Abstract
Metastasis is the most devastating attribute of breast cancer (BC) that leads to high mortality. It is a complex process of tumor cell migration, invasion, and angiogenesis. In this study, we evaluated the effect of ERA on BC metastasis and BC progression in vivo. The transwell invasion/migration and wound healing assays showed that ERA treatment significantly reduced the invasion and migration of BC cell lines. The expression of mesenchymal (E-cadherin and N-cadherin), matrix metalloproteinases (MMP2, MMP9), and stemness markers (Oct3) were down-regulated by ERA. Furthermore, ERA down-regulated angiogenic chemokines (CXCL1/2/3, CXCL5, and CXCL12) expression in the highly metastatic MDA-MB-231 cell line. The clonogenic survival of BC cells was also reduced by ERA treatment. Strikingly, ERA prevented DMBA-induced tumor growth in Swiss albino mice as depicted by a high animal survival rate (84%) in the ERA group and histopathological analysis. Conclusively, this study revealed that ERA possesses anti-metastatic potential and also reduces the growth of BC in vivo. Moreover, the GC–MS data revealed the presence of biologically active compounds (Lupeol, Phytol, phytosterol) and some rare (9, 19-Cyclolanost) phyto metabolites in ERA extract. However, further studies are suggestive to identify and isolate the therapeutic agents from ERA to combat BC and metastasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metastatic breast cancer (BC) refers to an advanced (stage IV) BC where cancer cells detach from the primary breast tissue and spread to other parts of the body most often to bones, lungs, liver, and the brain [1]. About 6% of BC patients are diagnosed with metastatic BC at the time of initial diagnosis whereas, 30% of BC survivors eventually develop metastasis [2]. Metastasis is proved to be a major underlying cause of death in the majority of BC patients. The 5-year survival rate of BC patients with distant metastasis declined to 22% in comparison to 80% in non-metastatic BC cancer patients [3].
Metastasis involves a complex interaction among tumor cells, extracellular matrix, blood or lymphatic vessels, and basement membrane surrounding these vessels [1]. The degradation of extracellular matrix (ECM) and epithelial to mesenchymal transition (EMT) are among the crucial steps for tumor cells' escape and invasion. Tumor cells break ECM by secreting proteases like matrix metalloproteinases (MMPs) most importantly MMP2 and MMP9 which can efficiently degrade collagen IV, the main component of ECM [4]. For the migration, the tumor cells undergo modifications at the genetic level that result in the transition from static epithelial cells into a migratory-mesenchymal state, and this process is called EMT. The EMT is controlled by the transcription factors Snail, Snug, and Twist which reduce E-cadherin and enhance N-cadherin in tumor cells in response to the external stimuli. This transition results in the enhanced ability of tumor cells to invade and migrate [5]. Growing tumor cells require more energy which is supplied by the formation of new blood vessels called angiogenesis. Tumor cells and microenvironment stimulate angiogenesis mainly by releasing vascular endothelial growth factor (VEGF) and chemokines which promote angiogenesis by several mechanisms. Some members of the CXC family called angiogenic chemokines with ELR motifs (CXCL1/2/3, CXCL5, and CXCL12) directly regulate the angiogenesis once induced by recruiting macrophages and neutrophils within the tumor microenvironment. The chemokines and chemokine receptors are good therapeutic targets because they intervene in disease progression, angiogenesis, and chemo-resistance development [6].
Therapeutic approaches for primary tumors have markedly improved over the last few years but systemic treatments to halt metastasis are still lacking. Finding natural anti-neoplastic agents that target the metastatic cascade at different steps has proved highly beneficial in this regard. The in vitro and in vivo anti-metastatic BC potential albeit by different mechanisms of various plants: Alisma canaliculatum [7], Camellia sinensis [8], Centipeda minima [9], Smilax china L. [10], and Solanum nigrum [11] has been reported in different studies. The ethanolic extract of Oldenlandia diffusa inhibited the BC cell's proliferation and metastasis by targeting MMPs and EMT pathways in both in vitro and in vivo models [12]. Nho et al. [10] revealed the anti-metastatic efficacy of Ampelopsis japonica (AJ) in signaling cascades of the highly invasive MDA-MB-231 cells by downregulating MMPs expression and up-regulating the tissue inhibitors of metalloproteinases (TIMP). Similarly, anti-migratory and anti-angiogenic activity of Cirsium japonicum var. maackii extract by inhibiting breast cancer cell viability, and endothelial cell differentiation and tube formation, are reported [13]. As reviewed by Park et al. [14], mixture extracts of different plants showing strong anti-metastatic activity have also been used in clinical studies of colorectal carcinoma patients. Hence, it enlightened the new perspective to use the anti-metastatic potential of plant extracts as a therapeutic strategy for BC patients with advanced disease stages.
Euphorbiaceae is a medicinally important family of angiosperms including genera Euphorbia, Phyllanthus, Jatropha, and Croton [15]. Euphorbia ryoleana Boiss (ERA), commonly called “sullu spurge” is a medicinally important species of Euphorbia. In Pakistan, it is locally known as “Dandathor” or “Dozakhimeva” [16] and is used as traditional medicine for various ailments like diarrhea, paralysis, jaundice, asthma, and pain. It is reported that its latex contains various secondary metabolites with major bioactive components of cycloartenol triterpene derivatives that exhibit anti-inflammatory and anti-arthritic properties [17]. The in vitro anti-cancer activity of ERA has also been studied against different cancer cell lines. It showed cytotoxicity in the hepatocellular carcinoma cell line (HepG2) and human colon cancer cell line (HCT116) [16]. We have previously reported the cell cycle arrest and caspase-dependent apoptotic activity of ERA against breast cancer cell lines (MDA-MB-231 and MCF-7) [18]. Herein, we intended to evaluate the anti-migratory and anti-metastatic potential of ERA against BC cell lines. This study revealed the anti-metastatic and anti-stemness ability of ERA ethanolic extract in vitro against BC cell lines. Moreover, ERA extract also delayed the DMBA-induced tumor progression in vivo.
Materials and methods
Cell lines and culturing condition
In this study two breast cancer cell lines were used; less metastatic MCF-7 (ATCC, Manassas, VA, USA) and highly metastatic triple -negative MDA-MB-231 (ATCC, USA) [19]. Both cell lines were serially passaged and stored in liquid nitrogen until for assays. The culture was maintained, in Dulbecco’s modified Eagle’s medium (Gibbco, Invitrogen) with 10% fetal bovine serum (FBS, Invitrogen), 1% of penicillin–streptomycin solution (10,000 U/mL) (Invitrogen) at 37 °C with 5% CO2 and 90% humidity. Cells were cultured in T25 and T75 cm2 flasks (SPL, life science) up to 70–80% confluency, and media were replaced every 3rd day. The morphological observation was recorded using Olympus IX83 microscope and cellSens imaging software.
