Abstract
A array of relevant cranial, postcranial, and dental morphologies are reviewed in an attempt to delineate shared derived features that would unite a group that includes extant humans and their fossil relatives to the exclusion of other hominoids. This group is now often referred to as tribe Hominini, but systematic practicality suggests that family Hominidae be retained, since the lower rank de facto limits even current, and certainly future, recognition of subclades. Potential hominid postcranial synapomorphies include a distinct angle at L5–S1, a long pubic ramus, a superoinferiorly short ilium that is roundedly expanded posteriorly, some thickening in the region of an iliac (crest) tubercle, a well-developed and knoblike anterior inferior iliac spine that lies noticeably superior to and somewhat back over the superior acetabular rim, a defined and deep greater sciatic notch, differential distribution of cortical bone of the femoral neck, anteroposteriorly long femoral condyles, and an outwardly slanted femoral shaft. Although “a weakly defined linea aspera” and “a concave rather than convex medial tibial condylar facet that lies level with the primitively concave lateral facet with the two facets being separated by a pair of distinct tibial tubercles” have been suggested as hominid apomorphies, this appears not to be the case – unless the apelike specimens commonly taken as hominid (e.g., from Hadar) are not. The only possible cranial feature appears to be alignment in the adult of the biporionic chord and basion (on the anterior margin of the foramen magnum). Derived dental features that might unite Hominidae also characterize an orangutan clade and thus must be explained away (e.g., as homoplasies) or dismissed as phylogenetically relevant in order to justify the former group. Of further note is the presence of Pongo clade-like facial features in australopiths and various specimens of Homo. These and the dental similarities suggest that focusing on Pan alone as the out-group from which to judge hominid-defining features is comparatively too narrow and, consequently, phylogenetically misleading. Within Hominidae, various subclades can be justified, suggesting that the relationships of various specimens referred to genus Homo lie within a clade that also subsumes “australopiths.” Much work remains before clade Hominidae can be more fully defined.
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
By the twenty-first century, one would think that paleoanthropology would long ago have left behind the legacy of Linnaeus’ (1735) ultra-vague and systematically useless definition of our species, Homo sapiens: nosce te ipsum (know thyself). Yet, in spite of the incredible number of discoveries of fossils attributed to our clade (i.e., the clade that includes H. sapiens but excludes apes) since the mid-to-late nineteenth century – when discovery of the Feldhofer Grotto Neanderthal and ultimately the Spy Neanderthals undermined the notion that humans were not antediluvian – the history of paleoanthropology contrasts with that of the centuries-old disciplines of vertebrate and invertebrate paleontology in its increasing rejection of taxonomic and systematic rigor. Consequently, the task of defining Hominidae is not as straightforward as one might imagine it should be.
Historical Background
In the first detailed attempt to support Linnaeus’ inclusion of humans within a specific group of mammals, and particularly in grouping humans with apes, Thomas Henry Huxley (1863b) sought evidence of this relationship not only in comparative hard- and some soft-tissue morphology but also in comparative development. Through ontogenetic comparison, he argued that if a monkey could be distinguished developmentally from other vertebrates as a mammal generally, and a primate more specifically, so, too, could humans. Hence, if a monkey was a primate, so, too, was a human. Huxley then turned to “man’s place in nature” within Primates. He began with the premise that humans were most similar to gorillas and then organized his comparisons first between gorillas and, when needed, other apes and then between them and monkeys. His rationale was if a morphological “gulf” existed between gorillas and monkeys, but not between gorillas and other apes, then humans could also be allied with the apes. But while Linnaeus claimed that, in essence, morphology barely distinguished humans from apes (Schwartz 1999), Huxley believed otherwise. Consequently, in spite of demonstrating that anatomies as distinctive as the human foot were basically comparable to the grasping feet of apes and monkeys, Huxley concluded that humans were still sufficiently unique to warrant their own taxonomic status apart from the great apes, all of which he relegated to a separate family. In hard-tissue morphology, Huxley remained as impressed by aspects of the postcranial skeleton as Aristotle had been of the human thumb and Johann Friedrich Blumenbach of the human pelvic girdle and foot.
With his emphasis on aspects of the human postcranium and dentition, Blumenbach should be regarded as the “father of paleoanthropology” inasmuch as the criteria he used to distinguish humans from other animals eventually became those that paleoanthropologists used to decide if a fossil qualified as a “hominid” (Schwartz 1999). Indeed, in 1795, in On the Difference of Man from Other Animals, Blumenbach (1969) emphasized various aspects of “the external conformation of the human body” as paramount to defining Homo sapiens: erect posture, broad and flat pelvis, two hands, non-divergent hallux, close-set and serially related anterior teeth, and some aspects of mandibular morphology.
With regard to erect posture, Blumenbach argued that, in contrast to other animals, this stance was natural and specific to H. sapiens as noted, for instance, in the ossification of tarsals before carpals. He claimed that only humans have a “true” pelvis, in which the broad and expanded ilia form a basin that cups the viscera. Like Aristotle, he regarded the human hand as special because of its long thumb. In addition, because of the uniqueness of the human foot, with its non-divergent hallux, Blumenbach believed that possession of only two “true” hands was significant. He also considered the human dentition distinctive in presenting orthally implanted lower incisors; canines not longer than, or separated from, the incisors; and molars with rounded cusps. He described the human mandible as quite short, bearing a prominent chin, and having a distinctive articulation with the skull (presumably referring to the depth of the articular fossa), which, he suggested, was correlated with human omnivory.
Although he recognized that humans differ from many mammals in lacking a distinct premaxilla, Blumenbach also, but mistakenly, believed that other primates were similar in this regard – a claim he then used to argue against separating humans from other primates taxonomically. [Goethe (1820) made a similar argument but did so on the incorrect belief that he could identify a premaxilla in humans.]
In addition to “the external conformation of the body,” and in keeping with concerns of eighteenth-century philosophers, Blumenbach (as well as Goethe) addressed the “internal conformation” of humans, i.e., the importance of reasoning (as well as other mental attributes) as a criterion by which to distinguish humans from other animals, including other primates. Although not stated in these terms, we might point to a focus on mental attributes as underlying the later emphasis in paleoanthropology on the size and external morphology of the brain [features that also attracted the attention of the eighteenth-century naturalists Buffon and Bonaparte (Schwartz 1987)].
Blumenbach’s criteria for distinguishing humans from other animals were imported into paleoanthropology with the discovery of Homo (Pithecanthropus) erectus from Trinil, Indonesia (Schwartz 1999). This historical twist is likely due to Huxley’s (1863a) argument that the Feldhofer Grotto Neanderthal was an extinct human whose cranial features extended into the past a “perceived” continuum of racial “brutishness” from the most “civilized” to the most “brutish” of living humans. In turn, these notions of ancientness equating with primitiveness and of a continuum that proceeded from a brutish primitiveness to human modernity came to inform much of paleoanthropology (Schwartz 1999).
The Trinil specimens, however, undermined Blumenbach’s H. sapiens-defining criteria. While the femur provided evidence of humanlike bipedalism (Blumenbach’s “erect posture”), the skullcap depicted an individual that had been less than fully human in its brain (and thus in its mental capacities). This unexpected combination of human and less-than-human features prompted Dubois to assign his new erectus first to the genus Anthropopithecus (the taxonomic alternative to Pan) (Dubois 1892) and then to Haeckel’s proposed genus for a hypothetical extinct, speechless human relative, Pithecanthropus (=“ape-man”) (Dubois 1894). The implication, of course, was that the emergence of erect posture and bipedalism preceded expansion and elaboration of the brain.
While lending itself to Darwin’s (1871) suggestion of a smooth transition from a semi-quadrupedal African ape to an erect bipedal human, this picture – bipedalism first, brain second – appeared contradicted with the discovery in the early 1900s at Piltdown, England, of a large, thin-boned, and rounded humanlike cranium; an apelike partial mandible preserving two molars; and an apelike lower canine. Under the presumption that these specimens were associated, the Trinil-based scenario of human evolution was turned around: early human relatives became human first in their brains and then in the rest of the body (as inferred from the mandible and teeth). That is, the brain enlarged prior to the attainment of fully erect posture and bipedal locomotion. It was not until the 1950s, when the Piltdown fraud was exposed, that this alternative notion of human evolution – brain first, body second – was rejected. Before then, however, the discovery of the Taung child and, more importantly, Dart’s (1925) interpretation of the specimen continued the intellectual trajectory Blumenbach had begun. But Dart conceived his scenario in the context of Darwin’s incorrect biogeographic premise of finding fossil evidence in Africa of intermediate forms that provided evidence of a morphological continuum between African apes and humans.
As Dart (1925, p. 196) summarized his overall impression of the preserved craniodental features of the Taung specimen, this individual represented “an extinct race of apes intermediate between living anthropoids and man.” Dart depicted specific features – such as the configurations of the brow, nasal bones, zygomatic regions, orbits, and upper and lower jaws as well as the inferred skull shape – as being of “delicate and humanoid character” (Dart 1925). Most central to his speculations were the size and potential details of the preserved partial endocast and also the forward position of the foramen magnum (as indicated by bone adherent to the endocast). Dart (1925, p. 197) assumed the latter was proof of this “humanoid’s” erect posture and then made the following extrapolations:
The improved poise of the head, and the better posture of the whole body framework which accompanied this alteration in the angle at which its dominant member was supported, is of great significance. It means that a greater reliance was being placed by this group upon the feet as organs of progression, and that the hands were being freed from their more primitive function of accessory organs of locomotion. Bipedal animals, their hands were assuming a higher evolutionary rôle not only as delicate tactual, examining organs which were adding copiously to the animal’s knowledge of its physical environment, but also as instruments of the growing intelligence in carrying out more elaborate, purposeful, and skilled movements, and as organs of offence and “defence”. The latter is rendered the more probable, in view, first of their failure to develop massive canines and hideous features, and secondly, of the fact that even living baboons and anthropoid apes can and do use sticks and stones as implements and as weapons of offence.
Regarding the Taung child’s brain (as represented by the endocast), Dart suggested that, since it was already as large as a chimpanzee’s and almost as large as a gorilla’s, it would have continued to enlarge, following a humanlike growth curve. In addition, as in humans but not apes, the Taung child’s brain was high and rounded, somewhat expanded in the temporal region, and apparently a posteriorly and inferiorly placed lunate sulcus. Believing his specimen to be more human- than apelike, Dart inferred that this “humanoid” had also been humanlike in its faculties of “associative memory and intelligent activity.” The expanded cerebral cortex (as indicated by the presumed lunate sulcus) also suggested to Dart that, in contrast to apes, the Taung “humanoid” had experienced increased sensory stimulation, both via vision (because of the forward position of the approximated orbits) and tactile sensation (because erect posture and bipedality supposedly freed the hands from involvement in locomotion). But the Taung child’s brain was not sufficiently enlarged in the temporal region for it to have reached the “necessary milestone in the acquisition of articulate speech” (Dart 1925, p. 198).
For Dart, the Taung child, the name bearer of his genus and species Australopithecus africanus, displaced both Piltdown’s Eoanthropus dawsoni and Trinil’s Pithecanthropus erectus as viable “links” between humans and their apelike ancestors. Indeed, in spite of Dart’s conceiving this extinct juvenile as intermediate between humans and apes, his interpretation reflected Blumenbach’s criteria for distinguishing Homo sapiens. In 1925, then, in spite of their differences, the three scenarios regarding human ancestry embraced the notion of an evolutionary continuum that proceeded from an apelike precursor, through an unknown series of intermediates, to the most modern looking of living humans.
In terms of the focus of this chapter – defining Hominidae – subsequent discoveries of potential extinct human relatives are less relevant than attempts to integrate these fossils into a systematic framework that had originally been based on living Homo sapiens. In this regard, after a decade-and-a-half of successful fossil hunting in the limestone caves of South Africa and in caves and deposits in Europe and Asia, and a proliferation of genus and species names, and debates over the relationships of what I will refer to as “australopiths” (based initially on South African specimens), Le Gros Clark (1940) was compelled to review the available evidence in order to determine the hominid status of any australopith.
In addition to echoing Huxley and Darwin’s assumption of a linear transformation from ape to human, Le Gros Clark based his conclusions on what he later called the “total morphological pattern” (Le Gros Clark 1955). From this perspective, he considered a fossil as being hominid not in terms of derived features it shared with humans but whether, overall, it resembled humans more than great apes. As will become obvious, this approach complicated matters further because the great apes were then considered evolutionarily united. Thus, a feature to compare with humans or potential hominid fossils could be extracted from any ape and deemed exemplary of the entire group, even if it only characterized the one ape [Gregory (1922) employed this device of “pick and choose” in arguing for an African ape-human relationship (Schwartz 2005)]. The irony of Le Gros Clark’s phenetic approach is that in 1955 he made one of the clearest statements about distinguishing between primitive and derived characters in generating hypotheses of relatedness.
Although Le Gros Clark (1940, p. 317) concluded that australopiths were “more human than simian” especially in their teeth, his comparisons then and in subsequent publications were biased toward the African taxa. If, however, he had included Pongo, he might have been struck by the similarities between this hominoid and australopiths in many details of facial and dental morphology (Schwartz 2004a, 2005). Perhaps then he might have expanded his comparisons to include at least small-bodied hominoids and some Old World monkeys, thereby providing paleoanthropology with the precedent of a more broad-based approach to phylogenetic reconstruction. Unfortunately, he did not, thus making his efforts to define Hominidae as useless as his definition of the order Primates [i.e., being characterized by their lack rather than sharing of derived feature/s (Le Gros Clark 1959)].
