Abstract
Purpose
The systemic inflammation response index (SIRI) and systemic immune-inflammation index (SII) are based on neutrophil, monocyte, platelet, and lymphocyte counts. The SIRI and SII are used to predict the survival of patients with malignant tumors. It is well known that the inflammatory immune response is closely related to cancer occurrence and progression. In the present study, we evaluated the potential prognostic significance of SIRI and SII in patients with primary central nervous system lymphoma (PCNSL).
Methods
Fifty-eight consecutive patients were enrolled in this study between November 2006 and May 2022. Among the 58 patients, 47 patients with sufficient blood test data and follow-up were analyzed. The patients with steroid intake at the time point of the blood test and higher C-reactive protein were excluded.
Results
The median follow-up and survival times were 31 and 36 months, respectively. The optimal cutoff SIRI value was based on the receiver operating characteristic curve (ROC) for overall survival (OS) and stratified patients into low (< 1.43 × 109/L, n = 22) and high (≥ 1.43 × 109/L, n = 25) SIRI groups. The optimal cutoff SII value based on the ROC for OS stratified patients into low (< 694.9, n = 28) and high (≥ 694.9, n = 19) SII groups. A low SIRI value was associated with longer OS (p = 0.006). Furthermore, a low SII value was associated with longer OS (p = 0.044). The prognostic factors associated with prolonged survival in univariate analysis using the Cox proportional hazard model were age < 65 years, low SIRI, and low SII. The multivariate analysis demonstrated that age < 65 years and low SIRI independently predicted longer OS.
Conclusion
Simple, less expensive, and routinely ordered preoperative blood count assessments such as SIRI and SII predict the OS of patients with PCNSL. This study demonstrated that PCNSL is associated with pre-treatment systemic immune-inflammation states.
Similar content being viewed by others
Introduction
Primary central nervous system lymphoma (PCNSL) is an aggressive extranodal non-Hodgkin lymphoma that accounts for 3–5% of all primary intracranial tumors [1, 2]. Whole-brain radiotherapy (WBRT) alone for PCNSL results in overall survival (OS) of only 12 months [3]. Given the limited results of WBRT alone, the addition of WBRT to chemotherapy with high-dose methotrexate (HD-MTX) is a basic treatment option for PCNSL instead of WBRT alone. The HD-MTX regimen produces a high objective response rate of 50–90% [4,5,6]. Furthermore, rituximab, HD-MTX, procarbazine, and vincristine (R-MPV) is one of the most promising induction regimens. Compared to HD-MTX, R-MPV combined with reduced-dose WBRT, with or without high-dose cytarabine, yielded favorable outcomes for PCNSL [7]. As a consolidation regimen, autologous stem cell transplantation (ASCT) for younger patients has become one of the standard treatments for preventing relapses and avoiding WBRT-induced neurotoxicity [8, 9].
Preoperative prognostic factors can provide meaningful information to neurosurgeons and neuro-oncologists treating PCNSL. The International Extranodal Lymphoma Study Group (IELSG) score and the Memorial Sloan-Kettering Cancer Center (MSKCC) score were proposed for risk stratification in PCNSL [10, 11]. According to the IELSG score, age > 60 years, Eastern Cooperative Oncology Group performance status (ECOG PS) > 1, elevated lactate dehydrogenase (LDH) serum level, high cerebrospinal fluid (CSF) protein concentration, and involvement of deep regions of the brain (periventricular regions, basal ganglia, brainstem, and/or cerebellum) are significantly and independently associated with worse survival [10]. Although CSF is currently not always measured as a pre-treatment test, a challenge to the IELSG score is that the pre-treatment protein concentration in CSF is a predictor of prognosis. Furthermore, the MSKCC scores are divided into three groups based on pre-treatment age and Karnofsky performance score (KPS), each correlating with OS [11]. A recent report demonstrated that adding the LDH–lymphocyte ratio (LLR) to the MSKCC score yielded a more accurate scoring system [12].The circulating tumor DNA level in pre-treatment plasma indicated significantly shorter progression-free survival and OS in PCNSL [13].
