5.1: The Periodic Table
- Page ID
- 366400
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
- Given the name or symbol of an element and a periodic table, identify if the element is a:
- metal, nonmetal, or metalloid
- main group element or a transition metal
- alkali metal, alkaline earth metal, halogen, noble gas, or none of the above
- Given the name or symbol of an element and a periodic table, identify the period and group number of the element.
In the 19th century, many previously unknown elements were discovered, and scientists noted that certain sets of elements had similar chemical properties. For example, chlorine, bromine, and iodine react with other elements (such as sodium) to make similar compounds. Likewise, lithium, sodium, and potassium react with other elements (such as oxygen) to make similar compounds. Why is this so?
In 1864, Julius Lothar Meyer, a German chemist, organized the elements by atomic mass and grouped them according to their chemical properties. Later that decade, Dmitri Mendeleev, a Russian chemist, organized all the known elements according to similar properties. He left gaps in his table for what he thought were undiscovered elements, and he made some bold predictions regarding the properties of those undiscovered elements. When elements were later discovered whose properties closely matched Mendeleev’s predictions, his version of the table gained favor in the scientific community. Because certain properties of the elements repeat on a regular basis throughout the table (that is, they are periodic), it became known as the periodic table.
Mendeleev had to list some elements out of the order of their atomic masses to group them with other elements that had similar properties.
The periodic table is one of the cornerstones of chemistry because it organizes all of the known elements on the basis of their chemical properties. A modern version is shown in Figure \(\PageIndex{1}\). Most periodic tables provide additional data (such as atomic mass) in a box that contains each element’s symbol. The elements are listed in order of atomic number.
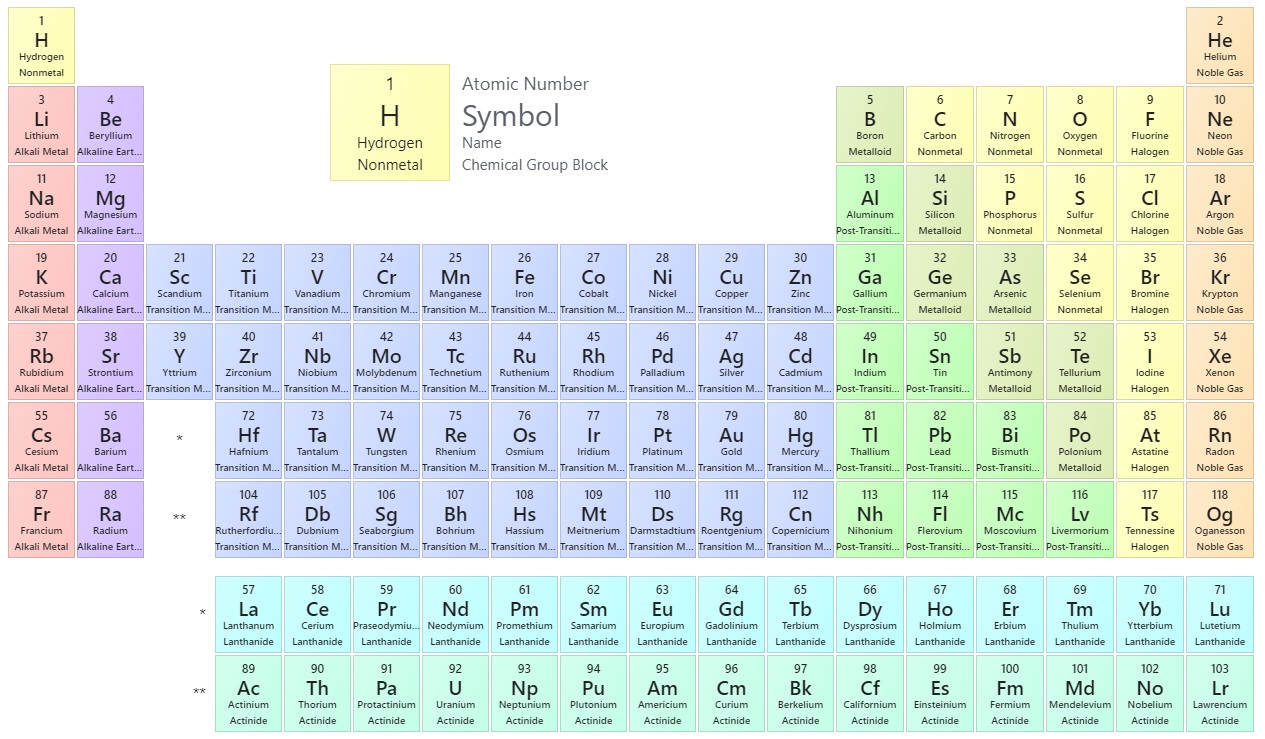
Features of the Periodic Table
Elements that have similar chemical properties are grouped in columns called groups (or families). As well as being numbered, some of these groups have names—for example, alkali metals (the first column of elements), alkaline earth metals (the second column of elements), halogens (the next-to-last column of elements), and noble gases (the last column of elements).