Mayr’s (1950) influential article on fossil hominids did not clarify the situation. Rather, on the grounds that all hominids were adaptively similar because they were bipedal, Mayr collapsed all named taxa into one genus, Homo. After claiming that because living humans are so diverse and occupy all available econiches the same had been true for all hominids (thus precluding the opportunity and prerequisite for speciation) (Mayr 1963), he subdivided his genus Homo into three time-sequential species: transvaalensis, erectus, and sapiens. Even though a much enlarged human fossil record later provoked Mayr (1953) to “accept” Australopithecus and acknowledge also Paranthropus, but as side branches that went extinct without issue, his concession did not elucidate how one determined in the first place if a specimen was hominid, especially if postcranial remains were unknown. This is, indeed, a problem. For while it may be true that some scholars (e.g., Le Gros Clark, Mayr, Washburn) decided “that the most important single factor in the evolutionary emergence of the Hominidae as a separate and independent line of development was related to the specialized functions of erect bipedal locomotion” (Le Gros Clark 1964, p. 14), the preponderance in the human fossil record of usually fragmentary skulls and jaws and isolated teeth makes impossible identifying a specimen as “hominid” on the basis of anatomical features believed to be reflective of bipedal locomotion. The cranial exception, of course, is the region of the foramen magnum and occipital condyles.
Toward a Definition of Hominidae
The task of defining Hominidae is twofold. First is a taxonomic decision. How expansive is the classificatory net Hominidae? To chimpanzees? Chimpanzees and gorillas? All great apes? Although Le Gros Clark (1955, 1964) wrote at a time when all great apes were relegated to the taxonomic family, Pongidae, his rationale for recognizing family Hominidae is, I believe, still viable and useful. Namely, Hominidae is a monophyletic group that subsumes extant humans and their fossil relatives, to the exclusion of any living relative. (It is in this sense that I use Hominidae/hominid/hominids throughout this contribution.) Accepting this proposition does not impinge on one’s preferred version of ape as closest living human relative. Further, it also allows more systematic space in which to accommodate the still taxonomically expanding human fossil record – which collapsing Pan and all hominids into genus Homo, on the grounds that this is “cladistic” (Goldberg et al. 2003), obviates. Indeed, the only thing “cladistic” about this, in the spirit of Hennig (1966), is translating a preferred scheme of relationship directly into a classificatory representation of it – which is not the same as generating the theory of relationship.
With this suggestion in mind, we can turn to the matter of defining Hominidae, but not from the perspective of looking for the “defining” moment in a transition from a presumed apelike condition to something seemingly hominid (either by a subtle hint of a supposed hominid trait or traces of a presumably primitive and retained feature). Rather, it seems logical and reasonable to return to Blumenbach’s list of criteria, to which other features have been added, as defining a clade that includes humans and their extinct relatives.
Defining Characters of Hominidae?
Traditionally Accepted Features of “Erect Posture”
As reviewed above, Blumenbach’s emphasis on “erect posture” and “two handedness” – or, as Le Gros Clark (1964, p. 14) put it, on “specialized functions of erect bipedal locomotion” – has remained central to considerations of our clade. Pilbeam (1972, p. 62), for example, summarized some of the “adaptations” apparently associated with these “specialized functions of erect bipedal locomotion”: a vertebral column with a distinct lumbar curve that is set at a sharp angle relative to the sacrum; a “carrying angle,” wherein the lateral femoral condyle is larger and more weight bearing than the medial condyle and the femoral shaft angles up and laterally away from the knee joint; a non-grasping foot with short toes and non-divergent hallux through which weight is transmitted during locomotion; and metacarpals in which the heads contact the substrate while the distal ends are elevated to form a springlike, transverse arch. We might also include Blumenbach’s description of the pelvic region as bowl shaped (i.e., broad and shallow) and having a short, potentially laterally flaring, posteriorly expanded, anteriorly truncated ilium; a somewhat forwardly oriented acetabulum (which is also reflected in the orientation of the proximal femur relative to the shaft); a defined greater sciatic notch; and a broad, short sacrum, wherein the alae are not remarkably small relative to the size of the lumbar facet (Schultz 1968). Clearly, these features distinguish living Homo sapiens from other extant primates. However, the degree to which these characteristics are expressed in what have been identified as fossil hominids, and whether the appropriate postcranial remains are known, is still up for debate.
For instance, among fossil specimens attributed to “anatomically modern” Homo sapiens that probably represent this taxon (Schwartz and Tattersall 2000a, b; Schwartz and Tattersall 2003, 2010), only Qafzeh 9 is known from a fairly complete, albeit extremely crushed, postcranium. Inasmuch as distortion of its skull and mandible compromises definitive identification of a bipartite brow with a “glabellar butterfly” and a mandible with a “true” chin with an inverted “T” configuration and thickened inferior symphyseal margin (Schwartz and Tattersall 2000a, b; Schwartz and Tattersall 2003, 2010), the pelvic region appears to present H. sapiens, not Neanderthal, morphology (Rak 1990) (personal observation). Other cranial and/or mandibular specimens conventionally attributed to “anatomically modern” H. sapiens and preserving critical morphology, especially Qafzeh 6 and all from Skhūl, do not present a bipartite brow or an inverted mandibular symphyseal “T” (Schwartz and Tattersall 2000a, b). Known Skhūl postcranial remains are incompletely representative and so crushed and poorly reconstructed that one can only sense their conforming to the abovementioned pelvic configurations (personal observations).
While Neanderthal postcranial morphology associated with bipedal locomotion differs from Homo sapiens in details of size, shape, and morphology [e.g., more posteriorly expanded ilia, superoinferiorly tall and anteroposteriorly compressed pubic symphyseal region, relatively long pubic ramus (and thus very wide/obtuse subpubic angle), smoothly “hook-shaped” greater sciatic notch, smaller and differently oriented iliac auricular region, relatively large proximal and distal femoral ends, very large acetabulum, truncated calcaneus (Rak 1990; Sawyer and Maley 2005; Trinkaus 1983; Trinkaus and Howells 1979)], they conform to the basic configurations summarized above, including a femoral “carrying angle” and a lumbar curve. Known Middle Pleistocene pelvic remains from Sima de los Huesos (Arsuaga et al. 1997), Arago (Day 1982) (personal observation), and Jinniushan (Rosenberg and Lu 1997) differ from Homo sapiens in some iliac details (e.g., flare, anterior superior iliac spine) but can otherwise be accommodated by Pilbeam’s pelvic criteria. The Sima de los Huesos femora also present a carrying angle (Day 1986).
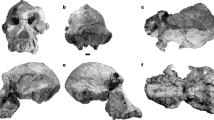
According to Rose (1984), os coxae KNM-ER 1808, KNM-ER 3228, and OH 28 are generally similar to Homo sapiens and H. neanderthalensis but differ in having relatively larger anterior iliac regions. Scrutiny of OH 28 (cast) reveals, however, that this specimen contrasts with H. sapiens as H. neanderthalensis does, e.g., in asymmetry of anterior versus posterior iliac proportions and curvature, thickness and anterior position of the external iliac pillar, thickness of the ilium posteriorly, and small size of the auricular surface (personal observation) (Fig. 1). Further, although Day (1986) claimed morphological similarity between OH 28 and Arago XLIV, the latter clearly differs not only in the massiveness of its ilium but in details such as a sigmoidally symmetrical and shallow anteroposterior iliac crest curvature, deep and uniform greater sciatic notch angle, large auricular surface, and huge acetabulum (personal observation) (Fig. 1). These inconsistencies raise questions not only about the suggested similarities between the pairs previously compared OH 28-Arago XLIV, KNM-ER 1808–3229, and Nariokotome KNM-WT 15000-Gona BSN49/P27 partial os coxae, but also about similarities claimed to exist among them all (Day 1986; Simpson et al. 2008; Walker and Leakey 1993).
Os coxae of Homo sapiens and various hominids with the ilium oriented laterally. Note differences in development of iliac pillar in H. sapiens, Arago XLIV, and OH 28, and virtual nondevelopment in “australopiths.” Also note differences in anterior and posterior iliac expression and especially in orientation of the acetabulum, which, if in the anatomical position, would reposition the ilium of all but H. sapiens posteriorly. See text for further discussion; not to scale; rev reversed (All specimens except H. sapiens and Sts 14 courtesy of the American Museum of Natural History)
A preliminary reassessment of the comparability of these OH, Arago, KNM, and BSN pelvic specimens based on casts and published images proves potentially interesting. For example, when orienting the acetabula in anatomical position, the “inner” iliac blade surfaces of all specimens face forward/anteriorly, as they do in Sts 14 and Al 288-1, and not medially, as in H. sapiens, H. neanderthalensis, and SK 50. Further, reconstructions of KNM-WT 15000 (e.g., as in the Neanderthal Museum) and the Gona pelvis, BSN49/P27 (Simpson et al. 2008), portray the respective ilia tilting laterally outward with their “inner” surfaces turning somewhat upward, albeit not as markedly as in some australopiths (see below, e.g., Sts 14, Al 288-1).
Sts 14’s superoinferiorly low and squat, posteriorly rounded and expanded, and clearly anteroposteriorly long ilia incorporate a well-defined greater sciatic notch (Figs. 1 and 2). In these features, the small Hadar AL 288-1 left os coxa, juvenile Makapansgat ilia MLD 7 and 25, Malapa UW-88-133, the virtually reconstructed Ardipithecus ARA-VP-6/500, and apparently the Swartkrans right partial os coxa SK 50 are similar to Sts 14 (Kibii et al. 2011; Lovejoy et al. 2009b) (see Figs. 1 and 2). Adult ilia Sts 14, AL 288-1, and likely also SK 50 differ from earlier-discussed specimens in being oriented more laterally outward than vertically, with concomitantly greater superior exposure of the internal iliac surface and more subdued “S”-shaped iliac crests; further, these specimens bear only moderately thickened iliac (crest) tubercular regions and poorly developed iliac pillars (Day 1986; Johanson et al. 1982; Robinson 1972). The virtual reconstruction of Ardipithecus portrays a more vertically and anteriorly oriented ilium, more open and poorly delineated greater sciatic notch (especially regarding defined posterior superior and inferior iliac spines), and a longer pubic ramus than the virtual reconstruction of Al 288-1 (Lovejoy et al. 2009b).
Right os coxae of Homo sapiens, Sts 4, and Malapa MH1 (UW-88-133) to illustrate, e.g., differences between the former and the latter two in details of the pubic and ischial regions and especially iliac superoinferior height and lateral flare. See text for further discussion; not to scale; reproduced to same size (Cast of UW-88-133 courtesy of the American Museum of Natural History)
Australopith anterior ilia present a dichotomy of morphology. In the better-preserved left os coxa of Sts 14, this region appears to be roundedly expanded anteriorly (Robinson 1972), as in StW 431 (Toussaint et al. 2003) and AL 288-1 (Johanson et al. 1982). Thus, a definitive anterior superior iliac spine cannot be identified. But in SK 50, even though its iliac crest is damaged along much of its length, what is preserved continues forward to become a well-defined, beak-shaped anterior superior iliac spine that projects markedly anterior to a bluntly thickened, almost knoblike anterior inferior iliac spine (Day 1986; Robinson 1972). The smaller Makapansgat juvenile ilia (MLD 7 and 25) are similar to SK 50 (but not to Sts 14 and AL 288-1) in having a projecting, beaklike anterior superior iliac spine (Dart 1957). Although differing from SK 50 and MLD 7 and 25 in the region of the anterior superior iliac spine, Sts 14 and AL 288-1 are similar to them in developing a knoblike anterior inferior iliac spine that lies noticeably superior to and back over the superior margin of the acetabulum, as in other potential hominids surveyed above.
Although australopiths have traditionally been interpreted as postcranially intermediate between knuckle-walking great apes and Homo – as noted, for instance, in their developing a humanlike posterior iliac expansion while supposedly retaining an apelike anterior iliac distension – this scenario seems inappropriate: not all specimens present similarly configured anterior superior iliac spines. Indeed, only SK 50 and MLD 7 and 25 compare favorably with great apes in having a beaklike anterior superior iliac spine that continues forward the trajectory of the iliac crest. The rounded anterior expansion of this region in Sts 14, AL 288-1, and apparently also in Malapa UW-88-133, while absolutely and relatively large compared to Homo sapiens and other possible fossil hominids, is, nevertheless, derived in its own right.
The preserved pubic rami of Sts 14 and AL 288-1 are reminiscent of this region in Neanderthals and the Jinniushan specimen in being relatively long, but the symphyseal regions of the australopith specimens are not also superoinferiorly tall and anteroposteriorly compressed (Rosenberg 1998). A superior view of the articulated Sts 14 pelvis illustrates (in contrast to Homo sapiens) the relation of the elongate pubic rami to the relatively wide pelvic canal and the relatively posterior positioning of the outwardly flared ilia (Robinson 1972). Further, while in H. sapiens (and other Pleistocene specimens surveyed above) the curve of the iliac crest positions the anterior superior iliac spine just lateral to the parasagittal plane intersecting the posterior superior iliac spine, in Sts 14, the anterior portion of the ilium would have been situated well lateral to the plane of the sacroiliac articulation (Robinson 1972). The known left os coxa of AL 288-1 was likely similar to Sts 14 (Johanson et al. 1982). Although SK 80’s iliac crest is similar to Sts 14 in not being strongly “S shaped,” when their iliac blades are oriented in the same plane, SK 80’s acetabulum is similar to H. sapiens in facing laterally and slightly downward (Robinson 1972). In contrast, the fairly vertically aligned Sts 14 acetabulum faces forward. When compared in anatomical position, the Sts 14 ilium is again more outwardly and obliquely oriented, while SK 50 is more anteroposteriorly arranged, as in H. sapiens and various other specimens attributed to Homo.
