Barium Sulfate
Barium sulfate is an inorganic compound with the chemical formula BaSO₄, commonly found in the mineral barite. It is widely used in various industries, particularly in medical imaging to help doctors see more clearly during X-rays. This compound is known for its high density and ability to block X-rays. Despite its usefulness in imaging, barium sulfate is non-toxic and not absorbed by the body, making it safe for medical purposes. In chemistry, barium sulfate serves as a crucial component in testing and research due to its stability and insolubility in water.
What is Barium Sulfate?
Chemical Names and Formulas
| Property | Value |
|---|---|
| Formula | BaSO₄ |
| Hill Formula | BaO₄S |
| Name | Barium sulfate |
| IUPAC Name | Barium(+2) cation sulfate |
| Alternate Names | Actybaryte, Barium(+2) cation sulfate, Barium sulphate, Baryte, Barytes, Blanc fixe |
Structure of Barium Sulfate
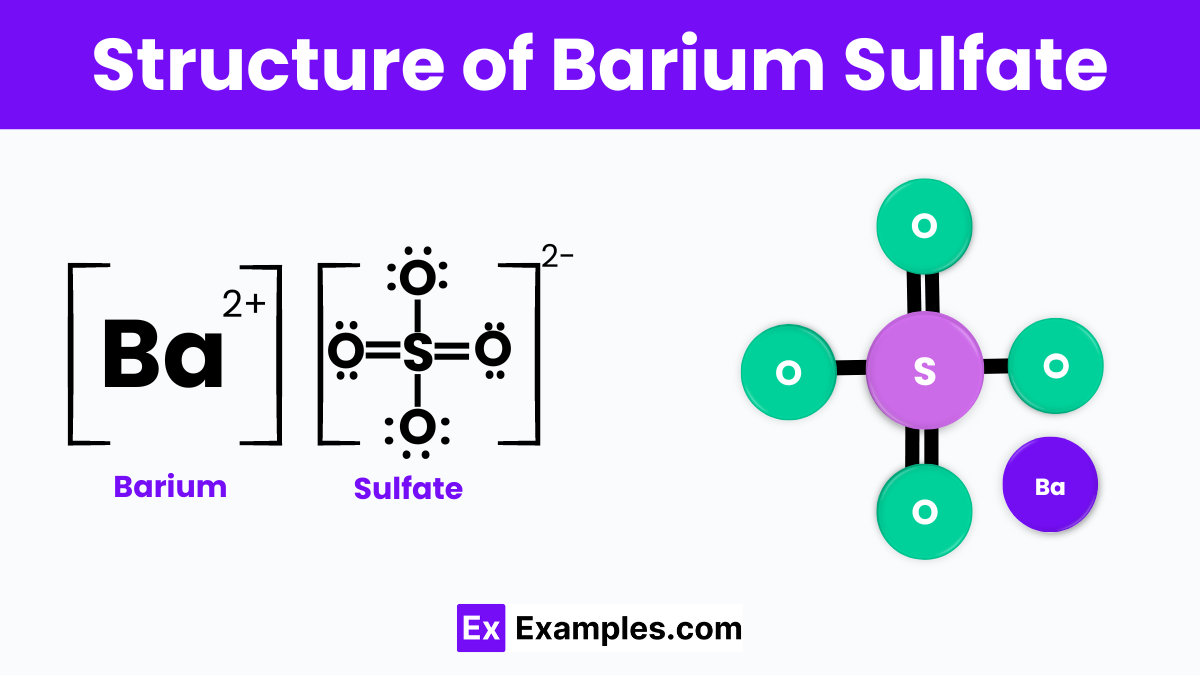
Barium sulfate has a simple structure where the barium ions (Ba²⁺) are linked to sulfate ions (SO₄²⁻). This forms a solid, crystal-like material that is white and powdery. In its structure, each barium ion is surrounded by sulfate ions, and this arrangement leads to its high stability and inability to dissolve in water. This structure makes barium sulfate very useful in many industries, such as medical imaging and manufacturing, where materials that do not change easily are needed. Its stable and non-reactive nature comes from this unique arrangement of ions.
