 0.6 |
| Delirium on admission | 216 (42.3%) | 20 (68.9%) | 2.8 | 1.3–6.1 | 0.005 |
| Previous admission for AWS | 95 (18.6%) | 6 (20,6%) | 1.1 | 0.4–2.7 | 0.7 |
| ICU in previous AWS | 39 (41%) | 4 (66%) | 2.7 | 0.5–14 | 0.2 |
| BPsa,d | 137.9 (20.4) | 131.4 (27.3) | 0.1 | ||
| BPdc | 79.7 (13.8) | 78 (15.8) | 0.4 | ||
| HRe | 99.4 (18.1) | 104.1 (25.3) | 0.1 | ||
| BT 24 hf | 37.5 (0.7) | 37.6 (0.8) | 0.1 | ||
RR, relative risk; 95% CI, confidence interval at 95%.
Mean (SD).
Percentage calculated on cases when available.
Systolic blood pressure at diagnosis (mmHg).
Diastolic blood pressure at diagnosis (mmHg).
Heart rate at diagnosis.
Highest body temperature in the first 24 h after diagnosis.
Univariate analysis of factors predictive of death: clinical variables
| . | Death . | RR . | 95% CI . | P . | |
|---|---|---|---|---|---|
| No (n = 510) . | Yes (n = 29) . | ||||
| Sex (male) | 466 (91.3%) | 26 (89.6%) | 1.2 | 0.3–3.8 | 0.7 |
| Agea | 45.4 (12) | 45.4 (12.5) | 0.9 | ||
| Reason for admission | |||||
| Withdrawal | 323 (63.3%) | 13 (44.8%) | 2 | 1.01–4.2 | 0.04 |
| Other reason | 185 (36.2%) | 16 (55.1%) | |||
| Withdrawal time (h)a | 55.6 (33.6) | 52.2 (33) | 0.6 | ||
| Grams of alcohol per daya | 39.3 (89.7) | 257.8 (93.9) | 0.3 | ||
| Smokerb | 6 (7.1%) | 12 (5.2%) | 0.7 | 0.3–1.9 | 0.5 |
| Seizures | 208 (40.7%) | 13 (44.8%) | 1.2 | 0.6–2.4 | 0.6 |
| Delirium on admission | 216 (42.3%) | 20 (68.9%) | 2.8 | 1.3–6.1 | 0.005 |
| Previous admission for AWS | 95 (18.6%) | 6 (20,6%) | 1.1 | 0.4–2.7 | 0.7 |
| ICU in previous AWS | 39 (41%) | 4 (66%) | 2.7 | 0.5–14 | 0.2 |
| BPsa,d | 137.9 (20.4) | 131.4 (27.3) | 0.1 | ||
| BPdc | 79.7 (13.8) | 78 (15.8) | 0.4 | ||
| HRe | 99.4 (18.1) | 104.1 (25.3) | 0.1 | ||
| BT 24 hf | 37.5 (0.7) | 37.6 (0.8) | 0.1 | ||
| . | Death . | RR . | 95% CI . | P . | |
|---|---|---|---|---|---|
| No (n = 510) . | Yes (n = 29) . | ||||
| Sex (male) | 466 (91.3%) | 26 (89.6%) | 1.2 | 0.3–3.8 | 0.7 |
| Agea | 45.4 (12) | 45.4 (12.5) | 0.9 | ||
| Reason for admission | |||||
| Withdrawal | 323 (63.3%) | 13 (44.8%) | 2 | 1.01–4.2 | 0.04 |
| Other reason | 185 (36.2%) | 16 (55.1%) | |||
| Withdrawal time (h)a | 55.6 (33.6) | 52.2 (33) | 0.6 | ||
| Grams of alcohol per daya | 39.3 (89.7) | 257.8 (93.9) | 0.3 | ||
| Smokerb | 6 (7.1%) | 12 (5.2%) | 0.7 | 0.3–1.9 | 0.5 |
| Seizures | 208 (40.7%) | 13 (44.8%) | 1.2 | 0.6–2.4 | 0.6 |
| Delirium on admission | 216 (42.3%) | 20 (68.9%) | 2.8 | 1.3–6.1 | 0.005 |
| Previous admission for AWS | 95 (18.6%) | 6 (20,6%) | 1.1 | 0.4–2.7 | 0.7 |
| ICU in previous AWS | 39 (41%) | 4 (66%) | 2.7 | 0.5–14 | 0.2 |
| BPsa,d | 137.9 (20.4) | 131.4 (27.3) | 0.1 | ||
| BPdc | 79.7 (13.8) | 78 (15.8) | 0.4 | ||
| HRe | 99.4 (18.1) | 104.1 (25.3) | 0.1 | ||
| BT 24 hf | 37.5 (0.7) | 37.6 (0.8) | 0.1 | ||
RR, relative risk; 95% CI, confidence interval at 95%.
