-
PDF
- Split View
-
Views
-
Cite
Cite
Christopher D. Paddock, John W. Sumner, James A. Comer, Sherif R. Zaki, Cynthia S. Goldsmith, Jerome Goddard, Susan L. F. McLellan, Cynthia L. Tamminga, Christopher A. Ohl, Rickettsia parkeri: A Newly Recognized Cause of Spotted Fever Rickettsiosis in the United States, Clinical Infectious Diseases, Volume 38, Issue 6, 15 March 2004, Pages 805–811, https://doi.org/10.1086/381894
Close - Share Icon Share
Abstract
Ticks, including many that bite humans, are hosts to several obligate intracellular bacteria in the spotted fever group (SFG) of the genus Rickettsia. Only Rickettsia rickettsii, the agent of Rocky Mountain spotted fever, has been definitively associated with disease in humans in the United States. Herein we describe disease in a human caused by Rickettsia parkeri, an SFG rickettsia first identified >60 years ago in Gulf Coast ticks (Amblyomma maculatum) collected from the southern United States. Confirmation of the infection was accomplished using serological testing, immunohistochemical staining, cell culture isolation, and molecular methods. Application of specific laboratory assays to clinical specimens obtained from patients with febrile, eschar-associated illnesses following a tick bite may identify additional cases of R. parkeri rickettsiosis and possibly other novel SFG rickettsioses in the United States.
Spotted fever group (SFG) rickettsiae are obligate intracellular, arthropod-borne bacteria that comprise at least 30 diverse genotypes, which include 15 distinct species currently recognized as pathogens in humans. In the United States, 3 species of SFG rickettsiae are recognized agents of human disease: Rickettsia rickettsii, the cause of Rocky Mountain spotted fever (RMSF); Rickettsia akari, the mite-borne agent of rickettsialpox; and Rickettsia felis, the cause of cat-flea SFG rickettsiosis [1]. R. rickettsii is the only 1 of these pathogens transmitted by ticks. Soon after the identification of R. rickettsii, investigators recognized the occurrence of other SFG rickettsiae and rickettsia-like bacteria in North American ticks [2]. Subsequent studies have shown that these other tick-associated rickettsiae are encountered far more frequently than R. rickettsii, even in areas where RMSF is endemic [3].
In 1939, entomologist and rickettsiologist R. R. Parker reported the isolation of a bacterium recovered from Gulf Coast ticks (Amblyomma maculatum) collected from Liberty County, Texas [4]. When Parker inoculated guinea pigs with “the maculatum agent,” the animals developed a mild febrile disease that resembled other recognized spotted fever rickettsioses, including RMSF and Mediterranean spotted (boutonneuse) fever [4, 5]. Investigators later characterized this bacterium as a unique SFG rickettsia, and the agent was designated Rickettsia parkeri [6, 7]. Speculation regarding the pathogenicity of R. parkeri in humans has followed this rickettsia since its discovery; to our knowledge, this report confirms for the first time the role of R. parkeri as the cause of a spotted fever rickettsiosis in a human.
Case Report
A 40-year-old, previously healthy man presented to an acute-care clinic during August 2002 with fever, mild headache, malaise, diffuse myalgias and arthralgias, and multiple eschars on his lower extremities. The patient observed these lesions 4 days earlier as small erythematous papules that developed into mildly tender pustules and quickly ulcerated. Two days later, he experienced rapid onset of fever with a maximum temperature of 39°C, which was accompanied by systemic symptoms. The patient lived in a suburban area adjoining a tidal estuary in the Tidewater region of southeast Virginia. He did not recall tick or mite bites or exposures to house mice in the month preceding his illness. However, he was frequently exposed to ticks and fleas through contact with several household dogs and cats and had recently walked his dogs in grassy fields near his residence. He often observed various rodents in these fields, including rats and nutria. There was no recent history of foreign or domestic travel. The patient was diagnosed with infected arthropod bites and was treated with amoxicillin–clavulanic acid. His symptoms progressed, and within 12 h he developed a nonpruritic, erythematous maculopapular rash on his trunk that soon spread to his extremities. Antibiotic therapy was changed to cephalexin, but the patient's symptoms persisted.