Plant collection and extraction
Euphorbia royleana Boiss. was collected from the botanical garden of University of the Punjab, Lahore, Pakistan. The plant was identified with the help of experts from the Institute of Agriculture Sciences at the same university and a voucher specimen was submitted to the University herbarium under voucher number LLAH#286021CEur. The detailed methods of plant collection, drying, extraction, and storage are already explained in our previously published article [18].
Transwell invasion and vertical migration assay
The transwell invasion and vertical migration assays were performed to evaluate the invasion abilities of cells. Both assays were performed by following the modified protocol of Maskey et al. [20]. Briefly, the transwell chambers with 8 µm filters (SPL Insert™ cat #36,124) were washed with serum-free DMEM and placed into the wells of a 24-well plate. The lower chamber contained DMEM with 10% fetal bovine serum. About 5 × 104 cells (control and treated with ERA extract for 48 h) resuspended in 200 µL DMEM with 1% FBS were plated in the top chamber having laminin-coated membrane with 8 µm diameter pores. 60 µL of invasion inducer (cat # ab235885) was added to control wells. Plates were then incubated at 37 ˚C in 5% CO2. After 24 h, cells were fixed in 3.7% formalin for 5 min and washed with 1X PBS followed by staining with 0.5% crystal violet. Images of six randomly selected fields of view were captured (at 20X magnification) by inverted microscopy (Olympus, IX83) and cells were counted at a magnification of 10X.
For the vertical migration assay, the control and treated cells (2 × 104 cells per Transwell chamber) were placed in the top chamber without laminin coating. After 24 h, the cells were stained and fixed as described previously. After imaging, the migrated cells were lysed in glacial acetic acid and the solutions were transferred to an ELISA plate for the colorimetric reading of optical density at 570 nm.
Wound healing assay
To perform a wound healing assay, MCF-7 and MDA-MB-231 cells were seeded into 6-well plates at 5 × 105 cells/well and grown until 60–80% confluency. After that, a cell-free area (wound) was constructed using a 200 μL pipette tip in each well and washed gently with ice-cold 1X PBS three times. Cells were further treated with IC50 concentration of ERA extracts. Healing of the wound was observed after 24 h and 48 h under the light microscope (Olympus, Tokyo, Japan), and images were analyzed using Image J software (NIH, Bethesda, MD, USA).
Expression analysis of genes involved in metastasis
The MDA-MB-231 and MCF-7 cells were grown in T25 flasks (4 × 103 cells/cm2) and treated either with ethanolic extracts of ERA or with 0.1% DMSO and incubated for 48 h. After incubation, cells were harvested and total RNA was extracted by using TRIzol Reagent (Invitrogen; Thermo Fisher Scientific) and cDNA synthesis was carried out with revertAid cDNA synthesis kit according to the manufacturer’s protocol. The expression of metastatic genes (MMPs, EMT markers, angiogenic chemokines, and stemness markers) was measured by quantitative real-time PCR using conditions described by Naz et al. [21]. The primer sequences of the studied genes are shown in Table 1. The data was analyzed using PikoReal Software 2.2. Ct values were normalized to the housekeeping gene β-actin, and expression was calculated as relative fold changes.
Clonogenic survival assay
MDA-MB-231 and MCF-7 cells (4 × 104 cells/cm2) were treated with ERA ethanolic plant extracts and 0.1% DMSO as control. After 48 h treatment, the cells were digested with trypsin and resuspended homogenously. A total of 500 cells from each group were cultured in a 6-well plate for 15 days. The cells were then fixed with 4% paraformaldehyde and stained with a freshly prepared 0.1% crystal violet stain for 10 min. Following rinsing with distilled water, the colonies that had formed in each well were imaged and counted under the light microscope (Olympus research inverted microscope model IX83) using 4X magnification.
Spheroid formation assay
We performed this assay to evaluate the effect of ERA on the 3D culture growth of BC cells in vitro using a modified method described by Parveen et al. [22]. Briefly, 1.5% agarose was prepared in 1X PBS solution used to prevent the cells' attachment to the surface and mixed with equal volume (1:1) of DMEM culture medium. The culture medium was suspended in 6-well culture plates. About, 10,000 cells of control (MDA-MB-231 and MCF-7), and ERA-treated BC cells were seeded and incubated at 37 ºC in a humidified incubator, 5% CO2 for 10 days. After incubation, the colonies that appeared were observed under BF using 10X and 20X magnification. The analysis of images (no. of cells, size of spheroids) was done using ImageJ software (a tool for image processing and analysis).
Evaluating the effect of ERA on DMBA-induced carcinogenesis in Swiss albino mice
Female Swiss albino mice (n = 18) weighing 25–35 g and aged between 12 and 13 weeks were obtained from the Animal House of the School of Biological Sciences (SBS), University of the Punjab, Lahore. The procedure and conditions of animal handling were approved by the Ethical Review Board of University of the Punjab. Briefly, the animals were caged in groups and kept at constant environmental and nutritional conditions throughout the experimental period with room temperature 23 ± 5 ◦C, humidity (50–55%) with 12 h light/ 12 h dark cycle and were fed a standard pellet diet. All the animals were divided into 3 groups (6 in each group). The detail of the drug dose and treatment with ethanolic ERA extract is given in Table 2. After 30 days, blood was collected via cardiac puncture of mice, and serum was isolated. The levels of Carcinoembryonic antigen (CEA) and cancer antigen (CA) 15–3 were evaluated in the serum of both control and experimental groups. For the histopathological study, the mammary gland tissues were removed from sacrificed mice and immediately fixed in 4% paraformaldehyde. The tissues were then dehydrated in ascending series of alcohol, kept in a 1:1 mixture of absolute alcohol and benzene, and then in benzene for 1 h each. Finally, tissue pieces were embedded in paraffin wax and 7-micron thick sections were cut and spread on glass slides, stained with hematoxylin and eosin, and mounted in DPX. Stained slides were viewed under a light microscope and imaged at different magnifications (20X, 40X).