Mean (SD).
Percentage calculated on cases when available.
Systolic blood pressure at diagnosis (mmHg).
Diastolic blood pressure at diagnosis (mmHg).
Heart rate at diagnosis.
Highest body temperature in the first 24 h after diagnosis.
When the repercussions of comorbidity on clinical course of withdrawal were evaluated, an increased risk of dying during alcohol withdrawal was associated with all methods for evaluating the severity of underlying liver pathology analyzed: clinical diagnosis of cirrhosis, Child’s classification of cirrhosis, the Maddrey index and Mendenhall classification (Table 3). The presence of comorbidity other than liver disease (hypertension, heart disease, bronchial pathologies, diabetes, epilepsy) was significantly associated with risk of death [nine deceased patients out of 29 (31%) presented comorbidity other than hepatic, compared with 83 out of 510 survivors (16.2%); RR 2.2; 95% CI 1.02–4.6; P = 0.04].
Univariate analysis of factors predictive of death: underlying liver disease
| . | Exitus . | RR . | 95% CI . | P . | |
|---|---|---|---|---|---|
| No (n = 510) . | Yes (n = 29) . | ||||
| No liver disease | 346 (67.8%) | 13 (44.8%) | |||
| Steatosis | 116 (22.7%) | 10 (34.4%) | |||
| Cirrhosis | 48 (9.4%) | 6 (20.9%) | 0.02 | ||
| Childa | |||||
| B | 26 (49%) | 0 (0%) | 2.5 | 1.3–4.6 | 0.02 |
| C | 22 (41.5%) | 5 (50%) | |||
| I. Maddreya (SD) | 7.8 (10.1) | 22.4 (24.5) | 0.01 | ||
| Mendenhalla | |||||
| I or II | 393 (91.8%) | 16 (76.1%) | 6.7 | 2.7–16.3 | <0.001 |
| III | 14 (3.2%) | 5 (23.8%) | |||
| . | Exitus . | RR . | 95% CI . | P . | |
|---|---|---|---|---|---|
| No (n = 510) . | Yes (n = 29) . | ||||
| No liver disease | 346 (67.8%) | 13 (44.8%) | |||
| Steatosis | 116 (22.7%) | 10 (34.4%) | |||
| Cirrhosis | 48 (9.4%) | 6 (20.9%) | 0.02 | ||
| Childa | |||||
| B | 26 (49%) | 0 (0%) | 2.5 | 1.3–4.6 | 0.02 |
| C | 22 (41.5%) | 5 (50%) | |||
| I. Maddreya (SD) | 7.8 (10.1) | 22.4 (24.5) | 0.01 | ||
| Mendenhalla | |||||
| I or II | 393 (91.8%) | 16 (76.1%) | 6.7 | 2.7–16.3 | <0.001 |
| III | 14 (3.2%) | 5 (23.8%) | |||
RR, relative risk; 95% CI, confidence interval at 95%.
Means and percentages calculated on number of cases where they were available: Child (53 cases—10 deaths). I. Maddrey (406 cases—19 deaths). Mendenhall (428 cases—21 deaths).
Univariate analysis of factors predictive of death: underlying liver disease
| . | Exitus . | RR . | 95% CI . | P . | |
|---|---|---|---|---|---|
| No (n = 510) . | Yes (n = 29) . | ||||
| No liver disease | 346 (67.8%) | 13 (44.8%) | |||
| Steatosis | 116 (22.7%) | 10 (34.4%) | |||
| Cirrhosis | 48 (9.4%) | 6 (20.9%) | 0.02 | ||
| Childa | |||||
| B | 26 (49%) | 0 (0%) | 2.5 | 1.3–4.6 | 0.02 |
| C | 22 (41.5%) | 5 (50%) | |||
| I. Maddreya (SD) | 7.8 (10.1) | 22.4 (24.5) | 0.01 | ||
| Mendenhalla | |||||
| I or II | 393 (91.8%) | 16 (76.1%) | 6.7 | 2.7–16.3 | <0.001 |
| III | 14 (3.2%) | 5 (23.8%) | |||
| . | Exitus . | RR . | 95% CI . | P . | |
|---|---|---|---|---|---|
| No (n = 510) . | Yes (n = 29) . | ||||
| No liver disease | 346 (67.8%) | 13 (44.8%) | |||
| Steatosis | 116 (22.7%) | 10 (34.4%) | |||
| Cirrhosis | 48 (9.4%) | 6 (20.9%) | 0.02 | ||
| Childa | |||||
| B | 26 (49%) | 0 (0%) | 2.5 | 1.3–4.6 | 0.02 |
| C | 22 (41.5%) | 5 (50%) | |||
| I. Maddreya (SD) | 7.8 (10.1) | 22.4 (24.5) | 0.01 | ||
| Mendenhalla | |||||
| I or II | 393 (91.8%) | 16 (76.1%) | 6.7 | 2.7–16.3 | <0.001 |
| III | 14 (3.2%) | 5 (23.8%) | |||
RR, relative risk; 95% CI, confidence interval at 95%.