Preoperative blood test data are a factor of interest in PCNSL and are crucial due to their predictive value. Inflammatory cytokines and chemokines produced by both tumor and stromal cells contribute to malignancy progression. A high neutrophil–lymphocyte ratio (NLR) was associated with increased peritumoral macrophage infiltration and the upregulation of several cytokines [14]. Pre-treatment NLR affected prognosis after chemotherapy for PCNSL [15]. In addition to the NLR, other blood test data have been reported as prognostic markers in patients with cancer: the platelet–lymphocyte ratio (PLR), systemic immune-inflammation index (SII), systemic inflammation response index (SIRI), and the prognostic nutritional index (PNI). However, only a few studies examined the importance of preoperative blood test data, including the SII and SIRI, in PCNSL [4, 15]. In the present study, the relationship between SII, SIRI, and PCNSL prognosis was examined.
Materials and methods
Patient characteristics
The patients’ medical records were reviewed to extract clinical information (sex, age, preoperative KPS score, blood test data, OS data). The blood test data included neutrophil, lymphocyte, monocyte, and platelet counts. The patients’ clinical data were retrospectively analyzed to evaluate the prognostic value of PCNSL. The Nara Medical University ethics committee approved this retrospective study (approval no. 3741). Between November 2006 and May 2022, 58 consecutive patients were treated with chemotherapy and radiotherapy for PCNSL at our hospital. All patients newly diagnosed with PCNSL, whose histological diagnosis was confirmed as diffuse large B-cell lymphoma, were retrospectively enrolled. This study included patients with full blood count results available for analysis before initial treatment. Three patients without adequate blood test data within 1 month of initial treatment and patients lost to follow-up were excluded. Four patients with steroid intake at the time point of the blood test were excluded: one had Sjögren’s syndrome, one had suspected demyelinating disease prior to the PCNSL diagnosis, and two were referred to our hospital after steroids had been started at their previous hospital. Furthermore, three patients with higher C-reactive protein (CRP) in the blood test pre-surgery (CRP value outliers calculated using the Smirnov-Grubbs test) were also excluded. Immunocompromised patients were not included in this series. The individual blood cell count data were extracted from the full blood count. The hematological counts were used to calculate several combined variables: NLR (neutrophil count/lymphocyte count), platelet–lymphocyte ratio (PLR, platelet count/lymphocyte count], lymphocyte–monocyte ratio (LMR, lymphocyte count/monocyte count), SII [(platelet count × neutrophil count)/lymphocyte count], and SIRI [(neutrophil count × monocyte count)/lymphocyte count].
The most appropriate therapy was identified by evaluating each patient prior to initial treatment. The evaluation was conducted by the tumor board review on brain tumors, a multidisciplinary team comprising neurosurgeons, neuro-oncologists, neuroradiologists, and radiation oncologists.
Clinical and radiological follow-up
Follow-up contrast-enhanced magnetic resonance imaging (MRI) was performed every 3 months after the end of initial treatment if possible. Once disease progression was detected, the decision regarding additional treatment for disease progression was based on evidence of clinical deterioration and associated imaging progression evaluated by the abovementioned tumor board review. If PCNSL recurrence was diagnosed with follow-up MRI using International PCNSL Collaborative Group (IPCG) criteria, additional treatment (additional radiotherapy, or chemotherapy) was administered or the patient was moved to the best supportive care. The therapeutic responses were assessed according to the IPCG criteria [16].
Statistical analysis
The median survival time was calculated using the Kaplan–Meier method. The log-rank test was used for univariate analyses. The prognostic factors of hematological markers, age (≥ 65 vs. < 65 years), sex, and pre-treatment KPS score (≥ 70 vs. < 70) were analyzed. Cox proportional hazards analysis was used to identify survival-associated factors at the univariate and multivariate levels. A receiver operating characteristic (ROC) curve was generated, and the area under the curve (AUC) was calculated to evaluate the prognostic power of the hematological markers and age for OS. CRP value outliers were calculated using the Smirnov-Grubbs test. All analyses were performed using EZR software (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [17], and p < 0.05 was considered statistically significant.