Each row of elements on the periodic table is called a period. Periods have different lengths; the first period has only 2 elements (hydrogen and helium), while the second and third periods have 8 elements each. The fourth and fifth periods have 18 elements each, and later periods are so long that a segment from each is removed and placed beneath the main body of the table.
Certain elemental properties become apparent in a survey of the periodic table as a whole. Every element can be classified as either a metal, a nonmetal, or a metalloid (or semi metal), as shown in Figure \(\PageIndex{2}\). A metal is a substance that is shiny, typically (but not always) silvery in color, and an excellent conductor of electricity and heat. Metals are also malleable (they can be beaten into thin sheets) and ductile (they can be drawn into thin wires). A nonmetal is typically dull and a poor conductor of electricity and heat. Solid nonmetals are also very brittle. As shown in Figure \(\PageIndex{2}\), metals occupy the left three-fourths of the periodic table, while nonmetals (except for hydrogen) are clustered in the upper right-hand corner of the periodic table. The elements with properties intermediate between those of metals and nonmetals are called metalloids (or semi-metals). Elements adjacent to the bold line in the right-hand portion of the periodic table have semimetal properties.
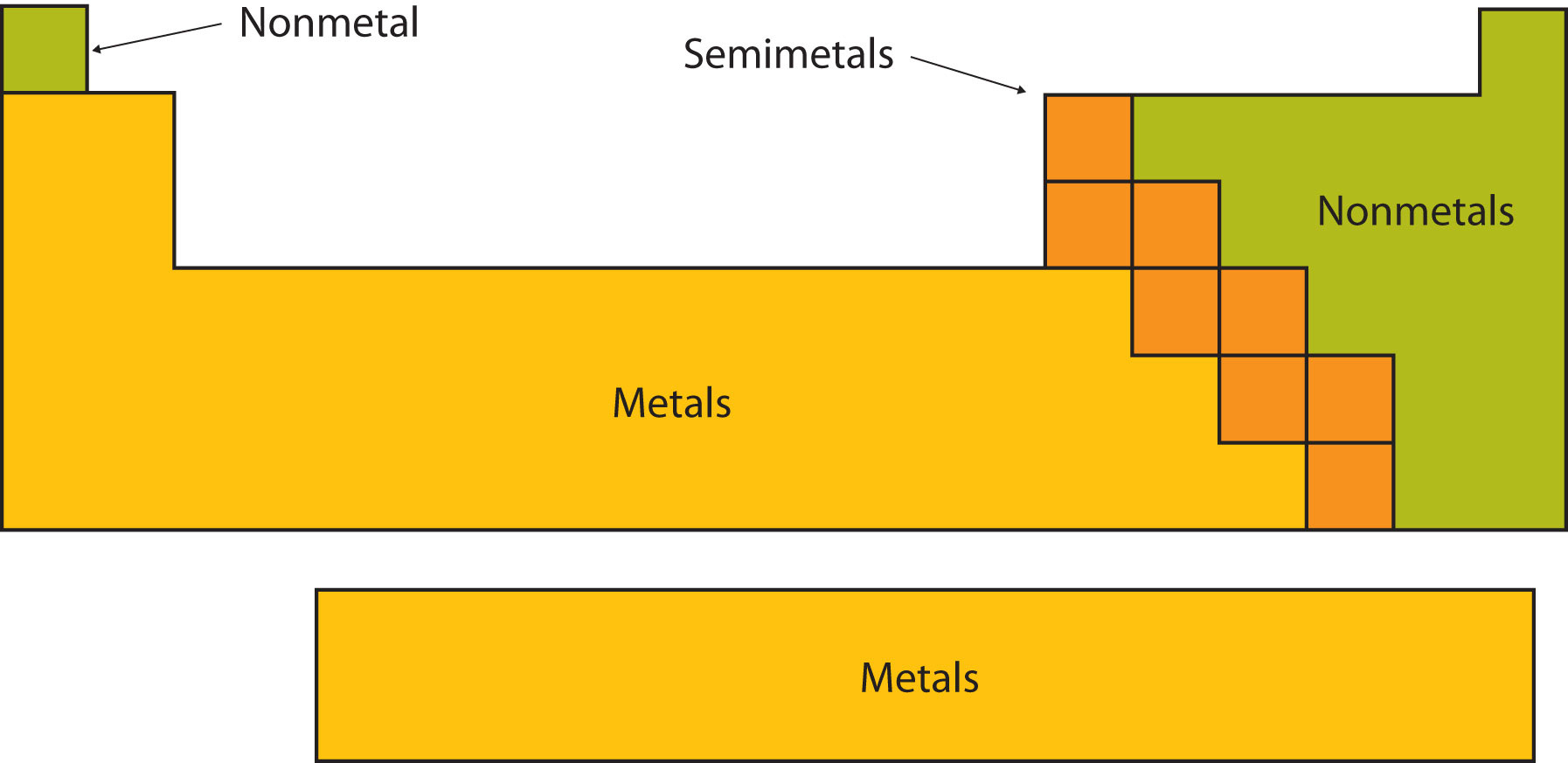
Based on its position in the periodic table, classify each element below as metal, a nonmetal, or a metalloid.