Although Malapa MH1 and MH2 are presented as having medially facing and vertically oriented ilia (Kibii et al. 2011), the largely complete right Australopithecus sediba ilium UW-88-133 (cast) can reasonably be oriented with more outward lateral flare (Figs. 1 and 2). This also appears to be the case with the reconstructed os coxa of Ardipithecus ramidus ARA-VP-6/500 (Lovejoy et al. 2009b) (see below).
Another feature of potential phylogenetic significance is the distance between the ischial tuberosity and the inferior acetabular lip, which is quite pronounced in great apes and apparently in catarrhines in general (Aiello and Dean 1999). The separation is marked in SK 50, shorter in Sts 14 and KNM-WT 15000, and minimal in Homo sapiens, in which a deep groove intervenes between the two structures. Perhaps further study of this region will prove enlightening, if not in defining features of clade Hominidae, perhaps in delineating a subclade/s within Hominidae.
Unfortunately, many details of the vertebral column in general are unknown. Of particular note is that while the lumbar region of KNM-WT 15000 is somewhat curved and angled inward at L5-S1, unlike H. sapiens, H. neanderthalensis, and large-bodied apes, this individual had six rather than five lumbar vertebrae (Walker and Leakey 1993).
Regarding femora, KNM-WT 15000, KNM-ER 1481 and 1472, Sts 34A, AL 129-1a, and UW 88-63 (casts) and D4167 (original) present carrying angles that set them apart from Sts 34B, AL 333-4, Trinil 3/Pith I, and Spy 1 (Table 1; see also Fig. 3) (Lordkipanidze et al. 2007; Walker and Leakey 1993). For the former seven specimens, medial distal femoral angles range between 103° and 110° and lateral angles between 70° and 77°; using Martin’s bicondylar angle [measured between the axis perpendicular to the distal articular plane and the lateral angle (Martin 1928)], the range of this sample is 13–20°. In contrast, distal femoral angles of the Sts 34B et al. specimens are lower, i.e., below 103° (medial), 77° (lateral), and 13° (Martin’s scale). Further, Sts 34A and Sts 34B also differ in size and detail of condylar shape and orientation (Table 1). As tabulated by Tardieu and Trinkaus (Tardieu and Trinkaus 1994), bicondylar angles for several living human populations (males and females pooled) range between 8.5° and 10.5° (Martin’s scale). The fact that two “groups” are distinguishable on the basis of distal femoral angle – with Homo sapiens falling into, or at least not with, one of them – raises doubt about the allocation to genus Homo of specimens preserving the distal femur (especially KNM-WT 15000, KNM-ER 1481 and 1472, and D4167) as well as specimens presenting pelvic as well as vertebral and other features not covered here that have been deemed australopith-like. Indeed, as will become clearer below, these inconsistencies redound, in broad perspective, on defining genus Homo on the basis of sapiens-like “striding bipedalism” (Wood and Collard 1999) (see also chapter “Defining the Genus Homo,” Vol. 3) and, more specifically, on assuming because of similar age and/or location, that specimens (e.g., those from Dmanisi) must represent not only the same taxon but a paleodeme of it that morphological difference represents not taxi diversity but merely individual variation (Lordkipanidze et al. 2013; Margvelashvili et al. 2013). In the case of the Dmanisi specimens, if, as the skulls and mandibles have been designated, D4167 represents H. erectus, then, at least with regard to distal femoral angle, there is no basis for taxonomically discriminating between any of the specimens discussed here where similar distinctions are demonstrable (see below).
Femora of Homo sapiens, TM 1513, Dmanisi D4167, KNM-WT 15000, and Sts 34B illustrating similarities in all but H. sapiens distally in bicondylar/carrying angle and proximally in lack of a distinct intertrochanteric line and posterior orientation of the lesser trochanter. See text for further discussion; not to scale (Copyright © J. H. Schwartz)
As Robinson (1972) long ago recognized, the distal femur presents much morphology of potential significance (see Fig. 4). For instance, as he saw it, when viewed from below, distal femora TM 1513 (left) and Sts 34 (right distal femur, now identified as Sts 34B) are generally similar to those of large-bodied apes in disparity and/or orientation of medial versus lateral condyle but differ in being deeper anteroposteriorly and more trapezoidal (mediolaterally narrower anteriorly) rather than rectangular in outline and in having a somewhat more concave and slightly asymmetrical patellar fossae, in concert with the lateral margin being more anteriorly distended than the medial margin (in other words, when viewed from below, the lateral condyle is anteroposteriorly longer than the medial, regardless of differences in configuration). Robinson’s enthusiasm notwithstanding, study of casts of these specimens reveals that while TM 1513 displays (slight) patellar fossa and condyle asymmetry, Sts 34B’s fossa is symmetrical and its condyles equally distended anteriorly (Table 1). Also, the curvature of the medial condyle is much more severe in TM 1513 than in Sts 34B, in which (uniquely for hominids studied here) it is “teardrop” shaped (Table 1). Interestingly, Sts 34B also differs markedly from Sts 34A in overall and specific distal femoral features – which is consistent with the craniodental nonuniformity of Sterkfontein fossils (Schwartz and Tattersall 2005).
Distal views of right femora of Pan troglodytes (=Pan trog.), Sterkfontein TM 1513, Homo sapiens, AL 333–4, KNM-ER 1472, AL 288-1a, and UW 88–63 (Malapa hominid 1) and left femora of KNM-ER 1482 and KNM-WT 15000. Note differences in, e.g., anteroposterior length, orientation and relative sizes of medial and lateral condyles, and relative depth and symmetry versus asymmetry of the patellar surface. Also note specific differences between AL 333–4 and AL 129-1a in, e.g., anteroposterior length, crispness of lateral patellar surface border, lateral epicondylar morphology, and divergence posteriorly of medial and lateral condyles; although AL 129-1a is damaged anteromedially, it is likely that this region would not have projected as far anteriorly as in AL 333–4. Of further note is that UW 88–63 resembles only KNM-ER 1481 and only in degree of asymmetry of the region of the patellar surface. See text for detailed discussion; not to scale; images reproduced to similar mediolateral width; r reversed (Except for TM 1513, all casts courtesy of the American Museum of Natural History)
Distal femora AL 129-1a and AL 333-4, of which the latter is the larger (McHenry 1986), also differ in morphological detail (Table 1). Lague (2002) acknowledges size and some morphological difference between the two specimens – especially the anterior projection of the medial margin of the patella fossa – but claims this merely reflects sexual dimorphic variation typical of extant populations of large-bodied hominoid species. If true, then a number of fossils that differ in ways similar to AL 129-1a and AL 333-4, or in other morphologies (Table 1), should be regarded as variants of the same subspecies. I am not suggesting that one ignore intraspecific variation, which includes sexually dimorphic differences. However, one can only address this topic after hypothesizing “groups” (e.g., morphs at least) within the morphological parameters of which individual variation can be assessed (Schwartz 2007b). Further, by focusing on general shape and outline, Lague overlooks differences between AL 129-1a and AL 333-4 not only in carrying/bicondylar angle but also in morphological detail (Table 1), beyond which are yet-to-be studied distinctions in, e.g., condylar side, epicondylar, epicondylar line, popliteal surface, and diaphyseal cross-section configurations (personal observations). Indeed, it appears that at present geometric-morphometric analyses are not capable of capturing the morphological details required of systematic analyses (e.g., see Lordkipanidze et al. 2013; see also Ulhaas 2007).
Turning to the proximal femur (Table 2), the most commonly reported “feature” is the femoral neck-shaft angle (measured inferiorly/medially at the intersection of the long central axes of the neck and shaft). As implied by DeSilva et al. (2013), Neanderthals display a moderate amount of variation and Homo sapiens considerable variation, the range of the latter encompassing virtually all other hominids. Gilligan et al. (2013) have argued that differences among sapiens populations in femoral neck-shaft angle are strongly correlated with differences in climate as well as in aspects of lifestyle, including types and use of clothing. Although neither the ontogeny of this angle nor its relation to the carrying/bicondylar angle has been studied, Gilligan et al.’s conclusion, at least with regard to the influence of lifestyle and clothing, is intriguing. For, if even partially correct, it suggests that the variability recorded for sapiens, and likely also for neanderthalensis, does not negatively impact the potential phylogenetic significance of the proximal femoral morphology of hominid species that were not geographically widespread and thus subject to disparate climates. Geographic and climatic restrictiveness also suggests that at least femoral neck-shaft angle differences are not due to climate-related clothing, if consideration of clothing of any sort is actually relevant. In other words, morphological variability in the species Homo sapiens, as well as in the species H. neanderthalensis, does not necessarily lead to the conclusion that variability is the sole explanation for difference among hominid specimens. Thus, while White (2003, 2012) remains steadfastly and zealously critical of paleoanthropologists who are “biased” toward identifying taxic diversity in the human fossil record, he is not exempt from bias toward explaining virtually all morphological difference as variation. Both claims should be regarded as hypotheses in need of testing (see also chapter “General Principles of Evolutionary Morphology,” Vol. 1).
Returning to the femoral neck-shaft angle, it is potentially noteworthy that non-sapiens/neanderthalensis specimens commonly regarded as Homo do not cluster together (Table 2). Rather, in this sample, the angle in the majority of specimens is 120°, in two it is 125°, and in most others it is 130°. Further, no “group,” even excluding those with 125° angles, conforms to any traditionally recognized hominid taxon or clade. Interestingly, Dmanisi D4167 has the lowest angle: 113°. The paucity of complete femora limits comparisons involving femoral shaft-neck and carrying/bicondylar angles, which, as seen, for instance, in D4167 (113°/15°), KNM-WT 15000 (120°/18°), KNM-ER 1472 (120°/13°), and Trinil 3/Pith I (120°/7°) (Tables 1 and 2) would appear informative for both systematic and locomotory deliberations.
Elsewhere, I (Schwartz 2007a) suggested that the posterior position/orientation of the lesser trochanter in South and East African “australopiths” and also KNM-WT 15000 (posterior) distinguished them not only from mammals in general but also from Homo sapiens, H. neanderthalensis, and from Orrorin, in which this structure points medially and is therefore visible when the femur is viewed anteriorly (Figs. 5, 6, and 7). Additional scrutiny of specimens (actual and casts) as well as review of the literature not only substantiates this observation but also demonstrates that, like KNM-WT 15000, many specimens allocated to Homo, including D4167 (Lordkipanidze et al. 2007), are “australopith”-like in having a somewhat-to-extremely posteriorly oriented lesser trochanter (Table 2; Fig. 6). These specimens are also “australopith”-like in presenting, for example, a relatively long, medially tapering, and somewhat anteroposteriorly compressed femoral neck; relatively small-to-moderate femoral head with little or no distension beyond the perimeter of the neck, especially proximally; and a weakly developed intertrochanteric line (see Table 2 for additional features).
Right proximal femora of Homo sapiens, Pan, and Macaca (top row, anterior; bottom row, posterior) illustrating typical primate/mammalian features, e.g., short neck, large rounded head, medially projecting lesser trochanter, and stout intertrochanteric line (arrows). See text for further discussion; not to scale (Copyright © J. H. Schwartz)
Proximal femora of various hominids: Dmanisi D4167 and casts of KNM-WT 15000, SK 97, and KNM-738 (top row, anterior; bottom row, posterior) illustrating atypical primate/mammalian features, e.g., long neck, small head, posteriorly projecting lesser trochanter (arrows), and weak intertrochanteric, spiral, and/or gluteal lines. See text for further discussion; not to scale (Copyright © J. H. Schwartz)
Since, as previously pointed out (Schwartz 2007a), some features seen in H. sapiens are typical of mammals, and essentially of all primates (e.g., Fig. 5), those that differ – as in “australopiths” and “australopith-like Homo” – are plausibly interpreted as derived or clade informative. In other words, such non-sapiens features would unite a group that consists of various specimens ascribed to Homo, to the exclusion of H. sapiens and sapiens-like hominids including, given preserved femoral morphology, Orrorin and Ardipithecus (see Tables 1 and 2). Consequently, because in the broad comparison “australopith”-like features emerge as derived relative to sapiens-like features, no “australopith”-like taxon (e.g., Australopithecus sediba represented by Malapa UW-88-04/05/39) should be considered “ancestral” to any specimens, and consequently taxa, that may constitute a Homo clade.
Further, as noted previously (Bartsiokas and Day 1993; Day 1973; Kennedy 1983), Trinil 3/Pith I (complete) and Trinil 6/Pith II (partial proximal) femora are morphologically dissimilar. More specifically, the former specimen presents sapiens- and the latter “australopith”-like features (Table 2). However, contrary to suggestions that all non-Trinil 3 (complete) femora constitute a group (Bartsiokas and Day 1993; Day 1973; Kennedy 1983), Trinil 3/Pith I (complete), Trinil 3/Pith III (shaft), and Trinil 9/Pith IX (shaft) are distinguished in diaphyseal shape and proximal morphological detail from Trinil 6/Pith III, and Trinil 8/Pith IV (shaft), which resemble each other morphologically (personal observations).