Results
Patient characteristics and survival factors
Overall, 47 patients were analyzed in this study. Table 1 summarizes the patients’ characteristics. There were 25 men and 22 women, with a median age of 67.6 years (range: 36–83 years). The median pre-treatment KPS was 66.2 ± 16.8. The PCNSL was in the frontal lobe (n = 14, 29.8%), parietal lobe (n = 13, 27.7%), basal ganglia (n = 13, 27.7%), temporal lobe (n = 11, 23.4%), cerebellum (n = 5, 10.6%), occipital lobe (n = 6.4%), brainstem (n = 1, 2.1%), intraventricular (n = 1, 2.1%), and disseminated (n = 4, 8.5%). Fifteen patients had multiple lesions. The median time from blood test to surgery was 8.9 ± 5.4 days. Chemotherapy was administered to 37 of 47 patients: 33 patients received HD-MTX (3.5 g/m), while four patients received chemotherapy consisting of rituximab, HD-MTX, procarbazine, and vincristine (R-MPV: rituximab, 375 mg/m2; MTX, 3.5 g/m2; procarbazine, 100 mg/m2; vincristine, 1.4 mg/m2). Forty-three of the 47 patients had received radiation therapy.
The median follow-up time was 31 months (range: 1–101 months). The median survival time (MST) was 36 months. The optimal NLR, PLR, LMR, SII, and SIRI cut-off values for OS were 1.93 (AUC = 0.629, sensitivity = 0.909, specificity = 0.359), 317 (AUC = 0.403, sensitivity = 0.117, specificity = 1.0), 3.0 (AUC = 0.525, sensitivity = 0.60, specificity = 0.571), 649.9 (AUC = 0.605, sensitivity = 0.484, specificity = 0.0.691), and 1.43 (AUC = 0.668, sensitivity = 0.705, specificity = 0.676), respectively (Table 2).
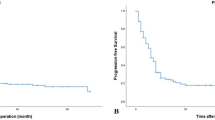
In the entire cohort, no significant differences by for OS using the Kaplan–Meier method were detected for sex (p = 0.247) and the pre-treatment KPS score (p = 0.596), NLR (p = 0.267), PLR (p = 0.274), and LMR (p = 0.148). There were significant differences for age (p = 0.002), SII (p = 0.044), and SIRI (p = 0.007) (Figs. 1, 2 and Table 1). The factors associated with survival were identified using Cox proportional hazards analysis at the univariate and multivariate levels. The Cox proportional hazards univariate analysis demonstrated that age < 65 years [hazard ratio [HR]: 0.69, 95% confidence interval (CI): 0.37–1.31, p = 0.254], low SII (HR: 1.90, 95% CI: 1.00–3.61, p = 0.049), and low SIRI (HR: 2.40, 95% CI: 1.24–4.62, p = 0.009) were significantly associated with longer OS. The Cox proportional hazards multivariate analysis demonstrated that age < 65 years (HR: 2.82, 95% CI: 1.33–5.95, p = 0.007) and low SIRI (HR: 2.21, 95% CI: 1.13–4.35, p = 0.021) were significantly associated with longer OS, while low SII tended to be associated with longer OS (Table 3).
SII
The SII cutoff value for OS was 694.9. The MST of patients with SII ≥ 694.9 (n = 19) and SII < 594.4 (n = 28) was 30 and 38 months, respectively (p = 0.044) (Fig. 2A). The Cox proportional hazards multivariate analysis that included age and SII as continuous values revealed that patients with higher SII values (HR: 1.83; 95% CI: 0.95–3.50; p = 0.07) adjusted by age (HR: 2.94; 95% CI: 1.41–6.17; p = 0.004) had a tendency of increased risk of death (Table 3).