Means and percentages calculated on number of cases where they were available: Child (53 cases—10 deaths). I. Maddrey (406 cases—19 deaths). Mendenhall (428 cases—21 deaths).
Table 4 shows the most relevant differences in biochemical analysis between the group of deceased patients and those who survived a withdrawal episode. No significant differences were observed in the variables directly related to alcohol consumption (mean corpuscular volume (MCV), gammaglutamyl transferase (GGT), aspartate aminotransferase (AST), alanine aminotransferase (ALT)). On the other hand, lower values in variables evaluating the patient’s basal health (hemoglobine (Hb), prothrombin index, albumin) were significantly associated with mortality risk.
Univariate analysis of factors predictive of death: biochemical variables
| . | Death . | P . | |
|---|---|---|---|
| No (n = 510) . | Yes (n = 29) . | ||
| Hemoglobin (g/dl)a | 13.3 (2) | 12.2 (2.1) | 0.001 |
| MCV (fl) | 101.3 (5.5) | 102.7 (6.2) | 0.1 |
| Leucocytes (/mm3) | 9.006 (3.556) | 8.217.8 (4.061) | 0.3 |
| Platelets (/mm3) | 162.373 (90.960) | 133.000 (61.218) | 0.1 |
| Prothrombin index (%) | 86.8 (19.3) | 71.7 (29.3) | 0.003 |
| Na (mEq/l) | 136.8 (4.2) | 136.1 (5.5) | 0.6 |
| K (mEq/l) | 3.7 (0.5) | 3.9 (0.6) | 0.2 |
| Creatinine (mg/dl) | 0.9 (0.3) | 1.1 (0.8) | 0.8 |
| Mg (mg/dl) | 1.6 (0.4) | 1.5 (0.8) | 0.3 |
| ALT (U/l) | 61.3 (48.8) | 49.7 (34.1) | 0.1 |
| AST (U/l) | 109.1 (149.6) | 120.2 (189.8) | 0.6 |
| GGT (U/l) | 419.4 (499.8) | 447.7 (434.5) | 0.3 |
| Albumin (mg/dl) | 3.6 (0.6) | 3.1 (0.6) | 0.002 |
| Bi total (mg/dl) | 1.8 (2.1) | 4.1 (5.7) | 0.2 |
| . | Death . | P . | |
|---|---|---|---|
| No (n = 510) . | Yes (n = 29) . | ||
| Hemoglobin (g/dl)a | 13.3 (2) | 12.2 (2.1) | 0.001 |
| MCV (fl) | 101.3 (5.5) | 102.7 (6.2) | 0.1 |
| Leucocytes (/mm3) | 9.006 (3.556) | 8.217.8 (4.061) | 0.3 |
| Platelets (/mm3) | 162.373 (90.960) | 133.000 (61.218) | 0.1 |
| Prothrombin index (%) | 86.8 (19.3) | 71.7 (29.3) | 0.003 |
| Na (mEq/l) | 136.8 (4.2) | 136.1 (5.5) | 0.6 |
| K (mEq/l) | 3.7 (0.5) | 3.9 (0.6) | 0.2 |
| Creatinine (mg/dl) | 0.9 (0.3) | 1.1 (0.8) | 0.8 |
| Mg (mg/dl) | 1.6 (0.4) | 1.5 (0.8) | 0.3 |
| ALT (U/l) | 61.3 (48.8) | 49.7 (34.1) | 0.1 |
| AST (U/l) | 109.1 (149.6) | 120.2 (189.8) | 0.6 |
| GGT (U/l) | 419.4 (499.8) | 447.7 (434.5) | 0.3 |
| Albumin (mg/dl) | 3.6 (0.6) | 3.1 (0.6) | 0.002 |
| Bi total (mg/dl) | 1.8 (2.1) | 4.1 (5.7) | 0.2 |
Mean (SD).