- Se
- Mg
- Ge
Solution
- In Figure \(\PageIndex{1}\), selenium lies above and to the right of the diagonal line marking the boundary between metals and nonmetals, so it should be a nonmetal.
- Magnesium lies to the left of the diagonal line marking the boundary between metals and nonmetals, so it should be a metal.
- Germanium lies within the diagonal line marking the boundary between metals and nonmetals, so it should be a metalloid.
Based on its location in the periodic table, do you expect indium to be a nonmetal, a metal, or a metalloid?
- Answer
- Indium is a metal.
Another way to categorize the elements of the periodic table is shown in Figure \(\PageIndex{3}\). The first two columns on the left and the last six columns on the right are called the main group elements. The ten-column block between these columns contains the transition metals. The two rows beneath the main body of the periodic table contain the inner transition metals. The elements in these two rows are also referred to as, respectively, the lanthanide metals and the actinide metals.
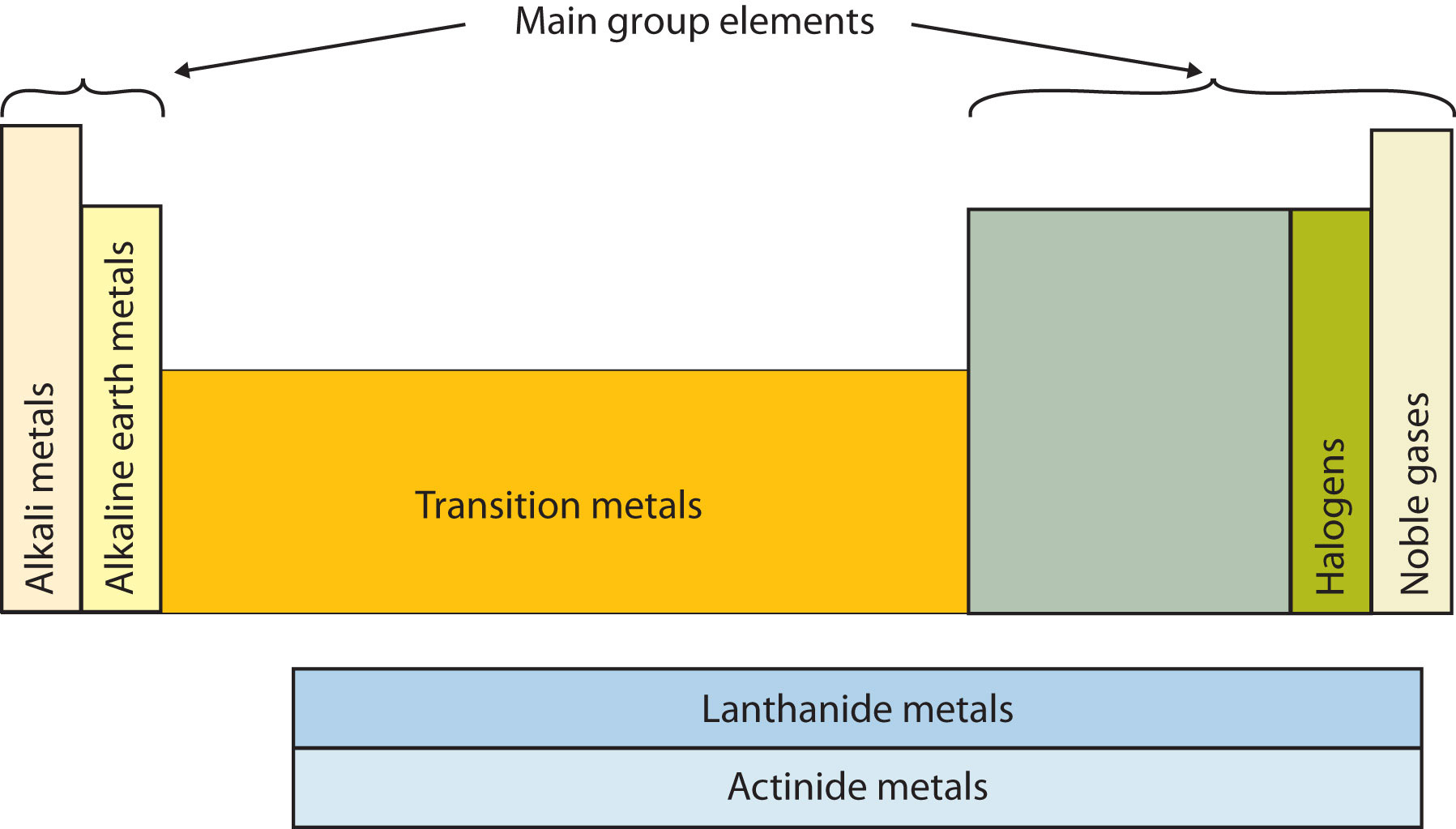
Descriptive Names
As previously noted, the periodic table is arranged so that elements with similar chemical behaviors are in the same group. Chemists often make general statements about the properties of the elements in a group using descriptive names with historical origins.