SIRI
The SIRI cutoff value for OS was 1.43. The MST of patients with SIRI ≥ 1.43 × 109/L (n = 25) and SIRI < 1.43 × 109/L (n = 22) was 30 and 39 months, respectively (p = 0.007) (Fig. 1B). The Cox proportional hazards multivariate analysis that included age and SIRI as continuous values revealed that higher SIRI values (HR: 2.21; 95% CI = 1.13–4.35; p = 0.021) adjusted by age (HR: 2.82; 95% CI = 1.33–5.95; p = 0.007) were independent prognostic factors of increased risk of death (Table 3).
Discussion
PCNSL is one of the most aggressive malignant brain tumors. WBRT alone for PCNSL cannot prevent recurrence and the OS is only approximately 1 year [3]. While patients treated with WBRT alone had a 2-year survival rate of only 30%, advances in methotrexate-based chemotherapy have improved the 5-year survival rate to 50–70% [1]. It is very important to know which group of patients can be expected to survive long-term when treating diseases with poor prognosis. Accordingly, various preoperative prediction systems have been proposed [10,11,12]. Some have argued that the IELSG score is not suitable in actual clinical practice as it requires the measurement of blood LDH and CSF protein [18]. The Taipei score is a new scoring system based on age, ECOG PS, and tumor location. The advantage of the proposed Taipei score is that it can predict the prognosis for both progression-free survival (PFS) and OS [19].
The immune and inflammatory cells include neutrophils, monocytes, lymphocytes, and platelets, which can be detected in circulating blood and might contribute to cancer invasion and metastasis [20, 21]. Pre-treatment blood test data predicted post-treatment prognosis in many malignancies, including colon, kidney, prostate, breast, lung, and bladder cancer [22,23,24,25,26,27]. As mentioned above, the NLR is the most widely used blood test data and is simple and easily obtained. Reports of the SII and SIRI in addition to the NLR have recently increased. The pre-treatment NLR affected the prognosis after chemotherapy for PCNSL [15]. Conversely, the NLR was not prognostic for OS after chemotherapy for PCNSL [4]. Berthelot et al. demonstrated that a low KPS and a high LDH rate were associated with poor OS. Subsequently, they evaluated the prognostic value of immune circulating factors such as the CD4 + –CD8 + ratio > 1.97, which was associated with poor PFS and tended to be associated with worse OS [28].
In this study, we examined the outcomes in patients with PCNSL and analyzed pre-treatment blood test data. We analyzed the pre-treatment blood test data, and determined that NLR, PLR, and LMR, which were previously reported to be useful, did not affect OS. One reason for this result is the very small sample size of this study. NLR, LMR, and PLR combine two blood test factors, while SII and SIRI combine three blood factors, which may reflect the patient’s status more accurately. The NLR can be measured via a blood test and has been studied as a potential biomarker in cancers, cardiovascular diseases, and autoimmune disorders. An elevated NLR may be a consequence of more neutrophils, fewer lymphocytes, or both. Many malignancies release myeloid growth factors, which subsequently increase neutrophil production. Meanwhile, circulating neutrophils contain and secrete vascular endothelial growth factor, tumor necrosis factor, and other cytokines that contribute to tumor progression. Therefore, a higher NLR leads to tumor angiogenesis and aids neoplasm proliferation. However, acute conditions (bacterial or viral infections) or drug treatments might overlap chronic inflammation and affect neutrophil and lymphocyte counts [14]. Furthermore, glucocorticoids can affect neutrophil counts, which affect the NLR, SIRI, and SII values [29]. Similarly, platelets are a critical source of cytokines, such as transforming growth factor β, platelet-derived growth factor, and vascular endothelial growth factor, which induce angiogenesis and cell invasion [29]. Monocytes are innate immune cells important in tumor progression, invasion, and metastasis and can be grouped into macrophages and myeloid-derived suppressor cells [29]. Preoperative evaluation that includes NLR, SIRI, and SII might lead to a more accurate prognosis in PCNSL.