Univariate analysis of factors predictive of death: biochemical variables
| . | Death . | P . | |
|---|---|---|---|
| No (n = 510) . | Yes (n = 29) . | ||
| Hemoglobin (g/dl)a | 13.3 (2) | 12.2 (2.1) | 0.001 |
| MCV (fl) | 101.3 (5.5) | 102.7 (6.2) | 0.1 |
| Leucocytes (/mm3) | 9.006 (3.556) | 8.217.8 (4.061) | 0.3 |
| Platelets (/mm3) | 162.373 (90.960) | 133.000 (61.218) | 0.1 |
| Prothrombin index (%) | 86.8 (19.3) | 71.7 (29.3) | 0.003 |
| Na (mEq/l) | 136.8 (4.2) | 136.1 (5.5) | 0.6 |
| K (mEq/l) | 3.7 (0.5) | 3.9 (0.6) | 0.2 |
| Creatinine (mg/dl) | 0.9 (0.3) | 1.1 (0.8) | 0.8 |
| Mg (mg/dl) | 1.6 (0.4) | 1.5 (0.8) | 0.3 |
| ALT (U/l) | 61.3 (48.8) | 49.7 (34.1) | 0.1 |
| AST (U/l) | 109.1 (149.6) | 120.2 (189.8) | 0.6 |
| GGT (U/l) | 419.4 (499.8) | 447.7 (434.5) | 0.3 |
| Albumin (mg/dl) | 3.6 (0.6) | 3.1 (0.6) | 0.002 |
| Bi total (mg/dl) | 1.8 (2.1) | 4.1 (5.7) | 0.2 |
| . | Death . | P . | |
|---|---|---|---|
| No (n = 510) . | Yes (n = 29) . | ||
| Hemoglobin (g/dl)a | 13.3 (2) | 12.2 (2.1) | 0.001 |
| MCV (fl) | 101.3 (5.5) | 102.7 (6.2) | 0.1 |
| Leucocytes (/mm3) | 9.006 (3.556) | 8.217.8 (4.061) | 0.3 |
| Platelets (/mm3) | 162.373 (90.960) | 133.000 (61.218) | 0.1 |
| Prothrombin index (%) | 86.8 (19.3) | 71.7 (29.3) | 0.003 |
| Na (mEq/l) | 136.8 (4.2) | 136.1 (5.5) | 0.6 |
| K (mEq/l) | 3.7 (0.5) | 3.9 (0.6) | 0.2 |
| Creatinine (mg/dl) | 0.9 (0.3) | 1.1 (0.8) | 0.8 |
| Mg (mg/dl) | 1.6 (0.4) | 1.5 (0.8) | 0.3 |
| ALT (U/l) | 61.3 (48.8) | 49.7 (34.1) | 0.1 |
| AST (U/l) | 109.1 (149.6) | 120.2 (189.8) | 0.6 |
| GGT (U/l) | 419.4 (499.8) | 447.7 (434.5) | 0.3 |
| Albumin (mg/dl) | 3.6 (0.6) | 3.1 (0.6) | 0.002 |
| Bi total (mg/dl) | 1.8 (2.1) | 4.1 (5.7) | 0.2 |
Mean (SD).
Admission to the ICU was significantly related to risk of dying [18/29 deceased patients were transferred to intensive care (62%), compared to 11/29 not transferred (37.9%); RR 3; 95% CI 1.4–6.1; P = 0.002]. The need for mechanical ventilation was the circumstance that most impacted prognosis of patients transferred to the ICU, especially when related to the development of pneumonia (Table 5).
Correlation between admission to ICU, orotracheal intubation and presence of intra-ICU pneumonia with risk of dying
| . | Death . | P . | |
|---|---|---|---|
| No . | Yes . | ||
| No ICU | 337 (66%) | 11 (37.9%) | |
| ICU, no OTI | 58 (11.3%) | 0 (0%) | |
| ICU, yes OTI, no pneumonia | 75 (14.7%) | 8 (27.5%) | |
| ICU, yes OTI, yes pneumonia | 40 (7.8%) | 10 (34.4%) | <0.001 |
| . | Death . | P . | |
|---|---|---|---|
| No . | Yes . | ||
| No ICU | 337 (66%) | 11 (37.9%) | |
| ICU, no OTI | 58 (11.3%) | 0 (0%) | |
| ICU, yes OTI, no pneumonia | 75 (14.7%) | 8 (27.5%) | |
| ICU, yes OTI, yes pneumonia | 40 (7.8%) | 10 (34.4%) | <0.001 |
OTI, orotracheal intubation.
Correlation between admission to ICU, orotracheal intubation and presence of intra-ICU pneumonia with risk of dying
| . | Death . | P . | |
|---|---|---|---|
| No . | Yes . | ||
| No ICU | 337 (66%) | 11 (37.9%) | |
| ICU, no OTI | 58 (11.3%) | 0 (0%) | |
| ICU, yes OTI, no pneumonia | 75 (14.7%) | 8 (27.5%) | |
| ICU, yes OTI, yes pneumonia | 40 (7.8%) | 10 (34.4%) | <0.001 |
| . | Death . | P . | |
|---|---|---|---|
| No . | Yes . | ||
| No ICU | 337 (66%) | 11 (37.9%) | |
| ICU, no OTI | 58 (11.3%) | 0 (0%) | |
| ICU, yes OTI, no pneumonia | 75 (14.7%) | 8 (27.5%) | |
| ICU, yes OTI, yes pneumonia | 40 (7.8%) | 10 (34.4%) | <0.001 |
OTI, orotracheal intubation.