Regarding pedal morphology, foot bones attributed to Ardipithecus ramidus (Lovejoy et al. 2009c) and a specimen described as Ardipithecus-like (Haile-Selassie et al. 2012) lacked an arch, had fully opposable halluces, and did not “toe off” with this digit. Day and Napier (1964a, b) reconstructed the OH 8 foot bones with an arch and a first digit in alignment with the other digits; but restudy suggests that the hallux was probably semi-opposable and, by extension, there was an absence of an arch (Clarke and Tobias 1995). The latter two features characterized StW 573 (“Little Foot”) (Clarke and Tobias 1995). In contrast, tarsals attributed to Australopithecus afarensis (Latimer and Lovejoy 1990; Ward et al. 2011) and Au. sediba (DeSilva et al. 2013; Zipfel et al. 2011) are interpreted as presenting an arch, with other preserved pedal elements of Au. afarensis indicating a fully adducted hallux. Clearly, Pilbeam’s pedal features do not define a hominid clade.
To review, some features of the os coxa and femur, in contrast to at least other catarrhines, appear to distinguish a hominoid clade that we could call “hominid,” i.e., a long pubic ramus, a superoinferiorly short ilium that is roundedly expanded posteriorly, some thickening in the region of an iliac (crest) tubercle, a well-developed and knoblike anterior inferior iliac spine that lies noticeably superior to and somewhat back over the superior acetabular rim, a deep greater sciatic notch, a defined linea aspera, development of an obliquely oriented femoral shaft (producing a carrying/bicondylar angle), and a concave lateral tibial facet for the femur that is at the same level as the (also but primitively concave) medial facet, with the two facets being separated by well-developed tubercles. Within this potential clade, a subclade appears to be distinguishable even just on features of the proximal femur – e.g., long neck, posteriorly directed lesser trochanter, poorly delineated head/neck cervical region, and weakly defined intertrochanteric line – and another, which may be a subclade of the latter, in presenting a medially tapering neck.
More Recently Suggested Postcranial Features of “Erect Posture”
With the discovery at Kanapoi and Allia Bay, Kenya of specimens attributed to the species Australopithecus anamensis, Leakey et al. (1995) presented features of the proximal tibia (via right proximal tibia KNM-KP 29285A) they thought distinguished hominids from apes and, by extension, from other catarrhines. For example, in apes, the medial tibial condylar facet is convex and in posterior view situated slightly higher than the gently concave lateral condylar facet and, in frontal view, the shaft does not flare out smoothly and evenly to the margins of the proximal articular surface (i.e., the proximal articular surface extends especially medially and laterally farther than the respective margins of the shaft). Further in apes, the two condylar facets are separated by a single, blunt tubercle [see also Aiello and Dean (1999)]. In contrast, Leakey et al. suggested, in specimens attributed to “accepted” australopith taxa as well to species of Homo, both tibial condylar facets are at least somewhat concave, separated by a pair of well-defined tubercles and, in posterior view, level with one another. Further in hominids, in anterior view, the tibial shaft flares out to meet the medial and lateral margins of the proximal articular surface. Although these distinctions apply in general to apes and extant humans (in which the tubercles are actually variably distinct), they do not completely describe “australopiths” (Fig. 8).
Posterior views of right proximal tibiae of Homo sapiens, AL 288-1a, AL 129-1a, AL 333x-26, and KNM-KP 28295A and left proximal tibiae of Pan troglodytes (=Pan trog.) and KNM-ER 1481. See text for further discussion; not to scale; r reversed (Casts of AL 288-1a, AL 129-1a, AL 33x-26, and KNM-ER 1481 courtesy of the American Museum of Natural History)
For example, in right proximal tibia AL 288-1a (cast), the lateral condylar facet is slightly convex while the medial is slightly concave; viewed from behind, medial facet lies slightly below the level of the lateral facet (Fig. 8). Further, the apically indistinct tibial tubercles are separated by and incorporated into a mediolaterally wide blunt, loph-like structure that is anteroposteriorly obliquely oriented from the medial to the lateral side. Right proximal tibia AL 129-1b (cast) is similar to Al 288-1a in overall size, concaveness versus convexity and disparity in height of the condylar facets, and in having indistinct tubercles incorporated into a loph-like structure but differs in the shapes of the facets, a more posterior placement of a taller intertubercular loph, and in a different orientation of the shaft. In the large right proximal tibia AL 333x-26 (cast), the cuplike condylar facets are markedly concave (more so than any other suspected hominid) and, as seen from behind, lie at the same level; also the tibial tubercles are symmetrically distinct at their tips and incorporated into a tall loph that is mediolaterally oriented and situated farther posteriorly. As seen in a cast of the large right proximal tibia KNM-KP 29285A, which is part of the published hypodigm of Australopithecus anamensis (Leakey et al. 1995), the lateral condylar facet is faintly while the medial facet is a bit more obviously concave; the two facets are separated by indistinct tubercles that present themselves as a single blunt, high-rising structure that is posteriorly situated on the proximal surface. From behind, the posteriorly sloping lateral condylar facet lies slightly higher than the medial facet. Finally, in left proximal tibia KNM-ER 1481 (cast), both condylar facets are somewhat concave; when viewed from behind, the medial facet lies slightly above the level of the lateral facet. In all of these tibial specimens, KNM-KP 29285A included, the medial margin of the shaft flares out toward the proximal articular region below which the profile actually swells (either slightly as in KNM-KP 292895A, AL 288-1am AL 129-1b, and H. sapiens or noticeably as KNM-ER 1481 and AL 333x-26). Also in these specimens, with the notable exception of H. sapiens, the lateral border of the shaft flares out slightly before terminating below the proximodistally relatively tall, somewhat squared-off overhang of the lateral portion of the proximal articular region with which the fibula articulates (Fig. 8). Interesting, in H. sapiens, the medial and lateral borders of the shaft fan out more or less symmetrically as they approach the proximal end with greater swelling occurring on the lateral rather than the medial side.
As is evident even from this small tibial sample, the possession of a concave lateral femoral condylar facet that lies level with the medial facet, a pair of apically distinct tubercles, and a more symmetrically flared proximal shaft does not fully characterize Homo sapiens (and H. neanderthalensis), and they certainly do not a hominid make. Indeed, the only tibial feature Leakey et al. (1995) discussed that could distinguish a hominid from an ape is the flaring and swelling of the medial profile of the shaft as it converges upon the proximal articular region – which, in its more swollen state would represent a more derived configuration. Features of the proximal tibia not detailed here (e.g., symmetry/asymmetry of condylar facet shape, orientation of condylar facets, configurations of intercondylar facet depressions, height of tubercular region) are also consistent with the picture of nonuniformity not only among this sample of “australopiths” but also among specimens from Hadar, among which the distinctiveness of AL 333x-26 alone makes clear that “australopith” systematics is far from being resolved (for another perspective, see chapter “The Species and Diversity of Australopiths,” Vol. 3). Further, proximal tibial as well as femoral morphology (see above) confirms observations based on dental morphology (Schwartz and Tattersall 2005), namely, that the Hadar specimens encompass more than one taxon . At a higher taxonomic level, since various “australopiths” retain primitive tibial features – e.g., convex medial condylar facet, medial facet higher than lateral facet, indistinct tibial tubercles, and asymmetrically flared proximal shaft – features Leakey et al. put forward as defining hominids appear instead to unite a subclade or subclades within it.
Since tibial and femoral morphologies reflect aspects of locomotion, the differences noted here between specimens suggests, as pedal morphology has begun to indicate, that “bipedalism” was enacted differently among different species or subclades of hominid (see also chapter “Origin of Bipedal Locomotion,” Vol. 3). Since no Hadar specimen is dentally comparable to the type specimen of Au. afarensis, Laetoli hominid (LH) 4, and all Laetoli specimens can reasonably be allocated to the same dental morph (Schwartz and Tattersall 2005), it would be interesting to know the postcranial morphology of the hominid – that is, of Au. afarensis – that left the footprints. Further, the singular degree to which the tibial condylar facets of AL 333x-26 are depressed and cuplike not only reflects a uniquely derived configuration but also likely a unique type of hominid locomotion.
In their description of the Lukeino proximal femur (BAR 1002’00), Pickford et al. (2002) commented on how slight the linea aspera is compared to specimens of australopiths and Homo (because the spiral line of BAR 1002’00 does not meet the gluteal/3rd trochanteric line to form a linea aspera of high relief). I agree with this general description (personal observation; Fig. 7). They (p. 202) then suggested, because apes do not present a muscle scar descending from the region of the gluteal tuberosity, that such “a precursor of the linea aspera” is a hominid-identifying feature related to bipedalism. A review of casts of some femora identified as australopith or Homo reveals, however, a more complex picture (Table 2). For instance, KNM-WT 15000, the supposedly first humanlike striding biped, lacks a spiral line altogether. Other specimens differ in presence and degree of expression of a gluteal line (Table 2), as well as in where each line, if it is identifiable, emerges relative to the associated trochanter and side of shaft; how and where they converge to form/not form a linea aspera; the shape and surface morphology of any supra-linea aspera triangle these lines might delineate between them; where on the shaft their merging produces a linea aspera; and the degree of development and distal course of the linea aspera (personal observation).
Pickford et al. (2002) also mentioned that in BAR 1002’00 the lesser trochanter is medially projecting and, as in humans, gorillas, and Pongo, also well separated from the femoral neck. Regarding the latter feature, images in Pickford et al. as well as, for instance, in Robinson (1972), Day (1986), Johanson et al. (1982), and Walker and Leakey (1993) illustrate that while notable separation of lesser trochanter and femoral neck may describe specimens such as SK 82 and 97, Sts 14, AL 288-1, OH 62, KNM-WT 15000, and KNM-ER 738, 1503, and 1547, this configuration does not characterize Homo sapiens (or, e.g., Pan). Perhaps this disparity is due to differences in femoral neck length – which might imply that separation of lesser trochanter and femoral neck is phylogenetically significant not for, but within, the clade Hominidae. As discussed above, the medial orientation in BAR 1002’00 of the lesser trochanter, although similar to Homo sapiens, is also broadly descriptive characteristic of primates (Swindler and Wood 1973) (personal observation; see above). In contrast, in specimens such as AL 128-1, 288-1, AL 333-95, AL 333-3, Maka VP 1/1, SK 82 and 97, OH 62, KNM-WT 15000, and KNM-ER 738 and 1547, the lesser trochanter is more posteriorly than medially if not strongly posteriorly directed (Table 2).
As contemplated above, interpretation of these different configurations is not straightforward. For instance, most mammals present a medially or more medially-than-posteriorly (i.e. medioposterior to posteromedial) oriented lesser trochanter. Based on commonality, the possession of this in apes and Homo sapiens could reasonably be interpreted as a primitive retention – in which case configurations to the contrary would be derived and thus potentially reflect a hominid subclade. On other hand, albeit less parsimoniously (but why should parsimony always dictate interpretation?), the apparent widespread distribution among possible hominids of a posteriorly oriented lesser trochanter (and other features as well) might be primitive within a hominid clade – which implies an “independent” development of the primitive mammalian condition in H. sapiens. Since, however, postcrania definitively associated with crania and teeth are rare indeed, the former interpretation might be the more likely.
Another feature that Pickford et al (2002; also Galik et al. 2004) suggested unites Orrorin via BAR 1002’00 with australopiths and at least Homo sapiens is differential distribution of femoral neck cortical bone, being thinner superiorly and thicker inferiorly (Ohman et al. 1997). Taxically broader study is required.
Non-postcranial Features of “Erect Posture”
Although other postcranial features might delineate clade Hominidae, I will turn now to another skeletal region from which “erect posture” or “bipedal locomotion” has been inferred: the cranial base. For ever since Dart’s (1925) discussion of the Taung child, an anteriorly placed foramen magnum with attendant occipital condyles has been central to the identification of hominoids as bipedal hominids. With Ardipithecus ramidus (White et al. 1994) and Sahelanthropus tchadensis (Brunet et al. 2002) being promoted as potential hominids, attention to basicranial morphology is crucial.
Interpretation of Ardipithecus (White et al. 1994) and Sahelanthropus (Brunet et al. 2002) as “hominid” was in part based on White et al.’s inference of bipedalism from the intersection of the bicarotid (foramen) chord and basion (=the anthropometric landmark in the midline of the anteriormost margin of the foramen magnum). The published photograph of the crushed and distorted basicranium of Sahelanthropus reveals, however, that what appears to be the anterior margin of the foramen magnum lies posterior to the bicarotid chord. It is obvious that in the undistorted state, the preserved left petrosal and thus the bicarotid chord were situated more anteriorly. Consequently, a forward position of the foramen magnum is not indicated.
Although not demonstrated, White et al.’s claim of an association between basion (i.e., the anterior margin of the foramen magnum) and the bicarotid chord, an anteriorly placed foramen magnum, and erect posture and bipedalism would seem intuitively reasonable. However, comparison of other potential fossil hominids, extant large-bodied hominoids, and various extant New and Old World monkeys, reveals a more complex picture (Schwartz 2004b). In juvenile anthropoids, including humans, basion, the bicarotid chord and the biporionic chord are essentially in alignment. This relationship is retained into adulthood in some anthropoid taxa, but, in others, during growth, the positions of basion and/or the bicarotid chord may change position relative to the biporionic chord. Consequently, it is the biporionic and not the bicarotid chord that better reflects the position of the foramen magnum. The alignment of basion and the two chords in the adult is, therefore, a neotenic feature that, while not defining clade Hominidae (Schwartz 2004b; Schwartz and Tattersall 2005), may be relevant to delineating relationships within it. More recently, in the context of comparison between an unspecified sample of extant catarrhines and extant sapiens, Kimbel et al. (2013) argued the hominid status of Ar. ramidus on the basis of reconstructing cranial base width and anterior foramen magnum position from missing basicranial elements in ARA-VP/500. Nevertheless, until the ontogeny of this general region in a diversity of anthropoids is understood, comparisons only between adult specimens are – as demonstrated above vis-à-vis ontogenetic changes – uninformative.