The SII is a prognostic predictor of various tumor types and was a useful prognostic factor in various cancers including renal cell carcinoma, gastric cancer, colorectal cancer, breast cancer, hepatocellular carcinoma, pancreatic cancer, and non-small cell lung cancer [27]. Univariate analysis demonstrated that the SII affected the PFS and OS after chemotherapy in PCNSL, but the results were not significant in multivariate analysis [15]. Another study reported that univariate and multivariate analysis revealed that the SII had a significant effect on OS at a cutoff value of 1016 [4].
The SIRI is calculated using neutrophils, monocytes, and lymphocytes and is used to predict the survival of patients with cancer. Furthermore, the SIRI can fully evaluate the balance between host immune and inflammatory conditions [21, 30, 31]. Similar to the SII, local and systemic inflammation are important promoters of tumorigenesis and tumor cell proliferation. Feng et al. demonstrated that the complete blood count score model predicted inferior prognosis in PCNSL. Subsequently, they proposed a new scoring system by combining four factors (NLR, PLR, SII, SIRI). In the present study, the SIRI was a promising prognostic factor of OS in PCNSL. In PCNSL, more accurate prognostic data can be obtained by combining monocytes and platelets than by NLR alone.
Limitations
This was a single-center retrospective analysis, which might have caused analytical bias. Furthermore, the sample size of patients with PCNSL was small. Additionally, the lack of pre-treatment blood cell count data in the excluded patients might have influenced the analysis. Moreover, preoperative signs of infection and fever were not considered, and there might have been bias due to acute inflammation.
Conclusion
The simple, less expensive, and routinely ordered preoperative blood count assessments such as the SII and SIRI predicted the OS of patients with PCNSL.
Data availability
No datasets were generated or analysed during the current study.
References
Mishima K, Nishikawa R, Narita Y, Mizusawa J, Sumi M, Koga T, Sasaki N, Kinoshita M, Nagane M, Arakawa Y, Yoshimoto K, Shibahara I, Shinojima N, Asano K, Tsurubuchi T, Sasaki H, Asai A, Sasayama T, Momii Y, Sasaki A, Nakamura S, Kojima M, Tamaru JI, Tsuchiya K, Gomyo M, Abe K, Natsumeda M, Yamasaki F, Katayama H, Fukuda H (2023) Randomized phase III study of high-dose methotrexate and whole-brain radiotherapy with/without temozolomide for newly diagnosed primary CNS lymphoma: JCOG1114C. Neuro Oncol 25:687–698. https://doi.org/10.1093/neuonc/noac246
(2017) Brain tumor registry of Japan (2005–2008). Neurol Med Chir (Tokyo) 57:9–102. https://doi.org/10.2176/nmc.sup.2017-0001
Nelson DF, Martz KL, Bonner H, Nelson JS, Newall J, Kerman HD, Thomson JW, Murray KJ (1992) Non-Hodgkin’s lymphoma of the brain: can high dose, large volume radiation therapy improve survival? Report on a prospective trial by the Radiation Therapy Oncology Group (RTOG): RTOG 8315. Int J Radiat Oncol Biol Phys 23:9–17. https://doi.org/10.1016/0360-3016(92)90538-s
Luo Q, Yang C, Fu C, Wu W, Wei Y, Zou L (2021) Prognostic role of blood markers in primary central nervous system lymphoma patients treated with high-dose methotrexate-based therapy. Front Oncol 11:639644. https://doi.org/10.3389/fonc.2021.639644