The development of intra-ICU pneumonia was significantly associated with orotracheal intubation [50 patients out of 133 with OTI (37.5%) compared to seven out of 58 without OTI (12%); RR 2.9; 95% CI 1.5–6.5; P < 0.001]. Intra-ICU pneumonia was the only infection associated with risk of death. The development of respiratory infections on the general hospital floor or of bacteremia, either in the ICU or on the floor, did not influence survival.
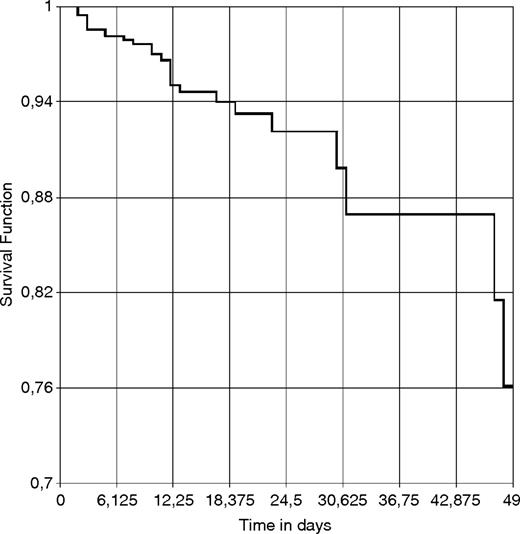
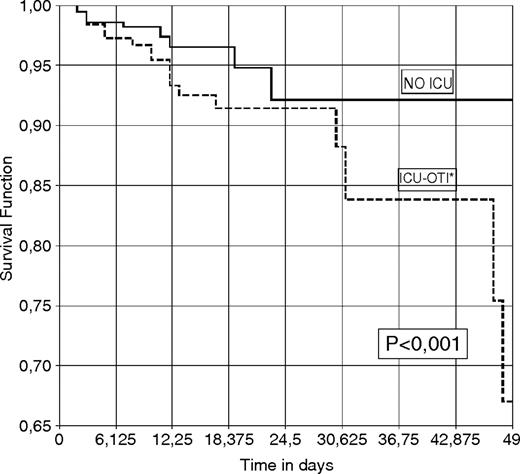
Figure 1 represents the estimated likelihood of surviving hospital admission for AWS. In Fig. 2, this likelihood is shown in function of whether the patient was transferred to the ICU and connected to mechanical ventilation or was not transferred to the ICU. There were no deaths among patients who were admitted to the ICU but were not intubated, which is why this possibility could not be represented in the graph.
Probability of surviving hospitalization for AWS up to 50 days post-admission.
Probability of surviving hospitalization for AWS up to 50 days post-admission in function of admission to ICU and connection to mechanical ventilation.
To obtain the best possible model of logistic regression, we used those factors that revealed themselves as significant in univariate analysis as well as those clinical variables that could impact the final result. The best model for multivariate analysis of the factors that conditioned death in patients with AWS included the following variables: severity of the underlying liver disease; the presence of delirium tremens at diagnosis; the existence of an underlying chronic pathology other than liver disease; and the need for orotracheal intubation, especially when intra-ICU pneumonia is added (Table 6). In order to evaluate the discrimination capacity of the model, an analysis was performed by means of an ROC curve, which yielded an area under the curve of 0.818 (95% CI 0.742–0.894).
Factors prognostic of death during hospitalization for AWS: multivariate analysis
| . | OR . | 95% CI . | P . |
|---|---|---|---|
| Underlying liver disease | 0.01 | ||
| No liver disease | |||
| Steatosis | 2.3 | 0.9–5.6 | 0.06 |
| Cirrhosis | 4.8 | 1.5–14.6 | 0.006 |
| Underlying pathology other than liver disease | 3.5 | 1.3–8.9 | 0.008 |
| Delirium tremens at diagnosis | 2.5 | 1–6.1 | 0.01 |
| Transfer to ICU | <0.001 | ||
| No ICU or ICU no intubation | |||
| ICU, yes OTI, no pneumonia | 2.9 | 1.1–7.9 | 0.03 |
| ICU, yes OTI, yes pneumonia | 8 | 3–21.3 | <0.001 |
| . | OR . | 95% CI . | P . |
|---|---|---|---|
| Underlying liver disease | 0.01 | ||
| No liver disease | |||
| Steatosis | 2.3 | 0.9–5.6 | 0.06 |
| Cirrhosis | 4.8 | 1.5–14.6 | 0.006 |
| Underlying pathology other than liver disease | 3.5 | 1.3–8.9 | 0.008 |
| Delirium tremens at diagnosis | 2.5 | 1–6.1 | 0.01 |
| Transfer to ICU | <0.001 | ||
| No ICU or ICU no intubation | |||
| ICU, yes OTI, no pneumonia | 2.9 | 1.1–7.9 | 0.03 |
| ICU, yes OTI, yes pneumonia | 8 | 3–21.3 | <0.001 |
OR, odds ratio; 95% CI, confidence interval at 95%; OTI, orotracheal intubation.