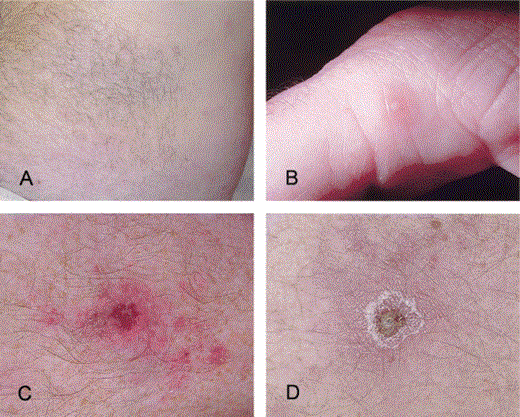
An infectious disease consultation was obtained 9 days into his illness. Physical examination revealed a mildly ill man with a temperature of 38°C and a faint, diffuse maculopapular rash, predominantly on his flanks and trunk (figure 1A). The rash also extended to his face and extremities, involving his palms and soles, and included a few scattered pustules on an erythematous base (figure 1B). Two eschars in different stages of development, each ∼1.5 cm in greatest dimension, were identified on the medial and proximal pretibial aspects of the right and left lower legs, respectively (figure 1C and 1D). A third lesion, an ulcerated papule, was observed on the distal pretibial aspect of the left lower leg. An enlarged, non-tender lymph node was palpable in the left inguinal region. The patient's leukocyte count was 3.3 × 103 cells/mm3 (neutrophils, 44%; bands, 5%; lymphocytes, 41%; and monocytes, 10%), his hemoglobin level was 13.5 gm/dL, and his platelet count was 149 × 103 cells/mm3. Other laboratory values were as follows: aspartate aminotransferase, 92 U/L; alanine aminotransferase, l95 U/L; alkaline phosphatase, 135 U/L; lactate dehydrogenase, 1502 U/L; and total bilirubin, 2.4 mg/dL. The patient received a diagnosis of rickettsialpox and was treated with doxycycline as an outpatient. The fever, arthralgias, and myalgias resolved within 2 days, the rash resolved within 1 week, and all laboratory values returned to normal within 1 month. During the diagnostic evaluation, biopsy and serum specimens were obtained and sent to the Centers for Disease Control and Prevention (CDC; Atlanta, GA) for confirmatory laboratory testing.
Cutaneous lesions in a patient infected with Rickettsia parkeri. A, A diffuse, pink macular rash involving the abdomen. B, A small pustule on the medial aspect of the first digit. C and D, Eschars located on the pretibial aspects of the right and left lower legs, respectively.
Methods
Serological testing. A serum specimen obtained during the acute phase of the patient's illness was evaluated for IgG antibodies reactive with various SFG rickettsiae by use of an indirect immunofluorescence antibody assay. Antigen suspensions were prepared from R. akari or R. rickettsii grown in chicken yolk sacs. Reactivity was determined using fluorescein isothiocyanate–conjugated goat anti–human IgG (γ-specific) (Kirkegaard and Perry Laboratories) at a dilution of 1 : 150.
Histopathological and immunohistochemical evaluation. A 5-mm punch biopsy specimen from the eschar on the proximal left lower leg was evaluated by light microscopy. Sections (3 µm) cut from formalin-fixed, paraffin-embedded tissue were stained with hematoxylin and eosin, and an immunoalkaline phosphatase technique was used to detect SFG rickettsiae [8]; polyclonal rabbit anti–R. rickettsii serum at a dilution of 1 : 500 was used as the primary antibody.
Isolation in cell culture. A 3-mm punch biopsy specimen from the lesion on the distal left lower leg was triturated using a polypropylene pellet pestle in a 1.5-mL centrifuge tube containing 250 µL of cell culture medium (RPMI 1640 supplemented with 5% fetal bovine serum and 2 mmol/L L-glutamine). Larger tissue fragments were allowed to sediment, and the remaining mixture was transferred to a confluent monolayer of Vero E6 cells in a 25-cm2 polystyrene cell culture flask and centrifuged at 140g for 30 min. Following centrifugation, the cells were overlaid with 5 mL of cell culture medium containing 10 U/mL penicillin G sodium and 10 µg/mL streptomycin sulfate and incubated at 34.5°C in a 5% CO2-enriched atmosphere. The medium was removed after 48 h and replaced with 5 mL of fresh cell culture medium containing no antibiotics. Thereafter, the medium was changed 1–2 times weekly. Cells were monitored for infection by examining cytocentrifuged preparations of cell culture supernatant fixed in absolute methanol and stained with a 0.01% solution of acridine orange at pH 3.5.
Electron microscopy. Infected Vero cells were examined by electron microscopy. Cells were fixed in situ at room temperature for 10 s with 2.5% glutaraldehyde in 0.1 mol/L phosphate buffer, scraped from the flask, and collected by centrifugation at 400g for 10 min. The pellet was fixed at 4°C for an additional hour, postfixed for 30 min in buffered 1% osmium tetroxide, dehydrated in a series of graded ethanol concentrations and propylene oxide, and embedded in an Epon substitute–Araldite mixture. Sections were stained with 4% uranyl acetate and Reynold's lead citrate.