Ferreri AJ, Cwynarski K, Pulczynski E, Ponzoni M, Deckert M, Politi LS, Torri V, Fox CP, Rosee PL, Schorb E, Ambrosetti A, Roth A, Hemmaway C, Ferrari A, Linton KM, Ruda R, Binder M, Pukrop T, Balzarotti M, Fabbri A, Johnson P, Gorlov JS, Hess G, Panse J, Pisani F, Tucci A, Stilgenbauer S, Hertenstein B, Keller U, Krause SW, Levis A, Schmoll HJ, Cavalli F, Finke J, Reni M, Zucca E, Illerhaus G, International Extranodal Lymphoma Study G (2016) Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol 3:e217-227. https://doi.org/10.1016/S2352-3026(16)00036-3
Ferreri AJ, Reni M, Foppoli M, Martelli M, Pangalis GA, Frezzato M, Cabras MG, Fabbri A, Corazzelli G, Ilariucci F, Rossi G, Soffietti R, Stelitano C, Vallisa D, Zaja F, Zoppegno L, Aondio GM, Avvisati G, Balzarotti M, Brandes AA, Fajardo J, Gomez H, Guarini A, Pinotti G, Rigacci L, Uhlmann C, Picozzi P, Vezzulli P, Ponzoni M, Zucca E, Caligaris-Cappio F, Cavalli F, International Extranodal Lymphoma Study G (2009) High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet 374:1512–1520. https://doi.org/10.1016/S0140-6736(09)61416-1
Yamaguchi J, Ohka F, Lushun C, Motomura K, Aoki K, Takeuchi K, Nagata Y, Ito S, Mizutani N, Ohno M, Suzaki N, Takasu S, Seki Y, Kano T, Wakabayashi K, Oyama H, Kurahashi S, Tanahashi K, Hirano M, Shimizu H, Kitano Y, Maeda S, Yamazaki S, Wakabayashi T, Kondo Y, Natsume A, Saito R (2023) CD79B Y196 mutation is a potent predictive marker for favorable response to R-MPV in primary central nervous system lymphoma. Cancer Med 12:7116–7126. https://doi.org/10.1002/cam4.5512
Houillier C, Dureau S, Taillandier L, Houot R, Chinot O, Molucon-Chabrot C, Schmitt A, Gressin R, Choquet S, Damaj G, Peyrade F, Abraham J, Delwail V, Gyan E, Sanhes L, Cornillon J, Garidi R, Delmer A, Al Jijakli A, Morel P, Waultier A, Paillassa J, Chauchet A, Gastinne T, Laadhari M, Plissonnier AS, Feuvret L, Cassoux N, Touitou V, Ricard D, Hoang-Xuan K, Soussain C, Lymphoma LOCNfC (2022) Radiotherapy or autologous stem-cell transplantation for primary CNS lymphoma in patients age 60 years and younger: long-term results of the randomized phase II PRECIS study. J Clin Oncol 40:3692–3698. https://doi.org/10.1200/JCO.22.00491
Ferreri AJM, Cwynarski K, Pulczynski E, Fox CP, Schorb E, La Rosee P, Binder M, Fabbri A, Torri V, Minacapelli E, Falautano M, Ilariucci F, Ambrosetti A, Roth A, Hemmaway C, Johnson P, Linton KM, Pukrop T, SonderskovGorlov J, Balzarotti M, Hess G, Keller U, Stilgenbauer S, Panse J, Tucci A, Orsucci L, Pisani F, Levis A, Krause SW, Schmoll HJ, Hertenstein B, Rummel M, Smith J, Pfreundschuh M, Cabras G, Angrilli F, Ponzoni M, Deckert M, Politi LS, Finke J, Reni M, Cavalli F, Zucca E, Illerhaus G, International Extranodal Lymphoma Study G (2017) Whole-brain radiotherapy or autologous stem-cell transplantation as consolidation strategies after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: results of the second randomisation of the International Extranodal Lymphoma Study Group-32 phase 2 trial. Lancet Haematol 4:e510–e523. https://doi.org/10.1016/S2352-3026(17)30174-6
Ferreri AJ, Blay JY, Reni M, Pasini F, Spina M, Ambrosetti A, Calderoni A, Rossi A, Vavassori V, Conconi A, Devizzi L, Berger F, Ponzoni M, Borisch B, Tinguely M, Cerati M, Milani M, Orvieto E, Sanchez J, Chevreau C, Dell’Oro S, Zucca E, Cavalli F (2003) Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol 21:266–272. https://doi.org/10.1200/JCO.2003.09.139