Proposed Craniodental Features of Being Hominid
Based on isolated teeth attributed to Ardipithecus, White et al. (1994) suggested that, in lateral profile, a hominid’s permanent upper canine should present subequally long and quite divergent mesial and distal edges terminating in “shoulder-like” basal swellings that create the impression of a superoinferiorly short crown. This does not, however, describe the permanent upper canine (C\) of Sahelanthropus (Brunet et al. 2002) or the majority of C1s of traditionally accepted Plio-Pleistocene and later hominids, including Homo sapiens (Schwartz and Tattersall 2005). Rather, White et al.’s description better captures the morphology of the C\s of adult female orangutans and the deciduous upper canines of juvenile orangutans and chimpanzees (Swarts 1988).
White et al. (1994; also Suwa et al. 2009) also described Ardipithecus’ C1 as “incisiform” but from the belief, also shared by Brunet et al. (2002), that a C1-C1-P1 honing complex had been lost in hominids via a decrease in size and projection of these teeth (particularly the canines) in concert with closure of presumed attendant diastema. According to this scenario, as canines became less caniniform, they became associated functionally with the incisors, ultimately assuming the morphology and function of these spatulate anterior teeth. Yet, both the right C1 allocated to Ardipithecus and the C1 in the skull of Sahelanthropus, although differing in buccal profile triangularity, are apically pointed. Indeed, Sahelanthropus’ trenchant C1 would likely have occluded with a much more “caniniform”-looking C1 than the very unprimatelike isolated element identified as this tooth (Schwartz 2004b). Although it appears from teeth in situ that Sahelanthropus lacked upper diastema, their absence is only inferred for Ardipithecus from isolated teeth. Nevertheless, if Ardipithecus did not have diastema, both it and Sahelanthropus would be more derived than geologically younger (and supposedly descendent) specimens with diastema [e.g., AL 200-1a and StW 252 (Schwartz and Tattersall 2005)] as well as the maxilla from Bouri Hata allocated to Australopithecus garhi [see photographs in Asfaw et al. (1999)]. Consequently, features associated with “reduction of a canine-premolar honing complex,” while not defining clade Hominidae, may delineate subclades within it.
Another approach to defining Hominidae is predicated on an a priori assumption of a human-chimpanzee relationship, which immediately constrains the hominid “out-group” to this single large-bodied hominoid. Thus, although White et al. (1994, p. 306) describe the dm1 of Ardipithecus as “apelike,” the only ape in their comparison is Pan . Yet, Hylobates and Gorilla dm1s are also very similar to Pan dm1s, while Pongo dm1s are most similar to those of traditionally accepted hominids, the major difference being more talonid cusp compression in the orangutan (Schwartz 2004b; Swarts 1988). Thus, Pongo , “australopiths,” and Homo dm1s conform best to White et al.’s (1994, p. 307) depiction of “apparently derived hominid features,” i.e., “buccolingual crown expansion, mesiolingually prominent metaconid, well-defined anterior fovea, and large talonid with well differentiated cusps” (White et al. 1994). In fact, the dm1s of Pongo and hominidsFootnote 1 are similarly derived relative to other extant hominoids (Schwartz 2007b; Schwartz and Tattersall 2002, 2003, 2005): e.g., the protoconid is more mesially situated; the less vertical and lingually facing anterior fovea (= trigonid basin) is noticeably smaller than the talonid basin and is enclosed by a distinct paracristid that courses somewhat mesially and then turns toward the lingual side; and the more horizontally oriented talonid basin is enclosed by a distinct hypocristid (Swarts 1988) (personal observation).
Only if Pongo (and members of its clade for which dm1s are currently unknown but for which this description would be predicted as applicable) is not the sister taxon of a potential hominid clade would these dental features be relevant to defining only clade Hominidae.
More broadly, Irish and Guatelli-Steinberg (2003) claim to have delineated features characteristic and also distinctive of the last common ancestor of clade Hominidae and also demonstrated that recent sub-Saharan Africans most closely resemble various Plio-Pleistocene hominids in these features. Their study is based on the ASU dental trait scoring system established decades ago by Turner et al. (1991) (see chapter “The Dentition of American Indians: Evolutionary Results and Demographic Implications Following Colonization from Siberia,” Vol. 3). Although seemingly objective (e.g., in matching individual dental features of a specimen with plaques of dental casts varying degrees of expression of that trait), the Turner scoring system has no relation to the terms and descriptive protocol that inform the study and systematic interpretation of mammalian teeth. Rather, Turner’s system focuses instead on predefined minutiae and often idiosyncratically defined variation. Thus, although hominids are toothed mammals, Turner and scions have so ignored the conventions of mammalian dental terminology and description that a mammalian systematist cannot understand or even divine the morphology of the teeth subjected to the ASU scoring system. Indeed, terms such as “lower molar deflecting wrinkle” and “lower molar seventh cusp” or referring to a (huge) protostyle as an element of a “twinned hypocone” demonstrate just how far from biological/systematic practice paleoanthropology is. In short, coding for one trait after another and their presumed degrees of expression informs neither about the relations of structures to one another nor, more importantly, about the shape of a tooth and the relative expressions of as well as the spatial relationships of the details of occlusal morphology. Further, rather than testing hypotheses of which specimens might represent which morph/taxon, these analyses begin by accepting prior taxonomic allocation of specimens [e.g., (Bailey 2002, 2004; Bailey and Wu 2010; Trinkaus et al. 2000)].
Unfortunately, the emphasis of the ASU approach on scoring individual traits with a focus on degrees of trait expression (e.g., larger, larger, small, smaller; distinct, more distinct, less distinct) rather than on their relationships informed the dental “study” of specimens identified as Australopithecus sediba (Irish et al. 2013). Although rooting their analysis in a gorilla out-group – without understanding that gorillas are unique among large-bodied hominoids in aspects dental morphology (Schwartz 1986) – Irish et al. (Irish et al. 2013) conclude that Au. sediba is dentally distinct from other “hominids” but similar in a phylogenetically meaningful way to their sample of Au. africanus and an assumed Homo habilis/heidelbergensis + H. erectus group. The latter larger grouping is based on four features, including the vague “identical LM1 cusp 7 expression.” Their conclusion, in conformity with interpretation of cranial and postcranial morphology, is that its “mosaic” of presumed primitive australopith and derived Homo features makes Au. sediba a good ancestor of genus Homo.
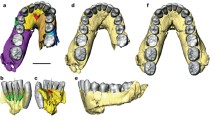
Although the taxonomic distinctiveness of Au. sediba has been challenged on the grounds that the skull upon which the species was based is that of subadult – and thus its adult morphology could be comparable to known specimens of australopith (Strauss et al. 2012, 2013) – tooth and mandibular morphology provide less speculative clues to understanding this hominid. Indeed, comparison with all known australopiths demonstrates that Au. sediba is remarkably similar to Kromdraai TM1517b, the mandibular holotype of Paranthropus robustus (Fig. 9).
Occlusal views (left to right) of right mandibles of Malapa hominid MH 2 (UW-88-54-128-129), Kromdraai TM1517b (type, Paranthropus robustus), and MH 1 (UW-88-8), illustrating numerous dental similarities, size being the major difference. See text for further discussion; not to scale but to relative size (Casts of MH 1 and MH 2 courtesy of the American Museum of Natural History)
For example, although the mandibular corpus of MH1 (UW-88-8) is more gracile, shorter superoinferiorly, and more swollen below the alveolar region – which may be because of its being subadult – both it and TM1571b are otherwise similar, including presenting two (top and bottom) mental foramina below P1. Further, in both specimens, the anterior root of the ramus originates below the region of M1-2, masking M3 entirely, and then swells only modestly before becoming crest-like as it ascends, inclining a bit backward. UW-88-129 presents a moderately steep postincisal plane, as also seen in TM1517b. Damage and incomplete restoration of TM1517b preclude assessing C1 orientation, but this tooth in UW-88-8 was relatively larger. Preserved alveoli in UW-88-8 suggest that the missing P1-2 were similar to TM1517b in shape: i.e., P2 was larger, especially in a distended hypocone region such that the buccal side of the tooth was mesiodistally somewhat shorter, while the buccal side of P1 was marginally but still visibly longer than the lingual side. The P2 of UW-88-129, although also smaller, is morphologically comparable to TM1517b. The M1s of the smaller UW-88-8 and TM1517b are otherwise distinctively similar, especially in the oblique orientation of the distal part of the crown, the presence of notches between buccal cusps, and a buccal cingulid on the protoconid. Further, for example, in both UW-88-8 and the TM1517b, M1-2 the hypoconids are compressed, they bear hypocristids, and their bases extend lingually across the midline of the crown, terminating approximately in the midline in a mesiodistally relatively long and internally squared-off end; also, small hypoconulids angle in toward the middle of the tooth, following the generally oblique orientation of their distal sides of the crowns. A groove on the internal face of the M1 metaconid base of TM1517b produces a “pillar-like” feature, which is seen on UW-88-8’s M2. Although the mesial part of TM1517b’s M2 is partially reconstructed, the distal extremity of a definitive protoconid buccal cingulid is clearly evident abutting the mesial side of this tooth’s hypoconid even in the presence of a notch between these two cusps. Similar morphological detail is noted in UW-88-8. Further, the M3s of TM1517b and UW-88-8 are generally similar in size and shape and also in presenting buccal cusp notches (also in UW-88-55a), distinct buccal cingulids on the distal part of the protoconid, a slight buccal enamel swelling below the hypoconulid, a distobuccally obliquely oriented metaconid base, and mesiodistally short trigonid basins confined to the inner portions of protoconid and metaconid bases.
If, as appears to be the case, these specimens represent the same hominid, they should be regarded as Paranthropus robustus, with the Malapa specimens’ smaller size likely reflecting sexual dimorphism: i.e., MH1 was female, not male (Berger et al. 2010). Since, however, dentally and even in bony morphology the partial face TM 1517a is relatable to a morph that includes the Taung child, and which therefore must regarded as Australopithecus africanus (Schwartz and Tattersall 2005; Fig. 10), only the lower jaw from Kromdraai TM1517b represents the holotype of P. robustus. In this regard, future comparisons of the upper dentition of MH1 with those of other “australopiths” should prove enlightening in sorting out which specimens actually do represent this taxon. Certainly, we now know a lot about the postcranial skeleton of this hominid.
Comparison of the Taung child specimen, the type of Australopithecus africanus, and a few other South African specimens that present similar facial and dental features of as well as variation within the Taung morph. Note that TM 1517a, which Broom referred to the holotype of Paranthropus robustus, compares favorably with this morph. Not to scale (Copyright © J. H. Schwartz)
Additional Defining Characters of Hominidae
Although some obviously derived features when considered in isolation might delineate clade Hominidae, they are also shared with Pongo and its potential extinct relatives. Consequently, in order to claim them as strictly hominid apomorphies, and their presence in an orangutan clade as autapomorphic for it and non-synapomorphic with hominids (i.e., features seen in hominid and orangutan clades are homoplastic), one must first embrace another theory of extant large-bodied hominoid relationship and then “explain away” the phylogenetic significance of the similarities between humans and orangutans, which is often done merely by declaring them homoplastic (e.g., Collard and Wood 2000, 2007; Diogo and Wood 2011; Lockwood and Fleagle 1999; Wood and Harrison 2011) as if homoplasy were identifiable without first presuming a theory of relationship based on characters deemed synapomorphic for other taxa (see discussion in Schwartz 2008; see also chapter “Homology: A Philosophical and Biological Perspective,” Vol. 1).
This, of course, is the current state of affairs in paleoanthropology, wherein the molecular similarities between Pan and Homo are taken as evidence of their close relationship (e.g., see Lockwood et al. 2004; Pilbeam 1986, 2000; Gibbs et al. 2000, 2002; Wood and Harrison 2011), even though most of the “favorable” comparisons involve only small portions of the 2–3 % coding (i.e., metabolically, not developmentally relevant) region of the genome and rarely include the orangutan and an array of other catarrhines (e.g., Grehan and Schwartz 2009; Grehan and Schwartz 2011; Schwartz 2009, 2011, 2012; Schwartz and Maresca 2006). Although Wood and Harrison (2011) assert there is also “overwhelming…morphological evidence for a ((Pan, Homo) Gorilla) Pongo) pattern of relationships” and that features suggesting a Pongo-Homo sister group were “selected” to do so (p. 470), neither of these statements is correct, especially, “overwhelming-morphological evidence for with regard to a ((Pan, Homo) Gorilla) Pongo) pattern of relationships.” Although Groves (1986) is consistently cited as having demonstrated a close human-chimpanzee relationship, scrutiny of his data demonstrates not only that most of the features he presented in support of this contention are not synapomorphic because they are found in other primates listed (Grehan and Schwartz 2009; Groves 1986; Schwartz 1988, 2005) but, more importantly, that numerous features are either ambiguously or incorrectly reported (Grehan and Schwartz 2009).