Factors prognostic of death during hospitalization for AWS: multivariate analysis
| . | OR . | 95% CI . | P . |
|---|---|---|---|
| Underlying liver disease | 0.01 | ||
| No liver disease | |||
| Steatosis | 2.3 | 0.9–5.6 | 0.06 |
| Cirrhosis | 4.8 | 1.5–14.6 | 0.006 |
| Underlying pathology other than liver disease | 3.5 | 1.3–8.9 | 0.008 |
| Delirium tremens at diagnosis | 2.5 | 1–6.1 | 0.01 |
| Transfer to ICU | <0.001 | ||
| No ICU or ICU no intubation | |||
| ICU, yes OTI, no pneumonia | 2.9 | 1.1–7.9 | 0.03 |
| ICU, yes OTI, yes pneumonia | 8 | 3–21.3 | <0.001 |
| . | OR . | 95% CI . | P . |
|---|---|---|---|
| Underlying liver disease | 0.01 | ||
| No liver disease | |||
| Steatosis | 2.3 | 0.9–5.6 | 0.06 |
| Cirrhosis | 4.8 | 1.5–14.6 | 0.006 |
| Underlying pathology other than liver disease | 3.5 | 1.3–8.9 | 0.008 |
| Delirium tremens at diagnosis | 2.5 | 1–6.1 | 0.01 |
| Transfer to ICU | <0.001 | ||
| No ICU or ICU no intubation | |||
| ICU, yes OTI, no pneumonia | 2.9 | 1.1–7.9 | 0.03 |
| ICU, yes OTI, yes pneumonia | 8 | 3–21.3 | <0.001 |
OR, odds ratio; 95% CI, confidence interval at 95%; OTI, orotracheal intubation.
Discussion
The main findings of this study are that the factors which determine survival after hospital admission for AWS depend both on the intensity of the clinical manifestations (DT, ICU) and the presence of associated comorbidity.
The death rate in our series (6.6%) is within the range of that observed in similar studies, where death rates of between 5 and 10% have been reported (Cushman 1987; Foy et al., 1997;,Horstmann et al., 1989), with a higher percentage of cases with alcohol withdrawal delirium, from general hospital units and with clinical comorbidity.
From a detailed analysis of the characteristics of patients with fatal outcomes in this study, several interesting findings can be highlighted: fatalities are concentrated almost exclusively within the alcohol withdrawal delirium group; over half of the deaths occurred in patients who had been admitted for diverse acute illnesses and developed DT subsequent to admission, and the majority of their pathologies are considered severe (sepsis, pancreatitis, multiple trauma, sigma volvulus, etc.); three out of every five died after being admitted to the ICU (all of them connected to mechanical ventilation); when the cause triggering death could be identified, in the majority of cases it was seen to be of infectious origin, principally intra-ICU pneumonia; the causes of death reveal a high frequency of multi-organ failure and respiratory distress in patients admitted to the ICU as well as a significant percentage of cardiogenic shock and sudden death.
The high concentration of AWS-related deaths in cases presenting DT has been described in previous studies (Ferguson et al., 1996; Salum, 1972; Tavel et al., 1961; Victor and Adams, 1953). It is reasonable to think that fatal outcomes of alcohol withdrawal must be associated with more severe states of deprivation. However, it is difficult to evaluate the role played by withdrawal itself in the survival and death rates of these patients. Initial clinical descriptions of the syndrome have described cases of death in the absence of other medical problems besides withdrawal and attributable only to alcohol deprivation per se (loss of fluids, hyperthermia, hypercatabolism) (Salum, 1972; Tavel et al., 1961; Victor and Adams, 1953). In contrast, the most recent studies point out that the majority of deaths occur once the withdrawal manifestations have been resolved, when the patient presents medical complications derived from treatment, hospitalization and comorbidity (Cushman, 1987; Foy et al., 1997;,Horstmann et al., 1989). The progressive improvement in treatment measures for these patients over the past few decades, combined with the advances achieved in critical patient care, has undoubtedly contributed to this change in trend.