Quantitative phytochemical analysis of ethanolic ERA extract
Analysis of TPC and TFC
The Folin-Ciocalteu assay was performed to estimate the total phenolic content (TPC) in the ethanolic ERA extract. Briefly, ethanolic plant extract (1 mg/mL concentration) was prepared by dissolving 5 mg plant extracts in 5 mL of 80% ethanol. 40 µL of ERA extract or standard solution of Gallic acid was mixed with 30 µL Folin-Ciocalteu reagent, 80 µL of 20% sodium carbonate, and 350 µL distilled water. The mixture was kept at room temperature for 30 min and measured the absorbance at 765 nm. The TPC was calculated from the standard curve and expressed as mg Gallic acid equivalent (mg GA)/g of ERA.
Likewise, aluminum chloride (AlCl3) method was used to quantify the total flavonoid content (TFC) in ERA. The 1 mg/mL plant extract was prepared in 80% ethanol as described previously. In 50 µL ERA extract /quercetin, different reagents (ethanol 150 µL, 10% AlCl3 10 µL, 1 M potassium acetate 10 µL, and distilled water 280 µL) were added. The mixture was kept at room temperature, and the absorbance was measured at 415 nm. All the concentrations were directed in triplicate. The TFC was determined from the tandard curve and expressed as mg quercetin equivalent (mg QE)/ g of plant extract.
GC–MS analysis
To identify the most abundant phytoconstituents in the ethanolic extract of ERA (aerial part) GC–MS analysis was implemented using Agilent (Agilent Technologies Inc., California, USA) equipped with DB-5MS column (30 mm, 0.25 mm, 0.25 µm) and mass detector with MS-mode: 35–500 m/z. Helium was the carrier’s gas at a flow rate of 1 mL/min. and instantaneously the injector was operated at 280 ºC and the oven temperature was programmed as follows; 50 ºC for 1 min, at 25 ºC/ min to 120 ºC (5 min), at 10 ºC/ min to 160 ºC, at 6 ºC/ min to 240 ºC, at 2 ºC/ min to 290 ºC, with total run time 51.13 min. The mass spectrometry settings included an Electronic Ionization (EI) source with electron bombardment energy of 70 eV, filament current of 100 μA, ion source temperature of 250 °C, and transmission line temperature of 280 °C. To identify compounds, MS (mass spectrometry) spectra were compared to the National Institute of Standards & Technology (NIST) library (https://www.nist.gov/nist-research-library) and data were analyzed by using MassHunter software. (https://www.agilent.com/en/product/software-informatics/mass spectrometry software).
Statistical analysis
All the assays were performed in triplicates in two different sets of experiments. The data represents the mean value of each experiment. The software GraphPad Prism program (v. 5.0; GraphPad, San Diego, CA, USA) was used for the analysis of data. To calculate the scientific significance of data, one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test and two-way analysis of variance (ANOVA) followed by Sidak’s post hoc test were used. The p-value ≤ 0.05 were considered significant.
Results
Optimal conditions for ERA’s extract activity
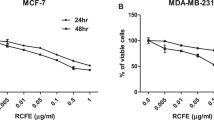
In our previous study, we initially screened the ethanolic extract of ERA for its anti-proliferative activity against two different breast cancer cell lines, MDA-MB-231 and MCF-7 using increasing concentrations and three different time points 24 h, 48 h, and 72 h. However, the optimal activity was observed at 48 h with IC50 conc. (80 µg/mL) [18]. All experiments described in this studywere conducted using IC50 conc. (80 µg/mL) of ERA and 48-h treatments.
ERA reduces the invasion abilities of the BC cells
The metastatic process involves the rearrangements of the cytoskeleton that result in the degradation of ECM and change in cellular morphology [23]. The results of the transwell invasion assay showed that the ERA treatment significantly decreased the invasiveness of both MDA-MB-231 and MCF-7 BC cells (Fig. 1A). Unlike control, only 12 and 5% of the MDA-MB-231 and MCF-7 cells population retained their invasive ability after ERA treatment (Fig. 1B). The gene expression analysis revealed that ERA extracts down-regulated the matrix metalloproteinases MMP2 and MMP9 to 0.59 and 0.05-fold in MDA-MB-231 while 0.01 and 0.14-fold in MCF-7 cells, respectively (Fig. 1C). The ERA extract modulated the expression of EMT markers i.e., the expression of N-cadherin was reduced to 0.2 and 0.015-fold in MDA-MB-231 and MCF-7, respectively. On the other hand, E-cadherin was 2.3-fold up-regulated in ERA-treated MCF-7 cells. However, E-cadherin expression was lost in MDA-MB-231 cells because of its mesenchymal characteristics (Fig. 1D). Altogether these results are evident that the ERA treatment reduced the invasion of BC cells by hindering the ECM degradation and EMT processes.
ERA inhibits the invasion of BC cells. A Effect of ERA extract on the invasion of BC cells. The images represent the results of the transwell invasion assay. B The bar graphs represent the number of invading cells of control vs ERA-treated BC cells. Invading cells were counted using six randomly selected areas, the graph represents the percentage value of invading cells. C The graph represents the gene expression of invasion markers; MMP9 and MMP2 in control vs ERA-treated cells. D Gene expression analysis of EMT markers before and after treatment of ERA. For statistical analysis, One-way ANOVA followed by Tukey's multiple comparisons was done to calculate the significant difference. (*P = 0.05, **P = 0.001, ***P = 0.002, ****P = < 0.0001). The graphical bars represent the mean values (n = 3 ± SEM)
ERA hinders the migration of the BC cells
The cell motility also represents the metastatic potential of BC cells. The effect of ERA on vertical and horizontal migratory properties of BC cells was analyzed by transwell migration and wound healing assay, respectively. These results revealed that ERA hindered the migration ability as measured by the low number of migrated cells in ERA-treated wells (Fig. 2A) and decreased optical density from 2 (of control cells) to 0.6 and 0.3 in ERA-treated MDA-MB-231 and MCF-7 cells, respectively (Fig. 2B). Likewise, a significant difference was observed in wound healing abilities of BC cells after ERA treatment. The highly aggressive MDA-MB-231 cells manifested almost complete (98%) wound closure whereas in ERA-treated MDA-MB-231 cells only 10% (P = 0.002) wound closure was observed after 48 h (Fig. 2C). On the contrary, less aggressive and less migratory MCF-7 cells manifested 46% wound closure after 48 h while only 3% (P = 0.002) wound healing was observed in ERA-treated MCF-7 cells (Fig. 2D). The results of these physiological assays strengthen the above data that anti-metastatic potential of ERA extract hindered the invasive and migratory abilities of BC cells.