Unfortunately, Groves’ work has been reiterated without question in subsequent publications claiming demonstration of a Homo-Pan relationship (e.g., see especially Lehtonen et al. 2011; Strait and Grine 2004; Collard and Wood 2000; Gibbs et al. 2000, 2002). Further, in light of Wood and Harrison’s claim of “overwhelming…morphological evidence for a ((Pan, Homo) Gorilla) Pongo) pattern of relationships,” Wood’s own work (Collard and Wood 2000; Gibbs et al. 2000, 2002), which was based in large part on Groves (1986), delineated essentially no hard-tissue and only a handful of soft-tissue features in support of a human-chimpanzee sister group. With regard to the criticism that features in support of a human-orangutan sister group were selected to demonstrate this, scrutiny of the relevant publications reveals that in all cases the comparative morphological, physiological, and developmental data cited was available in the literature (Grehan and Schwartz 2009; Schwartz 1983, 1988, 2004a, 2005). When new data was introduced, it was spelled out and, as with all data, presented for an array of primates, not just humans and large-bodied hominoids (Grehan and Schwartz 2009; Schwartz 1983, 1988, 2004a, 2005). In contrast to Groves’ claiming Homo-Pan synapomorphy not only in the rare instances when only these two hominoids shared features but more frequently when other primates shared features present in humans and chimpanzees, I hypothesized synapomorphy only when two taxa exclusively shared a feature. Consequently, in every publication, I presented all alternative theories of relationship, including Homo-Pan, even though they differed significantly in degree of robustness (= corroboration). The fact of the matter is, even if one used Groves’ data without eliminating ambiguous or incorrect data, and hypothesized synapomorphy on the basis of exclusivity of shared features, Homo-Pan emerges as the least and Homo-Pongo as the most highly supported theory of relationship (Schwartz 1988). With the addition of more features to the data base, and in the context of a broad comparison among primates, the same pattern of relationship emerges (Grehan and Schwartz 2011). If one were to cast the stone of “selectively” choosing features to support a particular human-ape theory of relationship, it would have to be toward those who maintain a Homo-Pan sister group through reiteration of faulty morphological datasets and ignore additional data that might lead to a conclusion other than their preferred conclusion.
Nevertheless, even if the latter phylogenetic hypothesis were true, this does not justify using Pan alone as the referent for defining clade Hominidae or for determining character polarity within this clade (e.g., see Asfaw et al. 1999; Brunet et al. 2002; Guy et al. 2005; Lovejoy et al. 2009b, c; Suwa et al. 2009; White et al. 1994, 2006; Wood and Harrison 2011). Rather, the broad comparative approach that Le Gros Clark might have pursued remains the more reliable way in which to hypothesize features that could distinguish a hominid from other primate clades and also determine character polarity within it. And, in doing so, one must also confront instances when humans, or hominids in general, compare more favorably with primates other than Pan, as, indeed, they often do with Pongo.
Pongo-like Dental and Palatal Features of Potential Hominids
An interesting case of “interpreter’s bias” lies in analyses of molar enamel thickness. For example, although Martin (1985) has been cited as demonstrating that the last common ancestor of large-bodied hominoids had thick molar enamel (which was retained in orangutan and hominid clades but secondarily reduced in African apes), he merely interpreted enamel thickness data in the context of an [orangutan (human-African ape)] theory of relatedness (Schwartz 1987). Otherwise he should, or at least could, have concluded that thick molar enamel united human and orangutan clades. Kelley and Pilbeam (1986) also interpreted molar enamel thickness in the context of an [orangutan (human-African ape)] theory of relatedness but presented two scenarios: Martin’s and one in which the last common ancestors of separate human and orangutan clades independently developed thick molar enamel. In the latter case, thick molar enamel would be a defining feature of Hominidae. In contrast, but embracing first a human-chimpanzee relationship, Suwa et al. (2009) could claim that thin enamel was secondarily derived in Pan.
More recently, Schwartz (2000) found that, while humans and orangutans both have thick molar enamel, humans have thicker enamel in some areas of their occlusal surfaces. He suggested that thick molar enamel had evolved independently in humans and orangutans (and, by implication, in the last common ancestors of separate human and orangutan clades) because of the demands of different diets. A more straightforward interpretation of G. Schwartz’s data is that human and orangutan clades shared a thick-molar-enameled common ancestor and that, within this clade, humans (and perhaps other hominids) are more derived. Only by accepting thick molar enamel as synapomorphic of traditionally accepted hominids can one embrace thin-enameled Ardipithecus as a sister of this clade (assuming that this relationship is based on synapomorphy). Of course, Brunet et al.’s (2002) suggestion that the thicker enameled Sahelanthropus is ancestral to Ardipithecus , and Suwa et al.’s (2009) argument based on a presumed hominid-Pan sister group that thin enamel in the latter must be secondarily derived, underscores the need for systematic rigor in paleoanthropology. For, indeed, explaining the distribution of morphology based on an already accepted theory of relationship does not substitute for the generation of that theory.
If thick molar enamel does not contribute to defining Hominidae, does dental morphology? Some aspects of it might (e.g., see discussion above on dm1 morphology) but, at present, delineating more than a handful of features would be a Herculean task. This difficulty derives from the longstanding notion of the Miocene being a time of an “ape radiation,” after which “hominids” emerged. Guided by this scenario, paleoanthropologists typically identified post-5.5 ma specimens with thick-enameled cheek teeth as “hominid” and pre-5.5 ma specimens as orangutan relatives. Nevertheless, study of fossils identified as hominid reveals that Pongo-like teeth have often been referred to species of Homo and Australopithecus (Grine and Franzen 1994; Schwartz 2004a; Schwartz and Tattersall 1996). Teeth allocated to Orrorin tugenensis (Senut et al. 2001), if truly thick-enameled, reinforce the question of how many Miocene “apes” are actually hominids and how many Plio-Pleistocene “hominids” are not. In addition, since “hominid” teeth are often worn flat, little attention has been paid to details of occlusal morphology. Thus, although most of the human fossil record has now been scrutinized at the level of morphs (Schwartz and Tattersall 2002, 2003, 2005), much work on testing these hypotheses remains before assigning specimens (other than, of course named type specimens) to specific genera and species.
Were it not for their presence in, for instance, Pongo, Sivapithecus, and Ankarapithecus, other features that could “define” Hominidae are the development of a single incisive foramen (Schwartz 1983, 1997) and a posteriorly thickened palate (as seen in midline cross section) (Schwartz 2004a). With regard to the palate, if the Hadar broken palate AL 200-1a does thin posteriorly, it would be the outlier among “accepted” hominids (Schwartz 2004a; Schwartz and Tattersall 2002, 2003, 2005). As with thick molar enamel and the morphological details of dm1, the simplest phylogenetic interpretation of these features is that they characterized the last common ancestor of human and orangutan clades.
Pongo-Like Features of “Australopiths”: Implications for Defining Hominidae
Another stumbling block to defining Hominidae is the degree to which “australopiths” and specimens traditionally allocated to Homo differ from one another, especially in craniofacial morphology, e.g., in orbital and nasal aperture outline as well as in the configuration of the supra- and infraorbital regions; elevation of the nasoalveolar clivus above the floor of the nasal cavity; height and orientation of the subnasal region; orientation, flatness, and height of the zygomatic region; development of a “snout” with/without facial pillars (vertical or medially inclined); and a canine fossa, and in the development and configuration of a mastoid process. Thus, while one might unite all of these potential hominids via various postcranial features, craniofacial morphology seems only to delineate possible hominid subclades. This may in part explain why one approach to associating living humans with Pan is to try first to link the latter with australopiths and then to assert, since australopiths and Homo form a clade, that humans and chimpanzees are closely related (Begun 1994; Begun et al. 1997). In the latter case, the primary feature of supposed synapomorphy between (an undefined) Australopithecus and Pan – bar-like supraorbital torus with sulcus behind – describes no “australopith” (e.g., Figs. 10 and 11) and only some specimens of Homo (e.g., Fig. 13; Clarke 1987; Schwartz and Tattersall 2002, 2003, 2005). In addition, not only is the markedly inferosuperiorly tall supraorbital region of Sahelanthropus not bar-like as in African apes [i.e., it is not both superiorly and slightly anteriorly projecting and bound posteriorly by a distinct posttoral sulcus; see Schwartz (1997) for discussion], it is so unusually tall that it must represent a derived, not primitive, configuration – which, together with its distinct dental morphology and not very thick molar enamel, makes determining its relationship to any hominid (or ape) difficult indeed (Schwartz 2004b).
While seeking connections between fossil hominids and African apes (especially Pan) has historical precedent (Johanson and White 1979), the most favorable comparisons are actually between Pongo (and its fossil relatives) and australopiths and some specimens attributed to Homo (Grehan and Schwartz 2009; Schwartz 2004a, 2005), e.g., rim-like superior orbital margins with a rather smooth transition into the frontal plane; often ovoid orbits; and forwardly facing, tall, and often vertical zygomatic regions (a further derived configuration, e.g., as in KNM-WT 17000, is a posteriorly tilted zygoma) (Figs. 10, 11, 12, and 13). Since the faces of Pongo, Sivapithecus, and Ankarapithecus as well as of most specimens taken as being hominid are relatively tall superoinferiorly and, even with anteriorly oriented zygomas, relatively narrow from side to side, those specimens with broad and short faces (in the orangutan clade, Lufengpithecus and among hominids, e.g., AL 444-1, SK 48, KNM-ER 406, KNM-WT 17000, KNM-ER 3883) emerge as potentially derived, which for hominids may reflect relatedness among some, if not all of them (see also Schwartz and Tattersall 2005; Fig. 14). Many australopiths are also similar to Pongo in having distinct facial pillars that emerge just above the canines and, together with a variably developed canine fossa, delineate a “snout” [Figs. 10 and 11; see also illustrations in Schwartz and Tattersall (2005)]. Interestingly, the Taung specimen has the long, slit-like single incisive foramen seen in Pongo and also preserved in Sivapithecus and Ankarapithecus (Schwartz 2005). While I am not advocating a special relationship between a Pongo clade and some or all australopiths, attention to these apparent synapomorphies is more relevant to the task of defining Hominidae, its subclades, and sister taxa than is currently appreciated.
Right lateral views of Pongo, Drimolen DNH 7, and Sts 5 (top row) and Pan, StW 505, and SK 48 (bottom row) illustrating, e.g., lack of Pan-like supraorbital, lower facial, and zygomatic morphology but orangutan-specific features in the hominids. See text for further discussion; not to scale (Copyright © J. H. Schwartz)
Comparison of various specimens of “robust australopith” to illustrate similarly derived laterally broad and superoinferiorly short faces in SK 48, KNM-ER 406, and KNM-WT 17000, which might reflect their membership in a hominid subclade that does not include specimens such as DNH 7 and OH 5. See text for further discussion; not to scale (Copyright © J. H. Schwartz)
Indisputable similarities between Pongo and australopiths exist (Schwartz and Tattersall 2005) and they should be accounted for in any critical evaluation of human-ape and within-hominid relationship. Further, these similarities make even more critical for defining a hominid clade and relationships within it broadening comparisons from solely between hominids and Pan alone, or between hominids and both African apes, to include as many catarrhine taxa as possible in order to provide the best foundation for delineating shared character similarity and determining character polarity – which are critical to phylogenetic reconstruction.
Conclusions
When comparing extant taxa, defining Hominidae is a simple matter. Through a curious historical twist, Homo sapiens is the only survivor of a clade whose dimensions we will never fully know. Take, for instance, the discovery of an apparent hominid from the late Pleistocene of Flores, Indonesia, best seen in LB1 (Brown et al. 2004). Its combination of morphologies would be unexpected no matter what its age.
LB1 has or had a moderately globular cranium (as in some hominids); somewhat thickened and anteriorly (but not superiorly) protruding but rim-like supraorbital margins with no sulcus behind (australopiths in part and orangutans and their relatives); tall, ovoid orbits (Pongo, Sivapithecus, some “australopiths”); flat nasal bones (most hominoids, including some Homo); forwardly facing and vertical yet superoinferiorly short zygomas (australopiths and orangutans and their relatives); well-developed mastoid processes (some “australopiths” and some Homo); a thick frontal with thick diploe (autapomorphic or pathological); no frontal sinuses (most primates, including bonobos); an anterior cranial fossa that does not extend fully over the orbital cones (most primates, including some Homo); a clivus that slopes gently away from the dorsum sellae (most primates, including some Homo); basion and bicarotid and biporionic chords in alignment (juvenile anthropoids, some adults); a broad incisive foramen that proceeds anteriorly as a expanding groove (a few “australopiths,” e.g., OH 5); marked separation of the nasoalveolar clivus and an anteriorly thin palate (African apes, some Miocene hominoids, some “australopiths”); a smoothly but narrowly curved mandibular symphyseal region (many anthropoids); a long retromolar space (various Homo); a broadly and smoothly rounded but somewhat truncated gonial angle (some Homo); a very anteroposteriorly long sigmoid notch (some Homo); a sigmoid notch crest that is deepest near the coronoid process (autapomorphic); very large cheek teeth and apparently relatively small anterior teeth (some “australopiths”); unusually mesiodistally short upper and lower molars with large mesial and truncated distal cusps (autapomorphic); a relatively long ilium (e.g., SK 50) with a beaklike anterior superior iliac spine (great apes, SK 50, MLD 7 and 25); a poorly defined iliac pillar (australopiths); a knoblike anterior inferior iliac spine that lies noticeably superior to above and somewhat back over the supra-acetabular rim (hominids as discussed here); an arcuate line that descends well before reaching the region of the acetabulum (some hominids); no ischial spine (most primates); posterior iliac expansion that defines a greater sciatic notch (hominids as used here); a “V”-shaped greater sciatic notch (most hominids, but not Neanderthals); a large femoral head (most primates); a long and anteroposteriorly compressed femoral neck (Orrorin, “australopiths” including BOU-VP-12/1, and some Homo, e.g., OH 62, KNM-WT 15000, D4167); a well-defined intertrochanteric crest (most primates, but not KNM-WT 15000, Orrorin, and most if not all “australopiths” or OH 62); a medially facing lesser trochanter (most primates, not “australopiths” or various Homo, e.g., OH 62, KNM-WT 15000, D4167); a weakly developed linea aspera (Orrorin and various “australopiths”); a femoral “carrying angle” (“australopiths” and Homo); a tibia that is much shorter than the femur (at least apes); poorly differentiated tibial tubercles (most primates); a medial tibial condylar facet that is situated higher than the lateral (at least apes); and a convex medial tibial facet (apes and some “australopiths,” e.g., AL 288-1a, AL 129-1b) (see Brown et al. 2004).