Delirious states have been only observed within the first 10 days after last alcohol consumption in an alcohol detoxification unit (Driessen et al., 2005). In our study, many deaths occurred after >10 days of treatment. Although considering that up to 10% of patients may show longer duration of AWS symptoms (Victor and Adams, 1953) and a possible prolongation due to severe somatic disorders (Wojnar et al., 1999), our data show that mortality has been influenced by medical complications occurring subsequent to admission after the symptoms of withdrawal have abated.
Four cases of death from cardiogenic shock were registered, one of which was after a myocardial infarction. Alcoholic patients frequently present with varying degrees of cardiopathy related to their toxic addiction, ranging from a subclinical and therefore unknown condition to a diagnosed dilated myocardiopathy (Fernández-Solá et al., 2000; Kajander et al., 2001; Urbano-Marquez et al., 1995). There are several factors that may negatively impact this situation during withdrawal. On the one hand, the existence of a hyperadrenergic state can lead to hyperdynamism and an increase in oxygen intake (Abraham et al., 1985). Finally, DT treatment often requires an intake of energy-providing food and liquids, which, in turn, can overload cardiac function. All these factors may be associated and collaborate in the development of this sort of heart-related complications.
This same context could be used to explain the cases of sudden death in some of these patients (13% in the series). Our study does not include necroscopic studies, with the exception of one case of massive pulmonary embolism, which could have occurred during other episodes. Nonetheless, the analysis of these cases ruled out the existence of potentially fatal electrolyte alterations or other possible causes such as respiratory arrest due to sedation. The cause of these ‘sudden’ deaths could also be the existence of heart-related complications. Arrhythmias are very common in alcoholic patients, even in the absence of any underlying cardiopathy (Ettinger et al., 1978). The presence of malignant ventricular tachycardia in DT patients has also been described (Ballas et al., 1982), on occasion in conjunction with a transitory extension of the QT interval during withdrawal, which is a product of the excess of catecholamines released into the bloodstream (Cuculi et al., 2006).
The factors associated with the risk of dying from an episode of AWS in a general hospital are the variables relevant to the presence of comorbidity, to the intensity of its manifestations (DT) and to the need of ICU and mechanical ventilation, whereas this association is not seen with the clinical variables characteristic of withdrawal itself (seizures, adrenergic hyperactivity, etc.).
Thus, the existence of both chronic comorbidity, aside from liver disease, and of acute comorbidity, that is to say, being admitted for a pathology other than AWS, was associated with the risk of dying. Something similar occurs with the existence of underlying cirrhosis and the different ways of measuring its severity. This last finding coincides with that of a previous paper (Puerta Louro et al., 2006) and with another classic study on autopsies in patients suffering from DT, in which mortality risk is significantly associated with the presence of liver disease (Tavel et al., 1961).
The influence of medical complications on these patients’ survival deserves special mention. The few published studies on death in AWS patients have highlighted the following factors as determinative of death: hyperthermia, bacteremia and respiratory infection (Puerta Louro et al., 2006; Tavel et al., 1961). Our results revealed no differences either regarding body temperature (although it must be kept in mind that temperature data were collected during the first 24 h post-diagnosis and we do not have data about its subsequent course) or the presence of bacteremia. Neither did we find any correlation between the manifestation of respiratory infection or with pneumonia on the general hospital floors and the risk of dying. However, when intra-ICU pneumonia developed, it was significantly associated with an elevated risk of dying.
Patients with alcohol dependency prove to be especially vulnerable in the ICU. Morbi-mortality of alcoholic patients admitted to the ICU is up to four times higher than that of non-alcoholics, and they present a high incidence of respiratory distress and multi-organic dysfunction (Jenkinson et al., 1983; Moss and Burnham, 2003). Alcohol dependency has also been associated with the risk of presenting with sepsis and dying while in the ICU (O'Brien et al., 2007) as well as with the risk of needing mechanical ventilation (De Wit et al., 2007), a circumstance that, in turn, significantly increases the risk of developing nosocomial pneumonia (Celis et al., 1988).
The impact of the ICU on patients with AWS has been less studied. A recently published study indicated that mechanical ventilation was used in clinical practice in 47% of cases (Gold et al., 2007), which is less than in our series (70%). Our results show, like those of other studies (De Wit et al., 2007; Gold et al., 2007), that orotracheal intubation in patients with DT is related to the development of nosocomial pneumonia, increased time spent in the ICU and prolonged hospital stay.