ERA hinders the migration ability of the BC cells. A Effect of ERA extract on the migration of BC cells. The images represent the transwell migration assay results. B The graph represents the optical density of migrating cells in both control and treated cells. The graphical bars represent the mean values (n = 3 ± SEM). C The bright-field microscopic images (at 10X) represent the wound closure ability of control and ERA-treated BC cells. D The graph shows the % wound closure ability of control vs ERA-treated MDA-MB-231 (above) and MCF-7 (below) cells. The data represent the means of three independent experiments in triplicates (n = 3 ± SD), whereas * represents the P values (ns = non-significant, **P = 0.001, ***P = 0.002)
Angiogenic chemokines expression is altered by the ERA treatment
ELR-Positive CXC-Family of chemokines is known to enhance angiogenesis by direct or indirect modulation of the tumor microenvironment (TME) [24]. As suggested by accumulated evidence CXCL chemokines (CXCL1/2/3, CXCL5, and CXCL12) involved in BC metastasis have > 500-fold expression in ER− (MDA-MB-231) cells as compared to ER+ (MCF-7) cells [25]. We assessed the regulatory effect of ERA on these CXC angiogenic chemokines in ER− (MDA-MB-231) cells. The ERA treatment impaired the gene expression of the various chemokines. In ERA-treated MDA-MB-231 cells; CXCL1/2/3, CXCL5, and CXCL12 were significantly down-regulated up to 0.22, 0.033, 0.28, 0.06, and 0.04-fold, respectively (Fig. 3). These results are suggestive that ERA down-regulated the expression of these angiogenic chemokines to reduce the metastatic progression directed via these soluble factors in TME.
ERA down-regulates the gene expression of angiogenic chemokines. The bar graph represents the effect of ERA extract on the transcription of a group of angiogenic chemokines. For statistical analysis, a two-way ANOVA followed by Sidak’s multiple comparison test was done. P ≤ 0.02 is significant. (***P = 0.001, ***P = 0.002, ****P = < 0.0001). The graphical bars represent the mean value of 3 independent experiments. (n = 3 ± SEM)
ERA impedes clonogenic survival of BC cells
The colony formation assay represents the metastatic seeding potential of BC cells in vitro. It measures the ability of a single invading cell to intravasate and grows as a tumor at the new target site. Our data showed that ethanolic extract of ERA reduced the colony formation ability of both MDA-MB-231 and MCF-7 cells in vitro (Fig. 4A). Only 31 colonies of MDA-MB-231 and 16 of MCF-7 cells were formed in plates containing ERA-treated cells, whereas, non-treated control BC cells produced abundant colonies (Fig. 4B). The pluripotency-related transcription factors Oct3 and Nanog are responsible for the breast cancer progression at invading site [26]. The expression analysis of these genes revealed that ERA treatment down-regulated Oct 3 and Nanog to 0.4 (P < 0.01) and 0.8-fold, respectively, in MDA-MB231 while 0.07 and 0.06-fold (P < 0.01) in MCF-7 cell line. The expression of Oct3 was more significantly reduced as compared to Nanog in MDA-MB-231 cells. Overall, the results of the colony formation assay depict that ERA extract reduced the pluripotency of the BC cells (Fig. 4C).
ERA extract affects the metastatic seeding ability of BC cells. A Bright-field microscopic images (at 4X) of crystal violet staining represent the clonogenic ability of control vs ERA-treated MDA-MB-231 and MCF-7 cells. B Graph showing the no. of colonies counted after 15 days of initial seeding of BC cells of control vs ERA-treated. Data represent the mean value of 3 independent experiments and each value is the average of three readings selecting three different areas during imaging at 4X. C The gene expression of stemness markers Oct3 and Nanog in control and treated BC cells. (n = 3 ± SEM). D The images represent the spheroids of MDA-MB-231 and MCF-7 cells at 4X and 20X magnification. The bar represents 100 µm distance. The average size of spheroids measured by ImageJ; MDA-MB-231 control 670 µm, ERA-treated MDA-MB-231 193 µm, while in MCF-7 control 408 µm, ERA-treated MCF-7 102 µm. E The bar graphs represent the average no. of cells in spheroids
ERA hindered the spheroid formation potential of BC cells in vitro
Spheroid formation assay is routinely used in vitro to assess tumor behavior, drug/chemotherapeutic responses, microenvironment interactions, and complex cell–cell signaling in a 3D context. Our data demonstrated that ERA extract-treated cells lost the potential of spheroid formation. As shown in Fig. 4 in control BC cells, multiple no. of intact 3D spheroids were observed. While in ERA-treated MDA-MB-231 and MCF-7 cells only 15 and 30 deshaped, small spheroids were detected, respectively. The average sizes of spheroids were; MDA-MB-231 control 670 µm, ERA-treated MDA-MB-231 193 µm, while in MCF-7 control 408 µm, ERA-treated MCF-7 102 µm as shown in Fig. 4D. There was a significant decrease in spheroids areas after in ERA-treated cells. Similarly, the estimated number of cells was 3380 ± 5 and1560 ± 5 in MDA-MB-231 and MCF-7 controls, while after ERA treatment only 740 ± 5 MDA-MB-231 and 230 ± 5 MCF-7 cells were observed in Fig. 4E.
Ethanolic extract of ERA prevented DMBA-induced carcinogenesis in Swiss albino mice
We further evaluated the preventive effect of ERA against DMBA-induced carcinogenesis in the Swiss albino mice. After 30 days of ERA treatment, the presence/absence of mammary carcinoma was evaluated by chemical and histological assays. In the tumor control group, 4 out of 6 animals died (77% mortality rate) during the 30 days of the establishment of the mammary carcinoma mice model. On the other hand, in the ERA-treated group, only 1 animal died (16% mortality rate) which depicts the significantly enhanced (84%) survival ability among these animals (Fig. 5A).