Group 1: The Alkali Metals
The alkali metals are lithium, sodium, potassium, rubidium, cesium, and francium. Hydrogen is unique in that it is generally placed in Group 1, but it is not a metal.
The compounds of the alkali metals are common in nature and daily life. One example is table salt (sodium chloride); lithium compounds are used in greases, in batteries, and as drugs to treat patients who exhibit manic-depressive, or bipolar, behavior. Although lithium, rubidium, and cesium are relatively rare in nature, and francium is so unstable and highly radioactive that it exists in only trace amounts, sodium and potassium are the seventh and eighth most abundant elements in Earth’s crust, respectively.
Group 2: The Alkaline Earth Metals
The alkaline earth metals are beryllium, magnesium, calcium, strontium, barium, and radium. Beryllium, strontium, and barium are rare, and radium is unstable and highly radioactive. In contrast, calcium and magnesium are the fifth and sixth most abundant elements on Earth, respectively; they are found in huge deposits of limestone and other minerals.
Group 17: The Halogens
The halogens are fluorine, chlorine, bromine, iodine, and astatine. The name halogen is derived from the Greek words for “salt forming,” which reflects that all of the halogens react readily with metals to form compounds, such as sodium chloride and calcium chloride (used in some areas as road salt).
Compounds that contain the fluoride ion are added to toothpaste and the water supply to prevent dental cavities. Fluorine is also found in Teflon coatings on kitchen utensils. Although chlorofluorocarbon propellants and refrigerants are believed to lead to the depletion of Earth’s ozone layer and contain both fluorine and chlorine, the latter is responsible for the adverse effect on the ozone layer. Bromine and iodine are less abundant than chlorine, and astatine is so radioactive that it exists in only negligible amounts in nature.
Group 18: The Noble Gases
The noble gases are helium, neon, argon, krypton, xenon, and radon. Because the noble gases are composed of only single atoms, they are called monatomic. At room temperature and pressure, they are unreactive gases. Because of their lack of reactivity, for many years they were called inert gases or rare gases. However, the first chemical compounds containing the noble gases were prepared in 1962. Although the noble gases are relatively minor constituents of the atmosphere, natural gas contains substantial amounts of helium. Because of its low reactivity, argon is often used as an unreactive (inert) atmosphere for welding and in light bulbs. The red light emitted by neon in a gas discharge tube is used in neon lights.
Provide the family or group name of each element.
- Li
- Ar
- Cl
Solution
- Lithium is an alkali metal (Group 1)
- Argon is a noble gas (Group 18)
- Chlorine is a halogen (Group 17)
Provide the family or group name of each element.
- F
- Ca
- Kr
- Answer a:
- Fluorine is a halogen (Group 17).
- Answer b:
- Calcium is a alkaline earth metal (Group 2).
- Answer c:
- Krypton is a noble gas (Group 18).
Classify each element as metal, nonmetal, or metalloid.
- Li
- Ar
- B
- Fe
Solution
- Lithium is a metal.
- Argon is a nonmetal.
- Boron is a metalloid.
- Iron is a metal.
Classify each element as metal, nonmetal, or metalloid.
- F
- Si
- Cu
- Answer a:
- Fluorine is a nonmetal.
- Answer b:
- Silicon is a metalloid.
- Answer c:
- Copper is a metal.
References
- Petrucci, Ralph H., William S. Harwood, F. G. Herring, and Jeffrey D. Madura. General Chemistry: Principles and Modern Applications. 9th ed. Upper Saddle River: Pearson Education, Inc., 2007.
- Sisler, Harry H. Electronic structure, properties, and the periodic law. New york; Reinhold publishing corporation, 1963.
- Petrucci, Ralph H., Carey Bissonnette, F. G. Herring, and Jeffrey D. Madura. General Chemistry: Principles and Modern Applications. Custom Edition for CHEM 2. Pearson Learning Solutions, 2010.
Contributions & Attributions
This page was constructed from content via the following contributor(s) and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality:
Henry Agnew (UC Davis)