Obviously, the need of ICU is clearly reflective of illness severity, so our data might be confounded by indication. Nevertheless, it has recently been shown that a therapeutic strategy of escalating the dosage of benzodiazepines and phenobarbital can reduce the need for mechanical ventilation, the time spent in the ICU and the incidence of nosocomial pneumonia in patients admitted to the ICU with DT (Gold et al., 2007). Although differences in mortality rate were not perceived, possibly due to the small number of cases, these findings show that the implementation of new therapeutic strategies can have a beneficial effect on the clinical course of these patients. In clinical practice, the transfer of a patient suffering from AWS to the ICU and the indication of IOT is a treatment measure that may sometimes depend on the skill and inclination of attending health care staff, the existence of specific treatment protocol, patient care and organizational problems within the hospital (i.e. number of health care staff on duty or call). It should be noted from our data that this is a factor in which medical staff can directly intervene, as the rest are related to the patient’s general health and basal characteristics (comorbidity, underlying liver disease and DT at diagnosis). So it might be reasonable to consider those therapeutic strategies that minimize, if possible, the impact of OTI in patients with AWS.
Our patients were treated with clomethiazole, which has been widely used in Europe in the treatment of AWS. Although its use is considered safe under strict medical supervision, it has some important side effects, including massive sedation and respiratory depression, especially when it is used intravenously and in combination with other sedative drugs (Morgan, 1995). In our series, two patients died of respiratory arrest due to sedation, which supposed one case per 270 treatments with clomethiazole. The importance of close monitoring in patients treated with this drug must be emphasized. Clomethiazole can cause other respiratory complications, mostly due to mucous congestion, leading to respiratory infections (Schied et al., 1986). It has been associated with pneumonia and prolonged intubation in ICU patients with AWS, although it did not influence survival in a randomized trial (Spies et al., 1996). Nevertheless, this point must be taken into account when analyzing the high incidence of ICU pneumonia described in our series, which, in turn, can determine the clinical outcome of patients with AWS.
Given that detailed analyses of the causes of death in AWS have been infrequently published, it is difficult to compare our data with series using drugs different from clomethiazole. Sometimes the treatment protocol is not specified (Puerta Louro et al., 2006) or is based on drugs that are not used at present, like paraldehyde (Tavel et al., 1961). Studies on similar populations that have used benzodiazepines do not specify the precise causes of death but report similar death rates to ours: 5 and 8.5%, respectively (Cushman, 1987; Foy et al., 1997). Both authors point out that the majority of deaths occurred once the delirious state had subsided and most patients had multiple medical problems.
Our data show a high proportion of patients with DT, suggesting rate of failure to contain the alcohol withdrawal syndrome. Although inadequate recognition and treatment could explain some of this, there are other considerations. Most patients admitted for AWS came from rural areas because of advanced symptoms and signs of alcohol withdrawal, many of them after an unsupervised voluntary detoxification. Also, we believe our patients are drawn from a population of extremely heavy drinkers, and are thus predisposed to the development of severe forms of withdrawal.
The retrospective nature of our study may have limited the quality of data collection (e.g. low frequency of smokers), although to palliate this bias as much as possible, all causes of death were established after prior review and consensus on the part of the research team. The long time span of the study period (16 years) may also have conditioned results in the sense that clinical care for AWS may have been modified during this period. Nevertheless, despite improvements implemented in critical patient care during this time span, the type of treatment and conditions related to health care staff attention on the hospital floors (number of medical and health care staff on the floor and on duty or call) have remained fairly constant during the period under study. Unfortunately, it was not possible to quantify the severity of symptoms according to the Clinical Institute Withdrawal Assessment of Alcohol Scale, revised (CIWA-Ar) scale, a validated, reliable scale for assessment of alcohol withdrawal severity (Sullivan et al., 1991), since it was not adopted in our center during the period of study. Nevertheless, although it is widely used to indicate when drug therapy is required and as an aid to assessment of withdrawal, the diagnosis of alcohol withdrawal and DT relies on DSM-IV criteria (Mayo-Smith et al., 2004).
In short, it is possible to identify with a good predictive capacity those patients at greater risk of dying following an episode of AWS in a general hospital. Patients with comorbidity, especially those with cirrhosis, cases with DT and those transferred to the ICU and subjected to mechanical ventilation, constitute a special risk group. We should center our efforts on improving diagnostic and treatment strategies for AWS in these cases, focusing our actions especially on those aspects of treatment that may be modifiable.
References
- alcohol withdrawal syndrome
- liver diseases
- liver cirrhosis
- alcohol drinking
- alcohol withdrawal delirium
- area under curve
- cause of death
- comorbidity
- habits
- health status
- intensive care unit
- pneumonia
- roc curve
- signs and symptoms
- spain
- terminally ill
- diagnosis
- mortality
- pathology
- orotracheal intubation