Preventive effect of ERA on in vivo DMBA-induced carcinogenesis. A Graphs show the 3 different groups of animals and their % survival rate. B Effect on level of tumor serum markers CEA (C) and CA15-3. TC, tumor control; NC, negative control (healthy); ERA, ERA-treated. C Histological, H & E staining of mammary glands of DMBA and ERA-treated Swiss albino mice. TC, the arrows indicate the neoplastic transformation of the epithelial cells of the mammary ducts, arrowhead mark inflammatory cell infiltration while asterisk shows proliferation of connective tissue and deposition of collagenous stroma with infiltration of inflammatory cells. NC, a small amount of the mammary tissue composed of mainly ductal tissue lined by small round cells with hyperchromatic nuclei and scanty cytoplasm (arrows) embedded in the abundant adipose tissue (asterisk) is visible. The collagenous stroma is negligible and there are no inflammatory cells. ERA, photomicrographs showing relatively normal-appearing ducts (black arrow), alveoli (white arrow), and terminal end buds (arrowhead). There is a small amount of stromal tissue having mild inflammation (asterisk)
Furthermore, the levels of serum tumor markers, CEA, and CA-15–3 were reduced to 0.2 ng/mL and 0.89 u/mL, respectively, in the ERA-treated group as compared to tumor control (TC) (Fig. 5B). The histological analysis by H and E staining revealed that in TC (tumor control) group, there was the neoplastic transformation of the epithelial cells of the mammary ducts, as well as the proliferation of connective tissue and deposition of collagenous stroma with infiltration of inflammatory cells was also observed. Atypical clusters of hyperchromatic malignant cells were abundant in the TC group. While in the ERA-treated group, normal-appearing ducts lined by hyperchromatic cells were observed, with a small amount of stromal tissue having mild inflammation more like the mammary gland tissues of normal healthy control NC (Fig. 5C). These results are suggestive that ethanolic extract of ERA enhanced the survival rate and restricts the development of DMBA-induced carcinogenesis in in vivo mode.
Phytochemical analysis of ERA
Phytochemical analysis of ERA measured TPC of ERA as 52.423 mg GAE/10 mg of plant extract. Similarly, TFC content detected is 49.405 ± 0.188 mgQE/10 mg of ERA extract (Fig. 6A). The data of GC–MS is shown in the form of chromatogram (Fig. 6B) it represents that the ERA ethanolic extracts contain > 50 components. The most abundant of them were; n-Hexadecanoic acid, C16H32O2 (19.07%), 7,10,13-Hexadecatrienoic acid, C16H26O2 (13.74%), Methyl 8,11,14-heptadecatrienoate, C18H30O2 (12.01%), 9,19-Cyclolanost-24-en-3-ol, C32H52O3 (5.59%), and beta.-Sitosterol, C29H50O (4.75%) (Table 3). These compounds were previously reported for anti-oxidant, anti-inflammatory, anti-viral, anti-diabetic, immunomodulatory, and specifically anti-cancer activities [27]. Furthermore, many other medicinally important phytochemicals such as Phytol, Vitamin E, Campesterol, Ledol, Caparratriene, gamma.-Sitosterol, and Lupeol were also identified in ERA ethanolic extract.
Quantitative phytochemical analysis of ethanolic extract of ERA. A The graph shows standard curve of quercetin with r2 = 0.9072 and gallic acid with r2 = 0.8012 while bar graphs represent the TFC (49.405 ± 0.188 mg QE/10 mg) and TPC (52.423 mg GAE/10 mg) of ERA. B Chromatogram of ethanolic extract of E. royleana obtained via GC–MS. The x-axis represents the RT (retention time) of each compound while y-axis represents their abundance
Discussion
Despite of high 5-year survival rate among BC patients after treatment, distant metastasis has long been the principal cause of mortality [28]. However, preventive measures against metastasis can reduce the chances of BC relapse and death rate. In this study, we evaluated the anti-metastatic potential of ethanolic ERA extract by its inhibitory effect against, invasion, migration, angiogenesis, and cancer cell stemness.
The matrix metalloproteinases (MMPs) play a vital role in ECM degradation and facilitate tumor progression. The MMPs, exclusively MMP2/9 are involved in the degradation of the basement membrane so that primary tumor cells can intravasate. Elevated expression of MMP2 and MMP9 have been strongly correlated with the invasiveness of BC cells [29]. Previous studies have shown the inhibitory effects of some natural compounds against BC metastasis by inhibiting MMPs (MMP2/9) expression [30, 31]. Similarly, this study revealed the inhibitory effect of ERA extract against MMP2 and MMP9 proteases in vitro.
A tumor cell undergoes genetic modification that resulted in a dramatic change in morphology before the invasion. EMT enhanced the cancer cell progression by changing its polarity, loss of cell-to-cell adhesion, and remodeling of the cytoskeleton. The tumor cells lost expression of E-cadherin (epithelial marker) and acquire N-cadherin, vimentin, etc. (mesenchymal marker). Once the invading cells extravagate the secondary site, they undergo mesenchymal to epithelial transition (MET) and re-expressed the E-cadherin [32]. Any agents that block or reverse EMT/ MET provide a therapeutic strategy to reduce invasion and migration. The expression of the mesenchymal marker (N-cadherin) was significantly reduced in both ERA-treated MCF-7 and MDA-MB-231 cells. The epithelial marker E-cadherin was significantly up-regulated in ERA-treated MCF-7 while the decreased expression of E-cadherin as observed in MDA-MB-231 cells might be due to DNA hypermethylation in the E-cadherin promoter region. Similarly, it was reported that polyphenols from Artemisia annua L. suppress the EMT process by downregulating mesenchymal markers expression without affecting E-cadherin in MDA-MB-231 cells. In another study, reduced E-cadherin expression was reported in curcumin-treated MDA-MB-231 cells, where curcumin inhibited EMT-mediated invasiveness in these cells [32]. It is important to conclude here that ERA extract hindered the EMT process by not only modulating the EMT gene expression but also inhibiting invasiveness and motility by inducing morphological changes as observed through cell-based assays.
Solid tumors > 3 mm3 in size interact with non-tumoral cells in the surrounding for their survival and progression. In the tumor microenvironment (TME), the inflammatory infiltrating cells are associated with metastasis. The soluble factors released by tumor cells recruit tumor-associated macrophages (TAMs) and monocytes to induce the release of pro-angiogenic chemokines. These angiogenic factors direct the complex process of angiogenesis. It involves a network of cytokines, chemokines, and proteolytic enzymes to degrade ECM, recruitment, and proliferation of endothelial cells for blood vessel formation by releasing chemoattractant in TME. As suggested by accumulated data angiogenic chemokines; CXCL1/2/3, CXCL5, and CXCL12 act as oncogenes and are associated with tumor progression during metastasis [33, 34]. The CXCL1 is known to promote the migration of BC cells in vitro, tumor growth, metastasis, as well as angiogenesis [35]. In a study, Minn et al. [36] revealed that down-regulation of CXCL1 and CXCL2 has a clear effect on the metastatic potential of MDA-MB-231 cells since silencing of each of the two cytokines reduce the expression of several pro-metastatic genes that are associated with the lung metastasis. Toulza et al. [24] reported that elevated expression of CXCL1 and CXCL5 in breast tumors not only provide chemotaxis for endothelial cell but also directly involved in neovascularization. Our data showed that ERA extract significantly inhibits the expression of these angiogenic chemokines to different levels. These results suggested a potent anti-angiogenic effect of ERA by blocking angiogenic chemokines in ER− MDA-MB-231 cells.