Molecular analyses. DNA was extracted from triturated biopsy tissue and from 7-day-old cell culture supernatant with the QIAamp DNA Mini Kit (Qiagen). Segments of the rickettsial 17-kDa genus–common antigen gene, citrate synthase (gltA) gene, and the rickettsial outer-membrane protein A (rompA) gene were amplified using single-stage or nested PCR assays. Primers R17122 and R17500 [9] were used in the primary amplifications, and primers TZ15 and TZ16 [10] were used in the nested amplifications of the 17-kDa antigen gene sequence. Primers RpCS.877p and RpCS.1258n [11] were used for single-stage amplification of the gltA sequence. Primers Rr190.70p [11] and 190–701 [12], and 190-FN1 (5′-AAGCAATACAACAAGGTC) and 190-RN1 (5′-TGACAGTTATTATACCTC) were used in primary and nested amplifications, respectively, of the rompA sequence. All reactions were performed using Ready-To-Go PCR Beads with puReTaq (Amersham Pharmacia Biotech), and final concentrations of primers at 1 µmol/L and MgCl2 at 1.5 mmol/L. Thermal cycling parameters consisted of an initial denaturation period of 5 min at 95°C followed by a standard 3-step cycling profile consisting of 40 cycles at 95°C for 30 s, annealing temperature for 30 s, and an extension period at 72°C for 1 min. The annealing temperatures were 55°C for the 17-kDa antigen gene assay and 60°C for the gltA and rompA assays. The cycling profile was followed by a final extension period at 72°C for 5 min.
Products of the correct size were isolated by electrophoresis in 1.6% low melting-point agarose gels containing ethidium bromide. Bands were excised, and the DNA was recovered using Wizard PCR Preps (Promega Corp.). The purified products were sequenced directly using a BigDye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems). Sequences were detected on an Applied Biosystems 310 automated sequencer. Nucleotide sequence homology searches were made through the National Center for Biotechnology Information BLAST network service.
Results
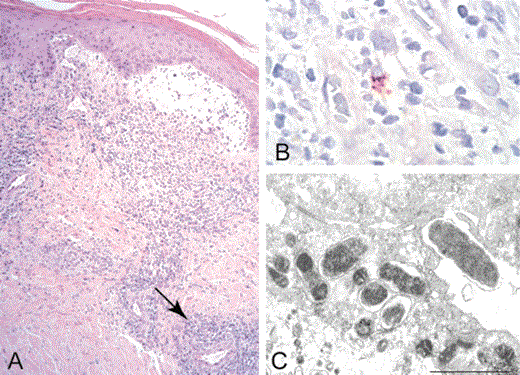
A serum specimen collected 11 days after the onset of the patient's illness revealed IgG antibodies reactive with R. rickettsii and R. akari, each at titers of 1024. Histopathological evaluation of the biopsy specimen obtained from the proximal left lower leg demonstrated focal ulceration and necrosis of the epidermis with adjacent subepidermal blistering and focal necrosis and hemorrhage of the superficial dermis. A dense, predominantly lymphohistiocytic perivascular infiltrate involved the dermis and extended focally to involve eccrine sweat glands, hair follicles, and subcutaneous adipose tissue (figure 2A). Immunohistochemical staining revealed SFG rickettsiae in the cytoplasm of a few cells in foci of perivascular infiltrates (figure 2B).
Histopathologic and immunohistochemical evaluation of a biopsy specimen from the margin of an eschar, and ultrastructure of Rickettsia parkeri (strain Portsmouth) isolated in cell culture. A, Lymphohistiocytic perivascular infiltrates (arrow, representative focus) involving the superficial and deep dermis, and subepidermal blistering at the periphery of the eschar represented grossly in figure 1D (hematoxylin and eosin stain; original magnification ×25). B, Immunohistochemical staining of SFG rickettsiae (red) in the cytoplasm of a cell in a focus of perivascular inflammation (immunoalkaline phosphatase with naphthol-fast red substrate and hematoxylin counterstain; original magnification ×250). C, Ovoid and rod-shaped bacteria in the cytoplasm of a Vero E6 cell (an electron micrograph; uranyl acetate and lead citrate stain; bar equals 1 µm).
Seven days after Vero cells were inoculated with the triturated biopsy specimen from the distal left lower leg, abundant intracellular and extracellular bacteria with diplobacillary, diplococcal, and coccobacillary morphology were identified in acridine orange-stained preparations of cell culture supernatant. Electron microscopic evaluation of infected cells demonstrated coccoid and rod-shaped bacteria, measuring to 1.5 µm in greatest length and 0.45 µm in greatest width, situated freely within host-cell cytoplasm (figure 2C).
