This is a summary of: Warren, A. L. et al. Structural pharmacology and therapeutic potential of 5-methoxytryptamines. Nature https://doi.org/10.1038/s41586-024-07403-2 (2024).
The problem
Psychedelics are psychoactive substances that have been used in shamanic rituals for millennia1, but are now attracting attention because of their potential in treating neuropsychiatric disorders, including depression and substance-use disorders. Psychedelics such as psilocybin2 (found in ‘magic mushrooms’), lysergic acid diethylamide (LSD) and 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT, found in the secretions of Colorado River toads, Incilius alvarius), have already entered clinical trials with promising early results. Yet it is still not fully understood how these drugs engage molecular targets in the brain, and how this leads to their therapeutic and hallucinogenic effects. Many psychedelics (including 5-MeO-DMT) are chemically similar to the neurotransmitter serotonin, and bind to several of the 12 human G-protein-coupled serotonin receptors3. Most studies have focused on the 5-HT2A serotonin receptor, but little is known about the role of other serotonin receptors, which might have a crucial role in the complex psychoactive and therapeutic effects of these drugs.
The discovery
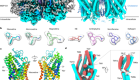
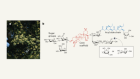
Using a highly interdisciplinary approach, we set out to investigate how the most prominent psychedelics — LSD, psilocybin, mescaline (peyote), N,N-dimethyltryptamine (DMT, found in the psychedelic brew ayahuasca) and 5-MeO-DMT — trigger biological signalling through receptors other than 5-HT2A. We found that 5-MeO-DMT is almost equally active at 5-HT2A and 5-HT1A, a serotonin receptor that has previously been implicated4 in the behavioural effects of 5-MeO-DMT. To understand the effects of the 5-HT1A activity of 5-MeO-DMT we implemented a medicinal chemistry programme, guided by the cryo-electron microscopy structures of psychedelics bound to 5-HT1A, to discover 5-MeO-DMT-like compounds that selectively act through 5-HT1A, and not through 5-HT2A. We then used the most 5-HT1A-selective compound in behavioural studies in mice to investigate the role of 5-HT1A in the hallucinogenic and antidepressant effects of 5-MeO-DMT.
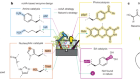
We first discovered that 5-MeO-DMT and serotonin bind to 5-HT1A in different ways — a finding that enabled the rational design of a series of 5-HT1A-selective compounds based on the structures of the receptor in complex with 5-MeO-DMT or serotonin. The most selective compound in this series binds to 5-HT1A in a similar way to clinically used antidepressants and anti-anxiety agents such as buspirone. Indeed, we found that a 5-HT1A-selective 5-MeO-DMT analogue produced no signs of hallucinations in animal models, but still showed potent antidepressant effects (Fig. 1). These findings suggest that receptors other than 5-HT2A not only modulate behavioural effects caused by psychedelics, but possibly also contribute substantially to their therapeutic potential.

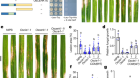
Figure 1 | An analogue of the psychedelic 5-MeO-DMT shows potent antidepressant-like activity in mice. a, Structure of 4-F,5-MeO-PyrT (4-fluoro-5-methoxy-N,N-pyrrolidinyl-tryptamine), which binds selectively to the serotonin receptor 5-HT1A. b, When tested in a behavioural assay that uses stress-induced avoidance of social interactions in mice as a model of depression, 4-F,5-MeO-PyrT was found to restore social interactions in stressed mice (compare first two sets of results); interaction ratio is a measure of social interactions; n is the number of mice per cohort; numbers over brackets represent P values. Co-administration of 4-F,5-MeO-PyrT and WAY-100635, a compound that blocks 5-HT1A receptors, reverses this effect (fourth set of results), indicating that the antidepressant-like effects of 4-F,5-MeO-PyrT are mediated by 5-HT1A.
The implications
Psychedelics cause a wide range of subjective effects in users, which vary even more greatly between different drugs5. Our studies further emphasize how serotonin receptors such as 5-HT1A probably modulate the subjective effects of the psychedelic experience, in addition to having a potentially pivotal role in their therapeutic effects. Given the heterogeneity and complexity of depression and psychiatric conditions in general, our studies strengthen the case that specific psychedelics will be most suitable for treating distinct types of depression.
Despite our finding that a 5-MeO-DMT derivative lacks hallucinogenic-like effects while retaining antidepressant-like activity in mice, it remains to be seen whether these results translate to humans. Furthermore, our studies focused solely on the role of 5-HT1A in the pharmacology of 5-MeO-DMT, but serotonin receptors other than 5-HT1A and 5-HT2A probably also contribute to the subjective and therapeutic effects of many psychedelics.
We are currently pursuing multiple lines of further research. For instance, we are investigating which brain circuitry is responsible for 5-HT1A-mediated modulation of psychedelic effects, and how the psychoactive profile of analogues of 5-MeO-DMT changes as their selectivity for 5-HT1A versus 5-HT2A increases. We are also looking into the role of other serotonin receptors in the molecular and behavioural pharmacology of psychedelics. — Daniel Wacker is at the Icahn School of Medicine at Mount Sinai, New York City, New York, USA, and Dalibor Sames is at Columbia University, New York City, New York, USA.
Behind the paper
D.S. and I had long known about each other’s work, but had never met. I was therefore excited when he reached out about a possible collaboration in 2020. We quickly discovered our shared opinion that there had been too much focus on 5-HT2A in psychedelics research, and that other receptors were under-investigated. That was the start of our joint study, even though neither of our laboratories has been able to secure funding for psychedelic work. However, D.S. and I had funding to study iboga alkaloids, which are structurally related to 5-MeO-DMT and serotonergic signalling. Perhaps the most difficult part of this work was the iterative synthesis and characterization of more than 100 compounds, which took several years. But it was that much more exciting when we found that the most 5-HT1A-selective 5-MeO-DMT analogue showed antidepressant-like effects without the 5-HT2A-related hallucinations. — D.W.

 Read the paper: Structural pharmacology and therapeutic potential of 5-methoxytryptamines
Read the paper: Structural pharmacology and therapeutic potential of 5-methoxytryptamines