Consistent with our previous results [18], colony formation assays confirmed the anti-neoplastic activity of ERA against BC cell lines. The cancer stemness markers Oct3 and Nanog are transcription factors responsible for maintaining the immortality of stem cells and the pluripotency in an embryo. Cancer cells use the high expression of these transcription factors to bypass natural cell death and maintain immortality. These two transcription factors also prevent lineage commitment in adult stem cells. The elevated expression of transcription factor Oct3 in both MDA-MB-231 and MCF-7 implies a direct link with tumorigenesis of these cell lines [26]. It is reported that pectolinarigenin from Cirsium japonicum Fisch. ex DC inhibits the BC cells' stemness and self-renewal ability by targeting Oct3/4 and Nanog [37]. The scattered expression of Nanog in BC cell lines revealed that only a small percentage of the cell population expresses this gene however, MCF-7 cells express a higher level of Nanog as compared to MDA-MB-231 [26]. Jeter et al. [38] reported that MCF-7 cells lost the colonial expansion after the expression of the Nanog was down-regulated. Similarly, ERA extract impedes the colonial regrowth of BC cells by downregulating the Oct3 and Nanog but, a non-significant difference in Nanog might explain the already low expression of this gene in MDA-MB-231 cells. Moreover, ERA extract hindered the spheroid formation in 3D culture might interpret its ability to damage cell–cell interaction and imbalance the TME required for metastatic cascade to colonize at secondary site [22]. These results support the notion that ERA restricts the metastatic seeding as well as pluripotent efficacy of BC cells.
In vitro results of the study proposed that ethanolic extract of ERA might be a source of therapeutic agents against BC as it strongly inhibits proliferation and metastasis in vitro. However, to find a potent therapeutic agent it is a prerequisite to find its activity using an animal model. Various techniques are used to induce BC tumors in mice/rats; xenografts, genetic engineering, or the use of chemicals such as DMBA. The use of DMBA to induce mammary glands tumor is the most common approach used in labs for screening anti-cancer drugs [30]. Therefore, the in vivo anti-BC potential of ERA was evaluated using DMBA-induced mammary carcinoma. The ERA extract did not show any signs of toxicity on the animals according to their food consumption and body weight compared with controls (NC/healthy) (Table 4). The neoplastic growth of mammary glands epithelial cells along with infiltration of infiltrating cells predict the tumor progression in the TC group.
However, in the ERA-treated group appearance of normal ducts and mild inflammation were suggestive of the anti-inflammatory and preventive effect of ERA against DMBA tumorigenesis. Accordingly, Alessandra-Perini et al. [39] reported the anti-angiogenic and anti-inflammatory effect of Euterpe oleracea extract that inhibits the DMBA-induced carcinogenesis of the mammary gland in an animal model as well as increasing the survival rate of animals in the experimental group.
The TPC and TFC revealed the presence of a high concentration of both phenols and flavonoids in ERA ethanolic extract. The quantitative analysis revealed the presence of 49 µg of quercetin and 52 µg of gallic acid per 10 mg of ERA extract. Quercetin and gallic acid have notable anti-metastatic, anti-migratory, and anti-stemness abilities proclaimed by various studies. Quercetin a flavonoid, is reported to reduce the colony formation ability of BC cells [40]. It inhibits MMPs and interferes with signaling pathways such as vascular endothelial growth factor (VEGF) formation, vital for tumor growth and angiogenesis, to restrict the invasion and angiogenesis [41]. It is reported that quercetin also impairs the stemness in hepatocellular carcinoma by downregulating Notch1 and Gli2 proteins in Notch signaling and Hedgehog pathway [42]. Likewise, gallic acid a polyphenol hindered metastasis by downregulating MMP2/9 activity, GRB2, PKC, p65, and NF-κB pathway [43]. It also exhibits anti-migratory potential by targeting Rho GTPases and focal adhesion kinase (FAK) signaling pathways that are involved in human oral cancer cell migration [44]. It is anticipated that the presence of quercetin and gallic acid in ERA extract enhanced its anti-metastatic and anti-migratory effect.
The GC–MS analysis showed that the ERA phytocomponents belong to different groups including fatty acids, essential oils, terpenoids, phytosterol, alkaloid, ethyl ester, anti-oxidants, phenols, and flavonoids. Many compounds detected in ERA already been reported in different studies possessing various biological activities. Some of the most important compounds were n-Hexadecanoic acid, Hexadecatrienoic acid, heptadecatrienoand ate, Phytol, gamma. -Sitosterol, beta.-Sitosterol, 9, 19-Cyclolanost, and Lupeol. The n-Hexadecanoic acid have been reported for their anti-oxidant, anti-microbial, and anti-inflammatory activities [45]. Likewise, Hexadecatrienoic acid, a plant metabolite is known for anti-bacterial effect [46]. Similarly, identified phytosterols i.e., gamma.-Sitosterol and beta.-Sitosterol are well known for their anti-diabetic, anti-inflammatory and immunosuppressive activities as well as used to combat rheumatoid, androgenetic alopecia and hypercholesteremia [27, 47]. Phytol and vitamin E, compounds with strong anti-oxidant, anti-nociceptive, anti-cancer [48] and immunomodulatory [49] potential were also present in ERA. Moreover, Lupeol familiar as magical drug with multiple biological activities [47] and a promising anti-cancer agent was also identified in ERA extract [50]. However, some rare phyto-metabolite such as methyl 8, 11, 14-heptadecatrienoate and 9, 19-Cyclolanost were also important phytoconstituents of ERA. This study enlightens the contribution of these compounds into anti-BC and anti-metastatic activities of ERA. Hence, the results demonstrate that ethanolic ERA extract can be used as a source of potent anti-neoplastic agents against BC, however in-depth analysis are further required.
Conclusively, the results of this study suggested significant in vitro anti-invasive and anti-metastatic potential of ERA ethanolic extract against BC. However, further studies are suggestive to decipher the mechanistic insights of BC inhibition.
Data availability
All data obtained during this study are included in the manuscript.