Is this Flores specimen – if the cranial and postcranial remains represent a single individual – a hominid? A gut reaction based on the external morphology of the skull is “yes,” but the internal morphologies are odd. The teeth are not necessarily hominid, the humeral shaft lacks the torque or “twist” characteristic of large-bodied hominoids (Morwood et al. 2005) and the morphology of the distal articular region is clearly not hominoid (Morwood et al. 2005; Schwartz 1986), the tibia is definitely not hominid, the femur is Orrorin-like, and the partial os coxa is somewhat “australopith”-like. So why is the composite Flores specimen considered a species of Homo when its affinities to a clade Hominidae are not entirely clear? Largely because there has been a history of allocating specimens to taxa based more on their geological age than on their morphology – which, in turn, has led to the general practice of trying to explain away “anomalous” morphologies in terms of variation (see also chapter “General Principles of Evolutionary Morphology,” Vol. 1). Methodologically, however, before one even contemplates referring a specimen to a genus and species, one should have to defend first why any specimen is a hominid and then a member of the nested subclades that subsume that species (see also chapter “Principles of Taxonomy and Classification: Current Procedures for Naming and Classifying Organisms,” Vol. 1). But in order to do so, we must have a working definition of this potential clade that is open to criticism, testing, and revision, rather than constructed in the context of a presumed theory of hominid-ape and then hominid-subclade relationship, wherein primitive features dominate the description and homoplasy assumed demonstrable (Lovejoy et al. 2009a; Wood and Harrison 2011). In this regard, there is still much work to be done.
Cross-References
Analyzing Hominin Phylogeny: Cladistic Approach
Fossil Record of the Primates from the Paleocene to the Oligocene
General Principles of Evolutionary Morphology
Historical Overview of Paleoanthropological Research
Hominoid Cranial Diversity and Adaptation
Homo ergaster and Its Contemporaries
Homology: A Philosophical and Biological Perspective
Phylogenetic Relationships of Hominids: Biomolecular Approach
Potential Hominoid Ancestors for Hominidae
Principles of Taxonomy and Classification: Current Procedures for Naming and Classifying Organisms
Species Concepts and Speciation: Facts and Fantasies
Notes
- 1.
Comparison comprises juvenile Homo, including Melka Konturé MK81 GAR IV (2), and “australopiths” (e.g., Hadar AL 333-43b; Koobi Fora KNM-ER 820, 1477, and 1507; Laetoli LH2 and 3q; Omo 227; Taung; Swartkrans SK 47; Kromdraai TM1536, TM1601a, and TM1604; KB5503 and KB5223; and Sterkfontein Sts24 and StW 104 and 151).
References
Aiello L, Dean C (1999) An introduction to human evolutionary anatomy. Academic, London
Arsuaga JL, Bermúdez de Castro JM, Carbonell E (1997) Special issue on the Sima de los Huesos hominids and site. J Hum Evol 33:105–421
Asfaw B, White TD, Lovejoy O, Latimer B, Simpson S, Suwa G (1999) Australopithecus garhi: a new species of early hominid from Ethiopia. Science 284:629–635
Bailey SE (2002) Neandertal dental morphology: implications for modern human origins. Ph.D. thesis, Arizona State, University, Tempe, AZ
Bailey SE (2004) A morphometric analysis fo maxillary molar crowns of Middle-Late Pleistocene hominins. J Hum Evol 47:183–198
Bailey SE, Wu L (2010) A comarative dental metrical and morphological analysis of a Middle Pleistocene hominin maxillar from Chaoxian (Chaohu), China. Quat Int 211:14–23
Bartsiokas A, Day MH (1993) Electron probe energy dispersive x-ray microanalysis (EDXA) in the investigation of fossil bone: the case of Java Man. Proc Biol Sci 252:115–123
Begun DR (1994) Relations among the great apes and humans: new interpretations based on the fossil ape Dryopithecus. Yearb Phys Anthropol 37:11–63
Begun DR, Ward CV, Rose MD (1997) Events in hominoid evolution. In: Begun DR, Ward CV, Rose MD (eds) Function, phylogeny, and fossils: Miocene hominoid evolution and adaptation. Plenum, New York, pp 399–415
Berger LR, de Ruiter DJ, Churchill SE, Schmid P, Carlson KJ, Dirks PHGM, Kibii JM (2010) Australopithecus sediba: a new species of Homo-like australopith from South Africa. Science 328:195–204
Blumenbach JF (1969) On the natural varieties of mankind (De Generis Humani Varietate Nativa). Bergman Publishers, New York
Brown P, Sutikna T, Morwood MJ, Soejono RP, Jatmiko, Saptomo EW, Due RA (2004) A new small-bodied hominin from the late Pleistocene of Flores, Indonesia. Nature 431:1055–1061
Brunet M, Guy F, Pilbeam D, Mackaye HT, Likius A, Ahounta D, Beauvilain A, Blondel C, Bocherens J-R et al (2002) A new hominid from the Upper Miocene of Chad, Central Africa. Nature 418:145–151
Clarke RJ (1987) The cranium of the Swartkrans hominid SK 847 and its relevance to human origins. University of the Witwatersrand, Johannesburg
Clarke RJ, Tobias PV (1995) Sterkfontein Member 2 foot bones of the oldest South African hominid. Science 269:521–524
Collard M, Wood B (2000) How reliable are human phylogenetic hypotheses? Proc Natl Acad Sci U S A 97:5003–5006
Collard M, Wood B (2007) Hominin homoiology: an assessment of the impact of phenotypic plasticity on phylogenetic analyses of humans and their fossil relatives. J Hum Evol 52:573–584
Dart R (1925) Australopithecus africanus: The man-ape of South Africa. Nature 115:195–199
Dart RA (1957) The second adolescent (female) ilium of Australopithecus prometheus. J Paleontol Soc India 2:73–82
Darwin C (1871) The descent of man and selection in relation to sex. John Murray, London
Day MH (1973) The Trinil femora. In: Day MH (ed) Human evolution: symposium of the Society for the study of human biology. Taylor and Francis, London, pp 127–154
Day M (1982) The Homo erectus pelvis: punctuation or gradualism? Congres International de Paleontologie Humaine 1:411–421
Day M (1986) Guide to fossil man, 4th edn. University of Chicago Press, Chicago
Day M, Napier JR (1964a) Hominid fossils from Bed I, Olduvai Gorge, Tanganyika. Fossil foot bones. Nature 201:967–970
Day MH, Napier JR (1964b) Fossil foot bones. Nature 201:967–970
DeSilva JM, Holt KG, Churchill SE, Carlson KJ, Walker CS, Zipfel B, Berger LR (2013) The lower limb and mechanics of walking in Australopithecus sediba. Science 340
Diogo R, Wood B (2011) Soft-tissue anatomy of the primates: phylogenetic analyses based on the muscles of the head, neck, pectoral region and upper limb, with notes on the evolution of these muscles. J Anat: 1–87. doi: 10.1111/j.1469-7580.2011.01403.x
Dubois E (1892) Palaeontologische onderzoekingen op Java [Paleontological investigations on Java]. Verslag van het Mijnwesen 3rd quarter, Leiden, The Netherlands, pp 10–14
Dubois E (1894) Pithecanthropus erectus, eine menschenähnliche Übergangsform aus Java. Landesdruckerei, Batavia
Galik K, Senut B, Pickford M, Gommery D, Treil J, Kuperavage AJ, Eckhardt RB (2004) External and internal morphology of the BAR 1002’00 Orrorin tugenensis femur. Science 305:450–453
Gibbs S, Collard M, Wood B (2000) Soft-tissue characters in higher primate phylogenetics. Proc Natl Acad Sci U S A 97:11130–11132
Gibbs S, Collard M, Wood BA (2002) Soft-tissue anatomy of the extant hominoids: a review and phylogenetic analysis. J Anat 200:3–49
Gilligan I, Chandraphak S, Mahakkanukrauh P (2013) Femoral neck-shaft angle in humans: variation relating to climate, clothing, lifestyle, sex, age and side. J Anat 223:133–151
Goethe JW (1820) Brief an J. G. von Herder. Sämtliche Werke, Propyläen-Verlag, pp 401–432
Goldberg A, Wildman DE, Schmidt TR, Hüttemann M, Goodman M, Weiss ML, Grossman LI (2003) Sister grouping of chimpanzees and humans as revealed by genome-wide phylogenetic analysis of brain gene expression profiles. Proc Natl Acad Sci U S A 100:5873–5878
Gregory WK (1922) The origin and evolution of the human dentition. Williams and Wilkins, Baltimore
Grehan J, Schwartz JH (2009) Evolution of the second orangutan: phylogeny and biogeography of hominid origins. J Biogeogr 36:1823–1844
Grehan J, Schwartz JH (2011) Evolution of human-ape relationships remains open for investigation. J Biogeogr 38:2397–2404
Grine F, Franzen J (1994) Fossil hominid teeth from the Sangiran Dome (Java, Indonesia). Cour Forsch Senckenberg 171:75–103
Groves C (1986) Systematics of the great apes. In: Swindler DR, Erwin J (eds) Comparative primate biology. Alan R. Liss, New York, pp 187–217
Guy F, Lieberman DE, Pilbeam D, Ponce de León M, Likius A, Mackaye HT, Vignaud P, Zollikofer C, Brunet M (2005) Morphological affinities of the Sahelanthropus tchadensis (Late Miocene hominid from Chad) cranium. Proc Natl Acad Sci U S A 102:18836–18841
Haile-Selassie Y, Saylor BZ, Deino A, Levin NE, Alene M, Latimer BM (2012) A new hominin foot from Ethiopia shows multiple Pliocene bipedal adaptations. Nature 484:565–569
Hennig W (1966) Phylogenetic systematics. University of Chicago Press, Chicago
Huxley TH (1863a) On some fossil remains of man, man’s place in nature. D. Appleton, New York
Huxley TH (1863b) On the relation of man to the lower animals. In: Huxley TH (ed) Man’s place in nature. D. Appleton, New York
Irish JD, Guatelli-Steinberg D (2003) Ancient teeth and modern human origins: an expanded comparison of African Plio-Pleistocene and recent world dental samples. J Hum Evol 45:113–144
Irish JD, Guatelli-Steinberg D, Legge SS, de Ruiter DJ, Berger L (2013) Dental morphology and the phylogenetic “place” of Australopithecus sediba. Science 340:164
Johanson DC, White TD (1979) A systematic assessment of early African hominids. Science 203:321–330
Johanson DC, Lovejoy CO, Kimbel WH, White TD, Ward SC, Bush ME, Latimer BM, Coppens Y (1982) Morphology of the Pliocene partial hominid skeleton (A.L. 288–1) from the Hadar Formation, Ethiopia. Am J Phys Anthropol 57:403–452
Kelley J, Pilbeam D (1986) The dryopithecines: taxonomy, comparative anatomy, and phylogeny of Miocene large hominoids. In: Swindler DR, Erwin J (eds) Comparative primate biology. Alan. R. Liss, New York, pp 1–41
Kennedy GE (1983) Some aspects of femoral morphology in Homo erectus. J Hum Evol 12:587–616
Kibii JM, Churchill SE, Schmidt P, Carlson KJ, Reed ND, de Ruiter DJ, Berger LR (2011) A partial pelvis of Australopithecus sediba. Science 333:1407–1411
Kimbel W, Suwa G, Asfaw B, White TD (2013) Aridipithecus ramidus and the evolution of the human cranial base. Am J Phys Anthropol 150:166–167
Lague MR (2002) Another look at shape variation in the distal femur of Australopithecus afarensis: implications for taxonomic and functional diversity at Hadar. J Hum Evol 42:609–627
Latimer BM, Lovejoy CO (1990) Hallucal tarsometatarsal join in Australopithecus afarensis. Am J Phys Anthropol 82:125–133
Le Gros Clark WE (1940) Observations of the anatomy of the fossil Australopithecinae. J Anat 81:300–333
Le Gros Clark WE (1955) The fossil evidence for human evolution. University of Chicago Press, Chicago
Le Gros Clark WE (1959) The antecedents of man: an introduction to the evolution of the primates. Edinburgh University Press, Edinburgh
Le Gros Clark WE (1964) The fossil evidence for human evolution, 2nd edn. University of Chicago Press, Chicago
Leakey MD, Feibel CS, McDougall I, Walker AC (1995) New four-million-year-old hominid species from Kanapoi and Allia Bay, Kenya. Nature 376:565–571
Lehtonen S, Sääksjärvi IE, Ruokolainen K, Tuomista H (2011) Who is the closest extant cousin of humans? Total evidence approach to hominid phylogenetics via simultaneous optimization. J Biogeogr 38:805–808