Preparation of Barium Sulfate
Barium sulfate is typically prepared by mixing solutions of barium ions and sulfate ions. When barium chloride (BaCl₂) is combined with sulfuric acid (H₂SO₄), a chemical reaction occurs. This reaction results in the formation of barium sulfate (BaSO₄), which appears as a white precipitate because it is insoluble in water. The chemical equation for this reaction is:
This simple method ensures that barium sulfate forms quickly and easily, making it accessible for use in various applications, including medical diagnostics and industrial manufacturing. The solid that forms is then collected and purified for use.
Physical Properties of Barium Sulfate
| Property | Description |
|---|---|
| Appearance | White crystalline solid |
| Solubility | Insoluble in water, slightly soluble in strong acids |
| Density | Approximately 4.5 grams per cubic centimeter |
| Melting Point | About 1580 degrees Celsius (does not melt but decomposes) |
| Molecular Weight | 233.39 grams per mole |
| Chemical Stability | Highly stable; non-reactive with most substances |
| Toxicity | Generally non-toxic, but can be hazardous if inhaled as dust |
Chemical Properties of Barium Sulfate
Insolubility in Water
Barium sulfate is highly insoluble in water, which means it does not dissolve when mixed with water. This property is crucial for its use in medical imaging, as it stays in the digestive system without being absorbed by the body.
Resistance to Acids
It is resistant to most acids, with the exception of sulfuric acid. This resistance to chemical reactivity makes it stable in harsh environments, suitable for various industrial applications.
Non-reactivity
Barium sulfate does not react with other elements or compounds under normal conditions. This non-reactivity makes it safe for use in a wide range of applications, including as a pigment in paints and plastics.
Thermal Stability
Barium sulfate decomposes when exposed to very high temperatures (above 1600°C). Upon decomposition, it can produce sulfur dioxide and oxygen.
Its thermal stability up to high temperatures is useful in ceramics and glass production.
Barium Sulfate (BaSO₄) Chemical Compound Information
Chemical Identifiers
| Property | Value |
|---|---|
| CAS Registry Number | 7727-43-7 |
| PubChem Compound ID | 24414 |
| PubChem Substance ID | 24852116 |
| SMILES Identifier | [O-]S(=O)(=O)[O-].[Ba+2] |
| InChI Identifier | InChI=1/Ba.H2O4S/c;1-5(2,3)4/h;(H2,1,2,3,4)/q+2;/p-2/fBa.O4S/qm;-2 |
| RTECS Number | CR0600000 |
| MDL Number | MFCD00003455 |
NFPA Label
| Property | Value |
|---|---|
| NFPA Health Rating | 0 |
| NFPA Fire Rating | 0 |
| NFPA Reactivity Rating | 0 |
Uses of Barium Sulfate
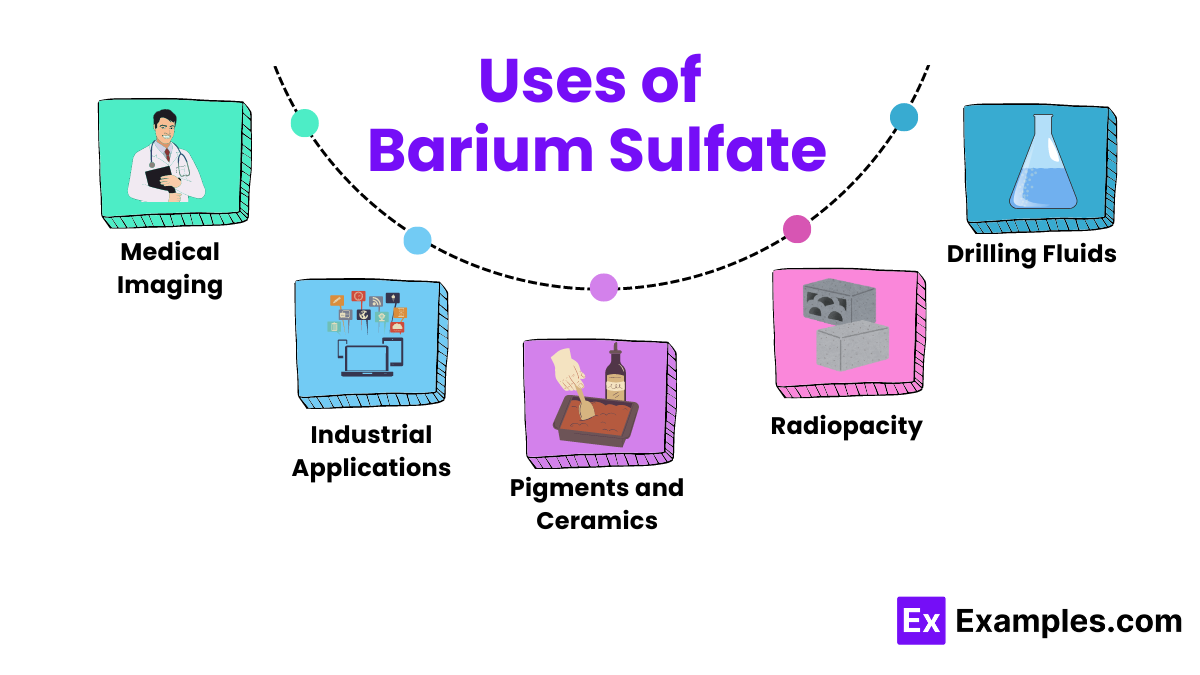
Medical Imaging