References
Riggio AI, Varley KE, Welm AL. The lingering mysteries of metastatic recurrence in breast cancer. Br J Cancer. 2021;124:13–26. https://doi.org/10.1038/s41416-020-01161-4.
Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69:363–85.
Liang Y, Zhang H, Song X, Yang Q. Metastatic heterogeneity of breast cancer: Molecular mechanism and potential therapeutic targets. In.: Elsevier; 2020, 14–27.
Serala K, Steenkamp P, Mampuru L, Prince S, Poopedi K, Mbazima V. In vitro antimetastatic activity of Momordica balsamina crude acetone extract in HT-29 human colon cancer cells. Environ Toxicol. 2021;36:2196–205.
Mrozik KM, Blaschuk OW, Cheong CM, Zannettino ACW, Vandyke K. N-cadherin in cancer metastasis, its emerging role in haematological malignancies and potential as a therapeutic target in cancer. BMC Cancer. 2018;18:1–16.
Palacios-Arreola MI, Nava-Castro KE, Castro JI, García-Zepeda E, Carrero JC, Morales-Montor J. The role of chemokines in breast cancer pathology and its possible use as therapeutic targets. J Immunol Res. 2014;2014.
Choi J, Ahn SS, Lim Y, Lee YH, Shin SY. Inhibitory effect of Alisma canaliculatum ethanolic extract on NF-κB-dependent CXCR3 and CXCL10 expression in TNFα-exposed MDA-MB-231 breast cancer cells. Int J Mol Sci. 2018;19:2607.
Luo K-W, Yue GG-L, Ko C-H, Gao S, Lee JK-M, Li G, et al. The combined use of Camellia sinensis and metronomic zoledronate in 4T1 mouse carcinoma against tumor growth and metastasis. Oncol Rep. 2015;34:477–87.
Lee MM-L, Chan BD, Wong W-Y, Qu Z, Chan M-S, Leung T-W, et al. Anti-cancer activity of Centipeda minima extract in triple negative breast cancer via inhibition of AKT, NF-κB, and STAT3 signaling pathways. Front Oncol. 2020;10:491.
Nho KJ, Chun JM, Kim DS, Kim HK. Ampelopsis japonica ethanol extract suppresses migration and invasion in human MDA-MB-231 breast cancer cells. Mol Med Report. 2015;11:3722–8.
Lai Y-J, Tai C-J, Wang C-W, Choong C-Y, Lee B-H, Shi Y-C, et al. Anti-cancer activity of Solanum nigrum (AESN) through suppression of mitochondrial function and epithelial-mesenchymal transition (EMT) in breast cancer cells. Molecules. 2016;21:553.
Yang B, Wang N, Wang S, Li X, Zheng Y, Li M, et al. Network-pharmacology-based identification of caveolin-1 as a key target of Oldenlandia diffusa to suppress breast cancer metastasis. Biomed Pharmacother. 2019;112: 108607.
Wrighton KC, Thrash JC, Melnyk RA, Bigi JP, Byrne-Bailey KG, Remis JP, et al. Evidence for direct electron transfer by a Gram-positive bacterium isolated from a microbial fuel cell. Appl Environ Microbiol. 2011;77:7633–9.
Park J, Jeong D, Song M, Kim B. Recent advances in anti-metastatic approaches of herbal medicines in 5 major cancers: from traditional medicine to modern drug discovery. Antioxidants (Basel). 2021;10:527. https://doi.org/10.3390/antiox10040527.
Mwine TJ, Damme VP. Why do Euphorbiaceae tick as medicinal plants? A review of Euphorbiaceae family and its medicinal features. 2011.
Ashraf A, Sarfraz RA, Rashid MA, Shahid M. Antioxidant, antimicrobial, antitumor, and cytotoxic activities of an important medicinal plant (Euphorbia royleana) from Pakistan. JFDA. 2015;23:109–15.
Ebrahim HY, Osman SA, Haffez HR, Hassan ZA. In-vitro screening of some plant extracts for their potential anticancer activity. Afr J Tradit Complement Altern Med. 2020;17:1–8.
Gull S, Farooq K, Tayyeb A, Arshad MI, Shahzad N. Ethanolic extracts of Pakistani euphorbiaceous plants induce apoptosis in breast cancer cells through induction of DNA damage and caspase-dependent pathway. Gene. 2022;824: 146401.
Aftab S, Khalid Z, Shakoori AR. Repression of Cell-to-Matrix Adhesion by Metformin Chloride Supports Its Anti-Metastatic Potential in an In Vitro Study on Metastatic and Non-Metastatic Cancer Cells. Critical Reviews™ in Eukaryotic Gene Expression. 2022;32.
Maskey N, Li D, Xu H, Song H, Wu C, Hua K, et al. MicroRNA-340 inhibits invasion and metastasis by downregulating ROCK1 in breast cancer cells. Oncol Lett. 2017;14:2261–7.
Naz H, Gull S, Bashir Q, Rashid N, Shahzad N. Thermococcus kodakarensis-derived L-asparaginase: a candidate for the treatment of glioblastoma. Biologia. 2021;76:1305–14.
Perveen S, Ashfaq H, Ambreen S, Ashfaq I, Kanwal Z, Tayyeb A. Methanolic extract of Citrullus colocynthis suppresses growth and proliferation of breast cancer cells through regulation of cell cycle. Saudi J Biol Sci. 2021;28:879–86.
Srinivasan A, Thangavel C, Liu Y, Shoyele S, Den RB, Selvakumar P, et al. Quercetin regulates β-catenin signaling and reduces the migration of triple negative breast cancer. Mol Carcinog. 2016;55:743–56.
Toulza F, Eliaou JF, Pinet V. Breast tumor cell soluble factors induce monocytes to produce angiogenic but not angiostatic CXC chemokines. Int J Cancer. 2005;115:429–36.
SenGupta S, Hein LE, Xu Y, Zhang J, Konwerski JR, Li Y, et al. Triple-negative breast cancer cells recruit neutrophils by secreting TGF-β and CXCR2 ligands. Front Immunol. 2021;12: 659996.
Ling G-Q, Chen D-B, Wang B-Q, Zhang L-S. Expression of the pluripotency markers Oct3/4, Nanog and Sox2 in human breast cancer cell lines. Oncol Lett. 2012;4:1264–8.
Rashed K. Beta-Sitosterol Medicinal Properties: A Review Article. Int J Sci Inventions Today, IJSIT, 2020, 9 (4). 2020:208–12.