Abrey LE, Ben-Porat L, Panageas KS, Yahalom J, Berkey B, Curran W, Schultz C, Leibel S, Nelson D, Mehta M, DeAngelis LM (2006) Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol 24:5711–5715. https://doi.org/10.1200/JCO.2006.08.2941
Gao Y, Wei L, Kim SJ, Wang L, He Y, Zheng Y, Bertero L, Pellerino A, Cassoni P, Tamagnone L, Theresa PK, Deutsch A, Zhan H, Lai J, Wang Y, You H (2021) A novel prognostic marker for primary CNS lymphoma: lactate dehydrogenase-to-lymphocyte ratio improves stratification of patients within the low and intermediate MSKCC risk groups. Front Oncol 11:696147. https://doi.org/10.3389/fonc.2021.696147
Mutter JA, Alig SK, Esfahani MS, Lauer EM, Mitschke J, Kurtz DM, Kuhn J, Bleul S, Olsen M, Liu CL, Jin MC, Macaulay CW, Neidert N, Volk T, Eisenblaetter M, Rauer S, Heiland DH, Finke J, Duyster J, Wehrle J, Prinz M, Illerhaus G, Reinacher PC, Schorb E, Diehn M, Alizadeh AA, Scherer F (2023) Circulating tumor DNA profiling for detection, risk stratification, and classification of brain lymphomas. J Clin Oncol 41:1684–1694. https://doi.org/10.1200/JCO.22.00826
Lin YJ, Wei KC, Chen PY, Lim M, Hwang TL (2021) Roles of neutrophils in glioma and brain metastases. Front Immunol 12:701383. https://doi.org/10.3389/fimmu.2021.701383
Feng Y, Liu Y, Zhong M, Wang L (2021) Complete blood count score model predicts inferior prognosis in primary central nervous system lymphoma. Front Oncol 11:618694. https://doi.org/10.3389/fonc.2021.618694
Abrey LE, Batchelor TT, Ferreri AJ, Gospodarowicz M, Pulczynski EJ, Zucca E, Smith JR, Korfel A, Soussain C, DeAngelis LM, Neuwelt EA, O’Neill BP, Thiel E, Shenkier T, Graus F, van den Bent M, Seymour JF, Poortmans P, Armitage JO, Cavalli F, International Primary CNSLCG (2005) Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol 23:5034–5043. https://doi.org/10.1200/JCO.2005.13.524
Kanda Y (2013) Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant 48:452–458. https://doi.org/10.1038/bmt.2012.244
Jahr G, Broi MD, Holte H Jr, Beiske K, Meling TR (2018) Evaluation of memorial Sloan-Kettering cancer center and international extranodal lymphoma study group prognostic scoring systems to predict overall survival in intracranial primary CNS lymphoma. Brain Behav 8:e00928. https://doi.org/10.1002/brb3.928
Liu CJ, Lin SY, Yang CF, Yeh CM, Kuan AS, Wang HY, Tsai CK, Gau JP, Hsiao LT, Chen PM, Liu YC, Hong YC, Ko PS, Liu JH, Lin CH (2020) A new prognostic score for disease progression and mortality in patients with newly diagnosed primary CNS lymphoma. Cancer Med 9:2134–2145. https://doi.org/10.1002/cam4.2872
Diakos CI, Charles KA, McMillan DC, Clarke SJ (2014) Cancer-related inflammation and treatment effectiveness. Lancet Oncol 15:e493-503. https://doi.org/10.1016/S1470-2045(14)70263-3
Jiang S, Wang S, Wang Q, Deng C, Feng Y, Ma F, Ma J, Liu X, Hu C, Hou T (2021) Systemic inflammation response index (SIRI) independently predicts survival in advanced lung adenocarcinoma patients treated with first-generation EGFR-TKIs. Cancer Manag Res 13:1315–1322. https://doi.org/10.2147/CMAR.S287897
Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ (2005) Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol 91:181–184. https://doi.org/10.1002/jso.20329