Medical professionals widely use barium sulfate in medical imaging, particularly for X-rays of the gastrointestinal tract. Because it is insoluble in water, it coats the inside of the stomach and intestines, providing a clear contrast that helps doctors diagnose conditions like ulcers, tumors, and blockages.
Industrial Applications
In the industrial sector, manufacturers use barium sulfate as a filler in plastics and rubbers to enhance their strength and resistance. It also finds use in paints and coatings to improve brightness and durability due to its high density and chemical inertness.
Drilling Fluids
In oil and gas drilling operations, companies add barium sulfate to drilling muds to boost the fluid’s density. This adjustment helps control the pressure in the well, preventing oil well blowouts and stabilizing the borehole.
Pigments and Ceramics
Paint and paper manufacturers use barium sulfate as a pigment in white paints and as a paper coating, enhancing whiteness and brightness. In ceramics, it acts as a flux and increases the glossiness of glazes.
Radiopacity
Manufacturers use barium sulfate in the production of concrete, bricks, and tiles to make them X-ray opaque. This feature proves particularly useful in hospitals and clinics, where walls must shield X-ray radiation.
Barium Sulfate: Effects on Human Body
- Diagnostic Use: When used as a contrast agent in X-rays, barium sulfate remains safe as it stays within the digestive tract without absorbing into the body. It provides clear images of the gastrointestinal area to assist in medical diagnoses.
- Inhalation Risks: If workers inhale barium sulfate dust, it can cause respiratory issues. Industries that handle barium sulfate in powder form should ensure workers wear protective gear to prevent inhalation.
- Non-Toxicity: Barium sulfate is non-toxic when it remains in the gastrointestinal tract or embedded in materials. However, accidental ingestion of large amounts beyond typical diagnostic use can lead to complications due to its physical presence and volume.
- Allergic Reactions: In rare cases, individuals may react allergically to barium sulfate contrast materials. Symptoms such as hives, itching, or difficulty breathing require immediate medical attention.
FAQs
Is Barium Sulfate Hazardous?
Barium sulfate is generally not hazardous when used as directed, particularly in specific contexts such as medical imaging and industrial applications.
What to Do After Drinking Barium Sulfate?
After consuming barium sulfate for an X-ray, drink plenty of fluids to help flush it out and follow your doctor’s instructions.
What Does Barium Sulfate Taste Like?
Barium sulfate often has a chalky taste, and manufacturers usually flavor medical solutions to improve their palatability.
How Long Does Barium Sulfate Stay in the Body?
The body typically excretes barium sulfate within 24 to 48 hours after someone consumes it.