DNA samples extracted from the distal left lower leg biopsy specimen and from the cell culture isolate were tested by PCR assays for various rickettsial gene targets. For each of the targets examined, nucleotide sequences obtained from the biopsy specimen and isolate were identical to existing sequences in GenBank for R. parkeri (strain Maculatum 20). Sequences of the 208-bp segment of the 17-kDa rickettsial antigen gene amplified from both specimens were identical to each other and to corresponding sequences of R. parkeri (U17008) and R. rickettsii (GenBank accession numbers M16486 and M28479), and differed from R. akari (AF445383) at 13 positions (6.3%) and from R. felis (AF195118) at 18 positions (8.7%). The sequences of the 540-bp rompA segment obtained by nested PCR of the biopsy specimen and the 590-bp rompA segment obtained by single-stage PCR of the isolate were identical to the corresponding segments of the rompA sequence (U43802) of R. parkeri. The corresponding rompA sequences (M31227 and U43804) of R. rickettsii differed at 27 positions (4.6%), and those of R. akari (L01461) and R. felis (AF191026) differed by at least 30%, depending on the alignment method used. A 341-bp segment of the gltA gene amplified from isolate was identical to the gltA sequence of R. parkeri (U59732) and differed from corresponding sequences of R. rickettsii (U59729), R. felis (U33922), and R. akari (U41752 and U59717), at 5 (1.5%), 13 (4.0%), and 16 (4.7%) nucleotide positions, respectively.
Discussion
For nearly a century following the discovery of the agent of RMSF [13], R. rickettsii was the sole tickborne rickettsia conclusively identified as a cause of human disease in the United States. The singularity of this situation became increasingly peculiar with successive discoveries of novel rickettsioses caused by distinct SFG rickettsiae in Europe, Africa, Australia, and Asia during the last 25 years of the 20th century [1]. Soon after the initial isolation of R. parkeri and during subsequent decades, investigators speculated a possible role of this bacterium as an agent of disease in humans [14, 15]. Parker and others identified similarities between “the maculatum agent” and the agents of other SFG rickettsioses, including boutonneuse fever (caused by R. conorii) and African tick-bite fever (caused by R. africae), by comparing clinical and serological characteristics of these infections in guinea pigs [4, 5, 7]. These early observations have been validated by contemporary phylogenetic analyses evaluating genotypic relationships among SFG rickettsiae that relate R. parkeri most closely to Old World, eschar-producing SFG pathogens, including R. conorii and R. africae [1, 12, 15].
The disease manifestations in the patient we describe (i.e., a relatively mild, febrile illness accompanied by multiple eschars and a maculopapular eruption) closely resemble clinical descriptions of African tick-bite fever. This patient's illness also shares some features with rickettsialpox and with spotted fever caused by R. felis [1]. Laboratory abnormalities identified in this patient, including mild leukopenia and elevated hepatic enzyme levels, and his rapid clinical response to therapy with doxycycline, are also features of these and other SFG rickettsioses.
Because extensive antigenic cross-reactivity exists among SFG rickettsiae, most available tests are only SFG-specific and cannot be used to ascribe etiology to a specific pathogen. In this context, the identities of unique rickettsial agents may be obscured when group-specific serological or immunohistochemical assays are used to confirm the diagnosis of a spotted fever rickettsiosis. As an example, the serum specimen of the patient described herein demonstrated an antibody titer of 2048 when reacted with R. parkeri as antigen (data not shown); this titer was not significantly different from the titers obtained using R. akari or R. rickettsii as antigens in the same serological assay. As exemplified by this report, establishing definitive etiological associations for SFG rickettsiae hinges on clinical foresight, procurement of appropriate specimens for confirmatory diagnosis, and application of traditional and contemporary laboratory methods during evaluations of patients with febrile, eschar-associated illnesses.
Because eschars are rarely described in patients with RMSF, the observation of this lesion at the site of tick bites in some patients with RMSF-like illnesses suggested to previous investigators that species of SFG rickettsiae other than R. rickettsii might elicit disease in the United States [16, 17]. Recent case reports commenting on the unusual occurrence of eschars in some patients with RMSF [18, 19] may have represented infections caused by R. parkeri or other SFG rickettsioses masquerading as RMSF. R. parkeri was perhaps isolated previously from some patients with what appeared to be RMSF, only to be misidentified as R. rickettsii by available diagnostic methods. In one study, investigators using restriction fragment-length polymorphism (RFLP) analysis to evaluate genetic similarities of various SFG rickettsiae identified a strain of "R. rickettsii" isolated from a patient with fatal spotted fever that had a genomic RFLP pattern identical to that of R. parkeri [20].