Keizman D, Ish-Shalom M, Huang P, Eisenberger MA, Pili R, Hammers H, Carducci MA (2012) The association of pre-treatment neutrophil to lymphocyte ratio with response rate, progression free survival and overall survival of patients treated with sunitinib for metastatic renal cell carcinoma. Eur J Cancer 48:202–208. https://doi.org/10.1016/j.ejca.2011.09.001
Chen J, Deng Q, Pan Y, He B, Ying H, Sun H, Liu X, Wang S (2015) Prognostic value of neutrophil-to-lymphocyte ratio in breast cancer. FEBS Open Bio 5:502–507. https://doi.org/10.1016/j.fob.2015.05.003
Sebastian NT, Raj R, Prasad R, Barney C, Brownstein J, Grecula J, Haglund K, Xu-Welliver M, Williams TM, Bazan JG (2020) Association of pre- and posttreatment neutrophil-lymphocyte ratio with recurrence and mortality in locally advanced non-small cell lung cancer. Front Oncol 10:598873. https://doi.org/10.3389/fonc.2020.598873
Gondo T, Nakashima J, Ohno Y, Choichiro O, Horiguchi Y, Namiki K, Yoshioka K, Ohori M, Hatano T, Tachibana M (2012) Prognostic value of neutrophil-to-lymphocyte ratio and establishment of novel preoperative risk stratification model in bladder cancer patients treated with radical cystectomy. Urology 79:1085–1091. https://doi.org/10.1016/j.urology.2011.11.070
Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, Zhang X, Wang WM, Qiu SJ, Zhou J, Fan J (2014) Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res 20:6212–6222. https://doi.org/10.1158/1078-0432.CCR-14-0442
Berthelot A, Bequet C, Harlay V, Petrirena G, Campello C, Barrie M, Appay R, Chinot O, Tabouret E (2022) Prognostic value of circulating lymphocyte subsets in primary central nervous system lymphoma. J Neurooncol 159:15–22. https://doi.org/10.1007/s11060-022-04032-5
Chen T, Tang M, Zhou Y, Wang Z, Li S, Wang H, Lu Y, Wang J, Shen W (2023) Pretreatment lymphocyte-to-monocyte ratio as a prognostic factor and influence on dose-effect in fractionated stereotactic radiotherapy for oligometastatic brain metastases in non-small cell lung cancer patients. Front Oncol 13:1216852. https://doi.org/10.3389/fonc.2023.1216852
Chen L, Kong X, Wang Z, Wang X, Fang Y, Wang J (2020) Pretreatment systemic inflammation response index in patients with breast cancer treated with neoadjuvant chemotherapy as a useful prognostic indicator. Cancer Manag Res 12:1543–1567. https://doi.org/10.2147/CMAR.S235519
Tang Y, Shao Y, Hu X, Ren S, Li X (2023) Validation and comparison of prognostic value of different preoperative systemic inflammation indices in non-metastatic renal cell carcinoma. Int Urol Nephrol 55:2799–2807. https://doi.org/10.1007/s11255-023-03724-9
Funding
There is no funding of any kind for this study.
Author information
Authors and Affiliations
Contributions
Conception and design: RMat, RMae, TM, TN; Acquisition of data: RMat; Analysis and interpretation of data: RMat, RMae, TM, TN; Drafting the article: RMat; critically revising the paper: All authors.
Corresponding author
Ethics declarations
Ethics approval
This retrospective study was approved by the Nara Medical University ethics committee (approval no. 3741).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Matsuda, R., Maeoka, R., Morimoto, T. et al. Pre-treatment systemic inflammation response index and systemic immune inflammation in patients with primary central nerve system lymphoma as a useful prognostic indicator. J Neurooncol (2024). https://doi.org/10.1007/s11060-024-04692-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11060-024-04692-5