Chen W, Zhou S, Mao L, Zhang H, Sun D, Zhang J, et al. Crosstalk between TGF-β signaling and miRNAs in breast cancer metastasis. Tumor Biol. 2016;37:10011–9.
Morini M, Mottolese M, Ferrari N, Ghiorzo F, Buglioni S, Mortarini R, et al. The α3β1 integrin is associated with mammary carcinoma cell metastasis, invasion, and gelatinase B (mmp-9) activity. Int J Cancer. 2000;87:336–42.
Al-Zharani M, Nasr FA, Abutaha N, Alqahtani AS, Noman OM, Mubarak M, et al. Apoptotic induction and anti-migratory effects of Rhazya stricta fruit extracts on a human breast cancer cell line. Molecules. 2019;24:3968.
Lee HS, Na MH, Kim WK. α-Lipoic acid reduces matrix metalloproteinase activity in MDA-MB-231 human breast cancer cells. Nutr Res. 2010;30:403–9.
Gallardo M, Calaf GM. Curcumin inhibits invasive capabilities through epithelial mesenchymal transition in breast cancer cell lines. Int J Oncol. 2016;49:1019–27.
Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–50.
Chen E, Qin X, Peng K, Xu X, Li W, Cheng X, et al. Identification of potential therapeutic targets among CXC chemokines in breast tumor microenvironment using integrative bioinformatics analysis. Cell Physiol Biochem. 2018;45:1731–46.
Bachmeier BE, Mohrenz IV, Mirisola V, Schleicher E, Romeo F, Höhneke C, et al. Curcumin downregulates the inflammatory cytokines CXCL1 and-2 in breast cancer cells via NFκB. Carcinogenesis. 2008;29:779–89.
Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–24.
Lu M, Xu X, Lu H, Lu Z, Xu B, Tan C, et al. Evaluation of anti-tumor and chemoresistance-lowering effects of pectolinarigenin from Cirsium japonicum Fisch ex DC in breast cancer. Trop J Pharm Res. 2016;15:547–53.
Jeter CR, Badeaux M, Choy G, Chandra D, Patrawala L, Liu C, et al. Functional evidence that the self-renewal gene NANOG regulates human tumor development. Stem cells. 2009;27:993–1005.
Alessandra-Perini J, Perini JA, Rodrigues-Baptista KC, de Moura RS, Junior AP, Dos Santos TA, et al. Euterpe oleracea extract inhibits tumorigenesis effect of the chemical carcinogen DMBA in breast experimental cancer. BMC Complement Altern Med. 2018;18:1–11.
Yousefnia S, Naseri D, Seyed Forootan F, Tabatabaeian M, Moattar F, Ghafghazi T, et al. Suppressive role of Viola odorata extract on malignant characters of mammosphere-derived breast cancer stem cells. Clin Transl Oncol. 2020;22:1619–34.
Liu Y, Li C-L, Xu Q-Q, Cheng D, Liu K-D, Sun Z-Q. Quercetin inhibits invasion and angiogenesis of esophageal cancer cells. Pathol Res Pract. 2021;222: 153455.
Salama YA, El-Karef A, El Gayyar AM, Abdel-Rahman N. Beyond its antioxidant properties: Quercetin targets multiple signalling pathways in hepatocellular carcinoma in rats. Life Sci. 2019;236: 116933.
Carrano R, Grande M, Leti Maggio E, Zucca C, Bei R, Palumbo C, et al. Dietary Polyphenols Effects on Focal Adhesion Plaques and Metalloproteinases in Cancer Invasiveness. Biomedicines. 2024;12:482.
Kuo C-L, Lai K-C, Ma Y-S, Weng S-W, Lin J-P, Chung J-G. Gallic acid inhibits migration and invasion of SCC-4 human oral cancer cells through actions of NF-κB, Ras and matrix metalloproteinase-2 and-9. Oncol Rep. 2014;32:355–61.
Boubaker J, Ben Toumia I, Sassi A, Bzouich-Mokded I, Ghoul Mazgar S, Sioud F, et al. Antitumoral potency by immunomodulation of chloroform extract from leaves of Nitraria retusa, Tunisian medicinal plant, via its major compounds β-sitosterol and palmitic acid in BALB/c mice bearing induced tumor. Nutr Cancer. 2018;70:650–62.
Alqahtani FY, Aleanizy FS, Mahmoud AZ, Farshori NN, Alfaraj R, Al-Sheddi ES, et al. Chemical composition and antimicrobial, antioxidant, and anti-inflammatory activities of Lepidium sativum seed oil. Saudi J Biol Sci. 2019;26:1089–92.
Izu GO, Adeyi AO, Erukainure OL, Islam MS. Gamma-sitosterol–rich fraction from the methanolic extract of Ficus exasperata restores diabetes associated pathophysiological alterations in an alloxan-induced diabetic rats. Biokemistri. 2022;33.
Santos CCdMP, Salvadori MS, Mota VG, Costa LM, de Almeida AAC, de Oliveira GAL, et al. Antinociceptive and antioxidant activities of phytol in vivo and in vitro models. Neurosci J. 2013;2013.
Galli F, Azzi A, Birringer M, Cook-Mills JM, Eggersdorfer M, Frank J, et al. Vitamin E: emerging aspects and new directions. Free Radical Biol Med. 2017;102:16–36.
Wal A, Srivastava R, Wal P, Rai A, Sharma S. Lupeol as a magical drug. Pharm Biol Eval. 2015;2:142–51.
Acknowledgements
We are obliged to the School of Biological Sciences (SBS), PU, and Dr. Muhammad Ali (in charge of animal house) to provide us animal house facility, to conduct in vivo study as well as other basic research facilities. We are thankful to Miss Kokab Farooq for performing the spheroid formation assay as a part of our revised manuscript.
Funding
We are thankful to Higher Education Commission (HEC) Pakistan for funding this research under National Research Program for Universities (NRPU) project (6758/Punjab/NRPU/R&D/HEC/2016).
Author information
Authors and Affiliations
Contributions
SG contributed to the study design and performed the experiments. FT and IA data interpretation, and manuscript preparation. MAS, AT, LA, MA, and MIA provided research material and performed the statistical analysis. NS Conceptualization, supervision, revising, and editing the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Research involving human participants
The study did not involve Human participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gull, S., Tasneem, F., Ahmed, I. et al. Ethanolic extract of Euphorbia royleana Boiss. reduces metastasis of breast cancer cells and inhibits tumor progression in vivo. Med Oncol 41, 152 (2024). https://doi.org/10.1007/s12032-024-02378-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-024-02378-6