It remains unknown whether any of the ∼200–1000 cases of RMSF reported each year represent cases of spotted fever attributable to R. parkeri. However, some anecdotal data are compelling enough to suggest that cases of a second disease entity (i.e., an “American boutonneuse fever” caused by R. parkeri) are embedded among surveillance data for RMSF. Review of diagnostic reports for rickettsial disease testing at the CDC from 1998 through 2002 identified at least 3 additional patients with fevers, rashes, and solitary or multiple eschars that appeared following tick bites. These patients, who were exposed to ticks in Mississippi and Florida, each had IgG antibodies reactive with R. rickettsii at titers ⩾1024, and SFG rickettsiae were identified by immunohistochemical staining of an eschar biopsy specimen of 1 of these patients (CDC, unpublished data). It is also possible that cases of R. parkeri rickettsiosis have been diagnosed previously as rickettsialpox. In this context, the laboratory confirmation of RMSF and rickettsialpox may require serological or immunohistochemical assays more specific than the conventional methods.
The distribution, natural history, and ecology of R. parkeri remain to be defined. Although a bite by a specific arthropod was not identified for the index patient, 1 likely vector of R. parkeri is the Gulf Coast tick. In the United States, A. maculatum is distributed predominantly in coastal regions of southern and southeastern states, including Virginia, that border the Gulf of Mexico and Atlantic Ocean. [21, 22]. Well-established inland populations also exist in regions of Kansas and Oklahoma [23]. R. parkeri has been isolated from Gulf Coast ticks collected in Alabama, Georgia, Mississippi, and Texas [4, 5, 24, 25], suggesting that the range of R. parkeri could encompass the distribution of A. maculatum. Because the Gulf Coast tick is also found in some countries of Central and South America and on some islands of the Caribbean [21], it is possible that R. parkeri rickettsiosis may also occur in other regions of the Western Hemisphere: a study recently described patients from Uruguay with an eschar-associated, tickborne rickettsiosis, attributed presumptively to R. conorii by use of serological assays [26]. The recent identification of R. africae rickettsiosis in the French West Indies [27], viewed in conjunction with data presented herein, suggests that various tickborne SFG rickettsiae endemic to the Western Hemisphere may cause clinically similar illneses.
Parasitism of humans by A. maculatum has been recorded in at least 8 southern and southeastern states [28], and Gulf Coast ticks accounted for 12% of all tick bites described in a recent survey in Mississippi [29]. R . parkeri has also been detected in lone star ticks (Amblyomma americanum) collected from Mississippi and Kentucky [30], and some data suggest that A. americanum can transmit R. parkeri to guinea pigs [31]. Because lone star ticks account for the majority of recorded bites in the southern and southeastern United States [28], the occurrence and distribution of R. parkeri rickettsiosis could be greater than currently appreciated if A. americanum is involved in the transmission of this pathogen.
Ticks collected throughout the United States, including many that parasitize humans, are hosts to many distinct SFG rickettsiae [3, 17, 32–35]. The historical paradigm that SFG rickettsiae other than R. rickettsii associated with North American ticks were nonpathogenic existed in part because these agents had never been isolated from patients and because most diagnoses of SFG rickettsioses are established using serological assays that do not identify a specific etiologic agent. Contemporary investigators have suggested that some of these SFG rickettsiae may cause disease in humans [18, 32, 33, 37]. Several of these bacteria, including Rickettsia rhipicephali [17] and the nonspeciated serotypes 364D [32] and Tillamook [34], cause mild-to-severe disease in rodents. An SFG rickettsia nearly identical to Rickettsia honei was detected recently in Amblyomma cajennense ticks collected in south Texas [33], and R. honei has been linked with cases of rickettsial disease in Thailand and Australia [36]. In this context, it is reasonable to assume that other SFG rickettsiae will be identified as etiological agents of human illness in the United States. After >60 years of speculation, the confirmation of clinical disease caused by R. parkeri lends support to this assumption. Culture-based and molecular-based diagnostic approaches, when used to evaluate skin biopsy specimens of patients with eschar-associated illnesses following tick bites, may identify additional tickborne rickettsioses in the United States and other countries of the Western Hemisphere.
Acknowledgments
We thank Drs. Gregory Dasch and Joseph McDade and 2 anonymous reviewers, for their careful review of the manuscript; Dr. Wun-Ju Shieh, for assistance with image formatting; and William Lee, for preparing specimens for electron microscopy.





Comments