Linnaeus C (1735) Systema naturae per regna tria naturae, secundum classes, ordines, genera, species cum characteribus, differentiis, synonymis, locis. Laurentii Salvii, Stockholm
Lockwood CA, Fleagle J (1999) The recognition and evaluation of homoplasy in primate and human evolution. Yearb Phys Anthropol 42:189–232
Lockwood CA, Kimbel WH, Lynch JM (2004) Morphometrics and hominoid phylogeny: support for a chimpanzee-human clade and differentiation among great ape subspecies. Proc Natl Acad Sci U S A 101:4356–4360
Lordkipanidze D, Jashashvili T, Vekua A, Ponce de León M, Zollikofer CPE et al (2007) Postcranial evidence from early Homo from Dmanisi, Georgia. Nature 449:305–310
Lordkipanidze D, Ponce de León M, Margvelashvili A, Rak Y, Rightmire GP, Vekua A, Zollikofer C (2013) A complete skull from Dmanisi, Georgia, and the evolutionary biology of early Homo. Science 342:326–331
Lovejoy AO, Suwa G, Simpson GG, Matternes JH, White TD (2009a) The great divides: Ardipithecus ramidus reveals the postcrania of our last common ancestors with African apes. Science 326:73
Lovejoy AO, Suwa G, Spurlock L, Asfaw B, White TD (2009b) The pelvis and femur of Ardipithecus ramidus: the emergence of upright walking. Science 326:71–76
Lovejoy CO, Latimer B, Suwa G, Asfaw B, White TD (2009c) Combining prehension and propulsion: the foot of Ardipithecus ramidus. Science 326
Margvelashvili A, Zollikofer CPE, Lordkipanidze D, Peltomäki T, Ponce de León M (2013) Tooth wear and dentoalveolar remodeling are key factors of morphological variation in Dmanisi mandibles. Proc Natl Acad Sci U S A 110:17278–17283
Martin R (1928) Lehrbuch der Anthropologie, 2nd edn. Fischer, Jena
Martin L (1985) Significance of enamel thickness in hominoid evolution. Nature 314:260–263
Mayr E (1950) Taxonomic categories in fossil hominids. Cold Spring Harb Symp Quant Biol 15:109–118
Mayr E (1953) Comments on evolutionary literature. Evolution 7:273–281
Mayr E (1963) The taxonomic evaluation of fossil hominids. In: Washburn SL (ed) Classification and human evolution. Aldine, Chicago, pp 332–346
McHenry H (1986) Size variation in the postcranium of Australopithecus afarensis and extant species of Hominoidea. In: Pickford M, Chiarelli B (eds) Sexual dimorphism in living and fossil primates. Il Sedicesimo, Florence, pp 183–189
Morwood MJ, Brown P, Jatmiko, Sutikna T, Wahyu Saptomo E, Westaway KE, Due RA, Roberts RG, Maeda T, Wasisto S, Djubiantono T (2005) Further evidence for small-bodied hominins from the Late Pleistocene of Flores, Indonesia. Nature 437:1012–1017
Ohman JC, Krochta TJ, Lovejoy AO, Mensforth RP, Latimer B (1997) Cortical bone distribution in the femoral neck of hominids: implications for the locomotion of Australopithecus afarensis. Am J Phys Anthropol 104:117–131
Pickford M, Senut B, Gommery D, Treil J (2002) Bipedalism in Orrorin tugenensis revealed by its femora. C R Paleontol 1:191–204
Pilbeam D (1972) The ascent of man: an introduction to human evolution. Macmillan, New York
Pilbeam D (1986) Hominoid evolution and hominoid origins. Sci Am 250:295–312
Pilbeam D (2000) Hominoid systematics: the soft evidence. Proc Natl Acad Sci U S A 97:10684–10686
Rak Y (1990) On the differences between two pelvises of Mousterian context from the Qafzeh and Kebara caves. Am J Phys Anthropol 81:323–332
Robinson JT (1972) Early hominid posture and locomotion. University of Chicago Press, Chicago
Rose MD (1984) A hominine hip bone, KNM-ER 3228, from East Lake Turkana, Kenya. Am J Phys Anthropol 63:371–378
Rosenberg KR (1998) Morphological variation in west Asian postcrania. In: Akazawa T, Aoki K, Bar-Yosef O (eds) Neandertals and modern humans in Western Asia. Plenum, New York, pp 367–379
Rosenberg KR, Lu Q (1997) The pelvis of the Jinniushan specimen: an East Asian perspective on Neandertal morphology. Am J Phys Anthropol Suppl 24:199–200
Sawyer GJ, Maley B (2005) Neanderthal reconstructed. Anat Rec Part B New Anat 283B:23–31
Schultz AH (1968) The recent hominoid primates. In: Washburn SL, Jay PC (eds) Perspectives on human evolution. Holt, Rinehart and Winston, New York, pp 122–195
Schwartz JH (1983) Palatine fenestrae, the orangutan and hominoid evolution. Primates 24:231–240
Schwartz JH (1986) Primate systematics and a classification of the order. In: Swindler DR, Erwin J (eds) Comparative primate biology. Alan R. Liss, New York, pp 1–41
Schwartz JH (1987) The red ape: orangutans and human origins. Houghton Mifflin, Boston
Schwartz JH (1988) History, morphology, paleontology, and evolution. In: Schwartz JH (ed) Orangutan biology. Oxford University Press, London, pp 68–85
Schwartz JH (1997) Lufengpithecus and hominoid phylogeny: problems in delineating and evaluating phylogenetically relevant characters. In: Begun DR, Ward CV, Rose MD (eds) Function, phylogeny, and fossils: Miocene hominoid evolution and adaptations. Plenum, New York, pp 363–388
Schwartz JH (1999) Sudden origins: fossils, genes, and the emergence of species. Wiley, New York
Schwartz GT (2000) Taxonomic and functional aspects of the patterning of enamel thickness distribution in extant large-bodied hominoids. Am J Phys Anthropol 111:221–244
Schwartz JH (2004a) Barking up the wrong ape – australopiths and the quest for chimpanzee characters in hominid fossils. Coll Antropol 28:87–101
Schwartz JH (2004b) Issues in hominid systematics. In: Baquedano E, Rubio Jara S (eds) Miscelánea en homenaje a Emiliano Aguirre. Museo Arqueológico Regional de la Comunidad de Madrid, Madrid, pp 360–371
Schwartz JH (2005) The red ape: orangutans and human origins. Westview, Boulder
Schwartz JH (2007a) Defining Hominidae. In: Henke W, Tattersall I (eds) Handbook of paleoanthropology. Springer, Berlin, pp 1379–1408
Schwartz JH (2007b) Skeleton keys: an introduction to human skeletal morphology, development, and analysis, 2nd edn. Oxford University Press, New York
Schwartz JH (2008) Cladistics. In: Regal B (ed) Icons of evolution. Greenwood Press, Westport, pp 517–544
Schwartz JH (2009) Organismal innovation. In: O’Brien MJ, Shennan SJ (eds) Innovations in cultural systems: contributions from evolutionary anthropology. MIT Press, Cambridge, MA, pp 53–67
Schwartz JH (2011) Developmental biology and human evolution. Hum Orig Res 1:7–14
Schwartz JH (2012) Molecular anthropology and the subversion of paleoanthropology: an example of the ‘emperor’s clothes effect’? Hist Philos Life Sci 34:237–258
Schwartz JH, Maresca B (2006) Do molecular clocks run at all? A critique of molecular systematics. Biol Theory 1:1–15
Schwartz JH, Tattersall I (1996) Who’s teeth? (comment on the Longgupo hominoids). Nature 381:201–202
Schwartz JH, Tattersall I (2000a) The human chin: what is it and who has it? J Hum Evol 38:367–409
Schwartz JH, Tattersall I (2000b) Variability in hominid evolution: putting the cart before the horse? In: Antunes M (ed) Últimos Neandertais em Portugal (Last Neandertals in Portugal) Odontologic and other evidence. Academia das Ciéncias de Lisboa, Lisboa, pp 367–401
Schwartz JH, Tattersall I (2002) The human fossil record, volume one, terminology and craniodental morphology of genus Homo (Europe). Wiley-Liss, New York
Schwartz JH, Tattersall I (2003) The human fossil record, volume two, craniodental morphology of genus Homo (Africa and Asia). Wiley-Liss, New York
Schwartz JH, Tattersall I (2005) The human fossil record, volume four: craniodental morphology of Australopithecus, Paranthropus, and Orrorin. Wiley-Liss, New York
Schwartz JH, Tattersall I (2010) Fossil evidence for the origin of Homo sapiens. Yearb Phys Anthropol 53:94–121
Senut B, Pickford M, Gommery D, Mein P, Cheboi K, Coppens Y (2001) First hominid from the Miocene (Lukeino formation, Kenya). Comptes Rendue des Académie de Sci (Paris) Ser IIa 332:137–144
Simpson SW, Quade J, Levin NE, Butler R, Dupont-Nivet G, Everett M, Semaw S (2008) A female Homo erectus pelvis from Gona, Ethiopia. Science 322:1089–1092
Strait DS, Grine FE (2004) Inferring hominoid and early hominid phylogeny using craniodental characters: the role of fossil taxa. J Hum Evol 47:399–452
Strauss A, Gunz P, Spoor F (2012) Late juvenile cranial growth in hominids. Proc Eur Soc Stud Hum Evol 1:177
Strauss A, Gunz P, Benazzi S, Spoor F (2013) Late juvenile cranial growth and the diagnosis of Australopithecus sediba. Proc Eur Soc Stud Hum Evol 2:218
Suwa G, Kono RT, Simpson SW, Asfaw B, Lovejoy CO, White TD (2009) Paleobiological implications of the Ardipithecus ramidus dentition. Science 326:94–99
Swarts JD (1988) Deciduous dentition: implications for hominid phylogeny. In: Schwartz JH (ed) Orangutan biology. Oxford University Press, New York, pp 263–270
Swindler DR, Wood CD (1973) An atlas of primate gross anatomy: baboon, chimpanzee, and man. R. E. Krieger, Malabar
Tardieu C, Trinkaus E (1994) Early ontogeny of the human femoral bicondylar angle. Am J Phys Anthropol 95:183–195
Toussaint M, Macho GA, Tobias PV, Partridge TC, Hughes AR (2003) The third partial skeleton of a late Pliocene hominid (Stw 431) from Sterkfontein, South Africa. S Afr J Sci 99:215–223
Trinkaus E (1983) The Shanidar Neandertals. Academic, New York
Trinkaus E, Howells WW (1979) The Neanderthals. Sci Am 241:118–133
Trinkaus E, Lebel S, Bailey SE (2000) Middle Paleolithic and recent human dental remains from the Bau de l’Aubesier, Monieux (Vaucluse). Bulletin et Mémoires de la Société d’Anthropologie de Paris 12:207–226
Turner CGI, Nichol CR, Scott GR (1991) Scoring procedures for key morphological traits of the permanent dentition: the Arizona State University dental anthropology system. In: Kelly MA, Larsen CS (eds) Advances in dental anthropology. Wiley-Liss, New York, pp 13–32
Ulhaas L (2007) Computer reconstruction: Technical aspects and applications. In: Henke W, Tattersall I (eds) Handbook of paleoanthropology. Springer, Berlin, pp 787–813
Walker AC, Leakey RF (1993) The postcranial remains. In: Walker A, Leakey R (eds) The Nariokotome Homo erectus skeleton. Harvard University Press, Cambridge, MA, pp 95–160
Ward CV, Kimbel WH, Johanson DC (2011) Complete fourth metatarsal and arches in the foot of Australopithecus afarensis. Science 331:750–753
White TD (2003) Another perspective on hominid diversity – response. Science 301:703
White T (2012) Paleoanthropology: five’s a crowd in our family tree. Curr Biol 23
White TD, Suwa G, Asfaw B (1994) Australopithecus ramidus, a new species of early hominid from Aramis, Ethiopia. Nature 371:306–312
White TD, WoldeGabriel G, Asfaw B, Ambrose S, Beyene Y, Bernor RL, Boisserie J-R, Currie B, Gilbert H, Haile-Selassie Y, Hart WK, Hlusko LJ, Howell FC, Kono RT, Lehmann T, Louchart A, Lovejoy CO, Renne PR, Saegusa H, Vrba ES, Wesselman H, Suwa G (2006) Asa Issie, Aramis and the origin of Australopithecus. Nature 440:883–889
Wood BA, Collard M (1999) The changing face of the genus Homo. Evol Anthropol 8:195–207
Wood B, Harrison T (2011) The evolutionary context of the first hominins. Nature 470:347–352
Zipfel B, DeSilva JM, Kidd RS, Carlson KJ, Churchill SE, Berger LR (2011) The foot and ankle of Australopithecus sediba. Science 333:1417–1420
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this entry
Cite this entry
Schwartz, J.H. (2015). Defining Hominidae. In: Henke, W., Tattersall, I. (eds) Handbook of Paleoanthropology. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-39979-4_45
Download citation
DOI: https://doi.org/10.1007/978-3-642-39979-4_45
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-39978-7
Online ISBN: 978-3-642-39979-4
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences