Abstract
The harlequin ladybird, Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae), is native to Asia but has been intentionally introduced to many countries as a biological control agent of pest insects. In numerous countries, however, it has been introduced unintentionally. The dramatic spread of H. axyridis within many countries has been met with considerable trepidation. It is a generalist top predator, able to thrive in many habitats and across wide climatic conditions. It poses a threat to biodiversity, particularly aphidophagous insects, through competition and predation, and in many countries adverse effects have been reported on other species, particularly coccinellids. However, the patterns are not consistent around the world and seem to be affected by many factors including landscape and climate. Research on H. axyridis has provided detailed insights into invasion biology from broad patterns and processes to approaches in surveillance and monitoring. An impressive number of studies on this alien species have provided mechanistic evidence alongside models explaining large-scale patterns and processes. The involvement of citizens in monitoring this species in a number of countries around the world is inspiring and has provided data on scales that would be otherwise unachievable. Harmonia axyridis has successfully been used as a model invasive alien species and has been the inspiration for global collaborations at various scales. There is considerable scope to expand the research and associated collaborations, particularly to increase the breadth of parallel studies conducted in the native and invaded regions. Indeed a qualitative comparison of biological traits across the native and invaded range suggests that there are differences which ultimately could influence the population dynamics of this invader. Here we provide an overview of the invasion history and ecology of H. axyridis globally with consideration of future research perspectives. We reflect broadly on the contributions of such research to our understanding of invasion biology while also informing policy and people.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The harlequin ladybird (or multicolored Asian lady beetle), Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae), is native to Asia and is a polymorphic, eurytopic species with a broad dietary range (Roy and Brown 2015). It was widely introduced as a biological control agent of pest aphids, but has spread to many countries within which it was not intentionally released (Brown et al. 2008a). The global invasion of H. axyridis has been rapid (Brown et al. 2011b). Concerns about the adverse impact of H. axyridis particularly on biodiversity but also to people have motivated research across the world. There have been a number of reviews considering the biology and ecology of H. axyridis (Kenis et al. 2008; Koch 2003; Koch and Galvan 2008; Majerus et al. 2006; Pell et al. 2008; Pervez and Omkar 2006; Roy and Brown 2015; Sloggett 2012) often from a regional perspective but this species has an almost global distribution. Therefore, it is timely to provide a world overview of research and perspectives. Indeed, the collaborative nature of the research from around the world on this species is inspiring. As concerns increased with respect to the threats posed by H. axyridis, two working groups were established through the International Organisation for Biological and Integrated Control (within the Global and Western Palaearctic Regional Sections) in recognition of the need for collaborative research on the benefits and risks of exotic biological control agents. One of the first outputs from these working groups was the publication of a special issue on H. axyridis including 19 manuscripts representing authors around the world (Roy and Wajnberg 2008). Harmonia axyridis as both a biological control agent and an invasive alien species (IAS) has informed a range of applied ecological themes, from risk assessment to processes of invasion; H. axyridis is considered by many as a model species for understanding processes of invasion (Roy and Wajnberg 2008).
Here we first consider H. axyridis in the context of biological control. We then provide a brief overview of interactions between H. axyridis and other species before exploring its current global distribution. We document, on a regional basis, the occurrence and invasion history of H. axyridis providing an insight into research priorities and knowledge gaps identified by scientists from around the world. Finally we qualitatively explore the observed traits of H. axyridis from both the native and invaded range reflecting on the potential for future collaborations on a global scale.
Harmonia axyridis and biological control
There has been a long history of using ladybirds as biological control agents against various pest insects around the world, beginning with the successful introduction of the vedalia ladybird, Rodolia cardinalis (Mulsant) to citrus groves of California (Caltagirone and Doutt 1989). Harmonia axyridis has a wide diet breadth (reviewed by Hodek et al. 2012) including many species of aphids, which are the main prey (Osawa 2000) but also other insect taxa (Ohgushi and Sawada 1998). It has been used extensively in classical, augmentative and conservation biological strategies around the world (Koch 2003; Lombaert et al. 2008). There has been considerable research focus on the pest control services provided by H. axyridis in the native range, particularly in China (Wang et al. 2007), but also recently in Japan where adults and larvae of flightless H. axyridis derived through artificial selection from a Japanese wild population have been used for biological control of aphids mainly in greenhouses (Seko et al. 2014).
Harmonia axyridis was first introduced as a biological control agent in the USA. It is among a number of introduced species of ladybirds that now dominate in many agroecosystems across the USA (Lucas et al. 2007; Obrycki et al. 2009) and H. axyridis is considered an important predator of aphid pests in several crops, including pecan (LaRock et al. 2003; Tedders and Schaefer 1994), apple (Brown and Miller 1998), citrus (Michaud 2002) and potatoes (Alyokhin and Sewell 2004). In particular, research has focused on the effect of H. axyridis on the suppression of the alien soybean aphid, Aphis glycines Matsumura (Hemiptera: Aphididae) (Ragsdale et al. 2011) in soybean, where H. axyridis has recently become one of the most abundant coccinellids (Costamagna and Landis 2007; Gardiner and Landis 2007; Gardiner et al. 2009a; Hesler 2014; Liere et al. 2014; Varenhorst and O’Neal 2012). This system, including the overwintering host of A. glycines, the European buckthorn Rhamnus cathartica L. (Rhamnaceae) and H. axyridis, has been cited as an example of an invasional meltdown, whereby multiple IAS interact synergistically (Heimpel et al. 2010). Harmonia axyridis is known to readily consume other predators and parasitoids of aphids (Chacón et al. 2008), but there is a lack of evidence that this impacts pest control in soybean fields (Costamagna et al. 2008).
From the mid 1990s, H. axyridis was commercialized by a number of biological control suppliers in Western Europe for augmentative biological control of aphid pests in greenhouse crops and urban ecosystems (Coutanceau 2006b; Poutsma et al. 2008). Most commercial suppliers in Europe stopped selling the beetle in late 2003 to mid 2004, with the first reports of nuisance problems and increasing concerns about adverse environmental effects of its establishment. In France, the original strain of H. axyridis used since 1995 for commercial biological control was replaced in 2000 with a flightless strain developed by INRA (Coutanceau 2006b; Tourniaire et al. 2000). The flightless strain was effectively used to control aphids in hops (Weissenberger et al. 1999). However, the species was never a major player on the European biological control market: at the peak of its commercialisation, it took perhaps 5 % of the market share of aphidophagous natural enemies (De Clercq and Bale 2011).
The role of H. axyridis in supressing pest insects in Europe has received less attention than in the USA. In the Czech Republic it has been reported that H. axyridis controls pear psylla, Cacopsylla pyri (L.) (Hemiptera: Psyllidae), in commercial orchards more effectively than other ladybird species (Nedvěd 2014). However, much of the research across Europe has considered the occurrence of H. axyridis in crop systems without detailed consideration of effects on aphid populations. In Belgium, the population dynamics of H. axyridis in crop systems (wheat, corn, broad bean and potato crops) was studied through field surveys. It has been shown that H. axyridis is recorded in such crops 7–8 days after the dominant native ladybirds (Jansen and Hautier 2008; Vandereycken et al. 2013). A 1-year study involving field observations in wheat and bean crops in southern England reported an absence of H. axyridis in wheat (aphid abundance was reported as low), but presence of H. axyridis co-occurring with other ladybirds in bean crops (Wells 2011). Harmonia axyridis was the most common aphid enemy species in bean crops and the presence of this species was correlated with high aphid abundance (Wells 2011), perhaps unsurprisingly since the prey biomass required by each single larva to reach the adult stage is very high (Soares et al. 2001, 2003, 2004).
Even though H. axyridis can be considered an effective biological control agent at least from the USA studies, the Insurance Hypothesis predicts that control will in the long term be better achieved with a diverse array of natural enemies (Loreau et al. 2003). Since H. axyridis tends to depress the diversity of coccinellid assemblages (see below), consistently effective biological control may be threatened by the invasion of H. axyridis. Further research is needed to unravel the role of H. axyridis as a biological control agent of insect pests. Comparison of aphid populations before and after the arrival of H. axyridis, or among places with higher and lower abundances of H. axyridis, may provide useful information in this respect. Importantly, landscape composition has been found to influence the effectiveness of biological control by H. axyridis and other predators. Indeed, soybean fields embedded within diverse landscapes receive a greater pest control service from aphid predators than fields within simplified agricultural landscapes (Gardiner et al. 2009b) compared to landscapes dominated by forests and grasslands where soybean field size was reduced (Woltz and Landis 2014). Similar studies have been carried out in Chile, in regions where H. axyridis dominated the aphidophagous communities and again it has been demonstrated that biological control was related to landscape composition with benefits seen through positive associations with the abundance of woodland and urban habitats, but not with fruit crops, in the landscape (Grez et al. 2014a).
Harmonia axyridis and declines of native ladybirds
The wide diet breadth and recognition that H. axyridis is a top predator (Pell et al. 2008) has driven predictions that H. axyridis has the potential to adversely affect aphidophagous guilds. A number of large-scale analyses have indicated that declines of native ladybirds correlate with the establishment of H. axyridis. Indeed declines of native ladybirds have been reported across the USA (Alyokhin and Sewell 2004; Bahlai et al. 2014; Colunga-Garcia and Gage 1998; Evans 2004; Harmon et al. 2007; Hesler and Kieckhefer 2008; Losey et al. 2014; Majerus et al. 2006; Wheeler Jr and Hoebeke 1995) and Europe (Roy et al. 2012b). Following the establishment of H. axyridis in Michigan a decrease in populations of three species of ladybird has been reported: Brachiacantha ursina (F.), Cycloneda munda (Say) and Chilocorus stigma (Say) (Colunga-Garcia and Gage 1998), followed more recently by a decline in Coleomegilla maculata (DeGeer) (Coccinellidae) (Bahlai et al. 2015). However, this last study also noted that declines of several species reported previously from the same site (Colunga-Garcia and Gage 1998) appear to have stabilized or reversed, having become statistically undetectable (Bahlai et al. 2015). Also in the USA, Michaud (2002) reported H. axyridis to be displacing Cycloneda sanguinea (L.) in Florida citrus orchards. In the United Kingdom (UK) there is a strong correlation between the declines of seven out of eight native species of ladybird assessed and co-occurrence with H. axyridis (Roy et al. 2012b). In Chilean alfalfa fields the abundance of native ladybird species declined after H. axyridis was first observed in this crop in 2008. Furthermore, total ladybird species and diversity in alfalfa have also declined during this period (Grez & Zaviezo unpublished data). It is thought that the spread of H. axyridis has caused the decline of native species, Adalia bipunctata (L.) and Propylea quatuordecimpunctata (L.) in the Ukraine (Verizhnikova 2011) and Coccinella septempunctata L. and A. bipunctata in Moldova (Iazloveţchii and Sumencova 2013), but this requires confirmation through further research.
In contrast, some long-term studies (Honek et al. 2016) have highlighted that some ladybirds native to Central Europe had already been declining before the arrival of H. axyridis. Indeed, the diversity of native ladybird communities were similar before (Honek et al. 2014) and after (Honek et al. 2016) the arrival of H. axyridis in the Czech Republic. Furthermore, in Switzerland, on-going long-term population studies have highlighted that, so far, only A. bipunctata has significantly declined since the arrival of H. axyridis (Kenis and Eschen, unpublished data) although risk assessments (Kenis et al. 2010) predicted that three other ladybirds sharing the same ecological niches with H. axyridis (Adalia decempunctata (L.) , Oenopia conglobata (L.) and Calvia decemguttata (L.)) were at risk.
In Belgium, high niche overlap between H. axyridis and generalist native species, particularly A. bipunctata and P. quatuordecimpunctata, was observed, suggesting a high potential for impact of H. axyridis on those species. Large-scale mapping data showed substantial range contraction of A. bipunctata, Adalia decempunctata (L.), Calvia quatuordecimguttata (L.), Exochomus quadripustulatus (L.) and P. quatuordecimpunctata after the arrival of H. axyridis. As a consequence of the invasion, systematic surveying was set up in Brussels using standardized beating of trees in parks, avenues and roadsides. With the exception of C. quatuordecimguttata, these abundance data reflected the reported large scale trends (Roy et al. 2012b). Adalia bipunctata exhibited a 57 % decline in its extent of occurrence in the last decade and according to a conservative application of the IUCN guidelines has now become a good example of species that was formerly widespread but now meets the criteria of a red list species (Adriaens et al. 2015). However, as for Czech Republic, A. bipunctata and P. quatuordecimpunctata were already in decline prior to the invasion.
The clear differences in the response of ladybird assemblages to the arrival of H. axyridis between countries highlight the need for comparative studies. In a recent study it was concluded that differences in species trends between central (Czech Republic) and western Europe (UK) could be attributed to suboptimal environmental conditions in the UK, which is the edge of the biogeographic range for many ladybirds, exacerbating the negative effects of H. axyridis (Brown and Roy 2015). Such interactions between drivers of change are undoubtedly important in population dynamics, and the negative effects of H. axyridis are likely to be the result of a complex range of interactions and processes including resource competition and intra-guild predation (IGP; Majerus et al. 2006).
Long-term and large-scale data on the distribution and abundance of ladybirds are critical for the detection of population changes. To support the collection of long-term survey data, citizen science programmes such as the UK Ladybird survey, Lost Ladybug Project and Buckeye Lady Beetle Blitz in the USA, Chinita arlequin in Chile and the harlequin ladybird survey in Norway have been established to track changes in ladybird populations (Gardiner et al. 2012; Losey et al. 2007; Roy and Brown 2015; Sæthre et al. 2010a, b).
Harmonia axyridis and direct competitive interactions
The majority of research examining interactions between H. axyridis and other aphidophagous species has focused on direct interference competition on other ladybirds, proposing that decline is due to strong asymmetric IGP of eggs and larvae in favour of H. axyridis (Pell et al. 2008). This hypothesis is supported by many laboratory and field cage studies around the world (Cottrell 2004; Gardiner and Landis 2007; Hoogendoorn and Heimpel 2002; Katsanis et al. 2013; Roy et al. 2008; Snyder et al. 2004; Soares and Serpa 2007; Ware and Majerus 2008; Ware et al. 2009; Yasuda et al. 2004). Under open field conditions in Ohio, USA, eggs of the exotic H. axyridis were subject to lower predation relative to the eggs of native species (Smith and Gardiner 2013). However, out of 342 attacks, video surveillance illustrated that only two were attributable to alien ladybirds (H. axyridis feeding on conspecific eggs). Instead, a diverse guild of predators (dominated by Opiliones, Tettigoniidae and the native C. maculata) were responsible for the majority of egg attacks (Smith and Gardiner 2013). Importantly, this study accounted only for egg predation but alien and native ladybird species may compete directly through consumption of other life stages. Other tools, such as alkaloid (defensive chemicals within coccinellids) sequestration analyses, frass analysis, and PCR-based gut content analyses, have been developed to quantify the actual extent of interference competition occurring among native and alien species (Brown et al. 2015; Davidson and Evans 2010; Gagnon et al. 2011; Hautier et al. 2008, 2011; Sloggett et al. 2009; Thomas et al. 2013). For example, Hautier et al. (2011) found that 20.5 % of 590 H. axyridis larvae in Belgium tested positive for native ladybird alkaloids. Thomas et al. (2013) detected the DNA of native ladybirds within H. axyridis in the UK, with 3.7–22.7 % of 156 H. axyridis found to have consumed native species over 3 years. Gagnon et al. (2011) used molecular gut content analysis to illustrate that IGP among larval ladybirds in soybean fields can be very high; 52.9 % of sampled ladybirds contained the DNA of one or more other ladybird species. PCR primers have been developed and used to track aphid and ladybird predation by H. axyridis fourth-instar larvae collected in lime trees in Italy, and it was found that 7 % of sampled individuals contained the DNA of one of two native ladybird species (Rondoni et al. 2015).
There has been less research on the interactions between H. axyridis and species within the aphidophagous guild beyond ladybirds (Pell et al. 2008). However, laboratory and field studies have also indicated direct interactions with non-coccinellid aphidophagous predators. Harmonia axyridis is an intra-guild predator of Episyrphus balteatus (DeGeer); (Diptera: Syrphidae) with the strength of this asymmetric interaction increasing with developmental stage of H. axyridis and decreasing in the presence of extraguild prey (Ingels and De Clercq 2011). The aphid-specific pathogenic fungus Pandora neoaphidis (Remaudiere and Hennebert) Humber (Zygomycota: Entomophthorales: Entomophthoraceae) is consumed by H. axyridis (Roy et al. 2008) whereas most other aphid predators avoid consumption of infected aphids (Roy et al. 1998). Interestingly cage experiments in Italy have been used to study predation on immature stages of H. axyridis, and it has been observed that ants exhibit high levels of predation (Burgio et al. 2008a). Further research has demonstrated the influence of many factors on IGP, including intrinsic (such as feeding history of the species) and extrinsic (such as habitat complexity), and highlighted the importance of addressing such factors when considering the ecological relevance and extent of IGP (Ingels et al. 2015).
Harmonia axyridis and indirect competitive interactions
Harmonia axyridis may also adversely affect aphidophagous species through exploitative competition for shared resources (Evans et al. 2011). Studies to date have failed to show that H. axyridis negatively affects native North American ladybirds through exploitative competition among larvae (Hoogendoorn and Heimpel 2004; Yasuda et al. 2004), however, field and microcosm studies reveal reduced fitness in the predatory bug Anthocoris nemoralis (F.) (Hemiptera: Anthocoridae) due to competition with H. axyridis (Howe et al. 2015, 2016). Evans (2004) proposed that following the introduction of alien competitors (specifically C. septempunctata), native species abandoned croplands due to increased competition for shared prey. Adults of native species were thought to be displaced to ancestral (refuge) habitats not heavily exploited by alien competitors (Evans 2004; Snyder 2009). Evans (2004) tested this hypothesis by increasing aphid abundance in a Utah alfalfa fields and found that native ladybirds were drawn back into the field, apparently from refuges maintained in the surrounding landscape. In Japan H. axyridis coexists with other predators within natural habitats, without adverse negative impacts on co-occurring species (Osawa 2011).
Alien ladybirds and landscape-scale processes
Landscape change can strongly influence species declines within a region (Lindborg and Eriksson 2004). Factors such as habitat degradation, reduction of habitat patch size, and increased isolation of habitats (loss of connectivity) can also lead to declines in the abundance and diversity of coccinellid species within a landscape (Gardiner et al. 2009a; Grez et al. 2013, 2014b). Ladybirds may forage across several habitats within a given landscape including forests, grasslands, and croplands. Landscape changes that alter the distribution of these habitats may affect ladybirds by influencing prey populations, overwintering habitats, or by facilitating the invasion of alien competitors. Observations from several countries suggest that a high proportion of native ladybirds occur in less disturbed habitats, while a high proportion of alien ladybirds (including H. axyridis) occurs in human-modified habitats (Grez et al. 2013; Panigaj et al. 2014).
Landscape composition and heterogeneity can also affect competitive interactions among coccinellids (Gardiner et al. 2009a). In the USA, alien species were more abundant within soybean fields embedded within forested landscapes, and native species more common in agricultural landscapes with significant forage and grassland habitat. A large-scale study assessing the influence of landscape factors on the spread of H. axyridis across the UK indicated that coniferous woodland may negatively affect the spread of this species (Purse et al. 2014). Further research is needed to examine how the composition of the surrounding landscape influences the stability of native populations in the invaded regions.
Harmonia axyridis as a household and agricultural pest
Many ladybirds in temperate regions migrate for overwintering to elevated and conspicuous elements in landscape, or even to hill tops (Hodek et al. 2012). There they seek crevices in rocks, in bark of prominent trees and other shelters including south-facing anthropogenic structures such as buildings and monuments (Koch and Galvan 2008; Roy and Brown 2015; Wang et al. 2011). Harmonia axyridis forms large aggregations during the winter months across the native and invaded range in natural areas but also within homes and other structures (Nalepa 2007; Roy and Brown 2015). A recent study from Poland highlighted the use of wind turbines as overwintering sites for H. axyridis (Dudek et al. 2015). It shows hypsotactic behaviour (moving towards prominent objects on the horizon) and a clear preference for contrasting visual elements; vertically positioned stripes being more attractive than horizontal ones (Nalepa et al. 2005; Obata 1986). The relative attractiveness of different surface colours has been studied; white is the most attractive colour followed by yellow and black then green then red and finally natural wood (Wang et al. 2011). Volatile aggregation pheromones are not involved in this orientation (Nalepa et al. 2000) but it is apparent that contact chemoreception is important in the establishment of large aggregations (Durieux et al. 2012).
Harmonia axyridis accounts for 97 % of observations from houses contributed by citizen scientists to the US Lost Ladybug Project (Ramsey and Losey 2012). Infestations in homes can cause staining damage to carpet and furnishings and cause allergic reactions (Goetz 2008; Koch and Galvan 2008), but most often are considered a nuisance. Additionally, although this species does not have a greater propensity to bite humans than other ladybirds, its propensity to aggregate in dwellings in high numbers has resulted in a significant increase in the number of ladybird bites reported (Ramsey and Losey 2012). After infestation of the intensive care unit in a large hospital in Austria, counteractive measures including relocation of patients and temporarily closure of the station generated considerable financial costs. Management tactics have been developed to mitigate such nuisance problems (Kemp and Cottrell 2015).
Harmonia axyridis has been reported to feed on many fruit crops in many parts of the world including grapes, stone fruit, apples, pumpkins and berry crops (Koch et al. 2004a). Depending on fruit type, this feeding damage includes primary injury as well as secondary feeding on wounded tissue. In China H. axyridis has been documented foraging on pollen, nectar and young plant tissues, occasionally causing serious damage to fruits (Guo and Wan 2001; Yang et al. 2006). In contrast in Japan H. axyridis does not consume orchard fruits and overwintering H. axyridis are not regarded as a household pest. However, economically, H. axyridis presents the largest threat as a contamination pest in wine grape production (Koch and Galvan 2008). Beetles can be found within vineyards largely between the onset of ripening and harvest (Galvan et al. 2008). Adults aggregate and feed on injured fruit clusters (Koch and Galvan 2008). When fruit are harvested and crushed, H. axyridis release methoxypyrazines (MPs) which create an unpleasant odour and taste in the wine produced (Botezatu et al. 2013; Galvan et al. 2008). In the Midwestern USA, the dramatic increase in H. axyridis in wine grapes generally follows population declines in nearby soybean and maize fields (Bahlai and Sears 2009; Galvan et al. 2006, 2008); however, more research is necessary to document this dispersal hypothesis.
A push–pull strategy including artificial injury of selected fruit clusters on the vineyard margin and spraying the adjacent rows with bisulphite has been proposed as a management option (Glemser et al. 2012; Nedvěd 2014). In addition, progress has been made in developing both natural and synthetic corks that can significantly reduce MP concentrations in wine (Pickering et al. 2010). However, more research is needed to evaluate this approach for additional varietal sources and cost effectiveness. The establishment of H. axyridis in regions where the production of wine is economically important, including Crimea, the Caucasus, Canada, Europe, South Africa and South America, continues to be a concern, and close monitoring of the populations in these countries is critical.
Global distribution and regional research priorities
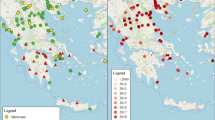
Harmonia axyridis has become one of the world’s most widely distributed ladybirds (Fig. 1) and is now found on all continents except Antarctica and notably is absent from a few large countries such as Australia. Outside of its native range (Asia), it has become very widely established in North America, South America and Europe (Fig. 2), and in limited parts of Africa. There is a small distance between the western edge of the native range and the eastern edge of the invaded range (see Russia and adjacent countries below), and it seems inevitable that these populations will meet in the near future.
In this section we document the distribution of H. axyridis around the world and its invasion history (summarized in Table 1), including the perspectives on regions where it has failed to invade. Additionally, current and future research priorities in different countries are presented.
Asia
Harmonia axyridis is native to East Asia (Mongolia, parts of China and Russia, northern Vietnam, Japan and Korea; Fig. 1).
China
Harmonia axyridis has a wide geographical range in China, especially in the north (Wang et al. 2007). Current research on H. axyridis in China is mainly focused on agricultural applications, including the development of artificial diet for H. axyridis to maximise production of this species for field release in augmentative biological control (Wang et al. 2007). Harmonia axyridis has also been used extensively for testing pesticides and specifically examining the sensitivity of natural enemies to pesticides (Tang et al. 2014). There is also ongoing research on the genetics of H. axyridis colour polymorphism, specifically how colour variation interacts with environmental factors.
Japan
In Japan, H. axyridis occurs on the islands of Hokkaido, Honshu, Shikoku, Kyushu, Tsushima, Iki and Gotoh, while its sibling species H. yedoensis (Takizawa) occurs on Honshu, Shikoku, Kyushu, Yakushima and the Ryukyus (Kurosawa et al. 1985). Future research in Japan will focus on intensive field studies to establish the mechanisms which enable coexistence of H. axyridis and other ladybird species in its native range.
Asian part of Russia, Kazakhstan and Kyrgyzstan
The northern part of the native range of H. axyridis occupies the south of Siberia and the Far East. The type locality of the species is in Siberia (Pallas 1771). The geographical distribution and colour variability of this species was first studied in the 1920s (Dobzhansky 1924). A recent map of the native range has been compiled (Orlova-Bienkowskaja et al. 2015). Harmonia axyridis populations west of Baikal Lake (in West Siberia and the western part of East Siberia) differ both genetically and morphologically from those living east of Baikal (Lombaert et al. 2011, 2014; Vorontsov and Blekhman 2001).
The native range of H. axyridis includes in the Altai Mountains, situated in north-eastern Kazakhstan and West Siberia. In some recent studies, the south-east of Kazakhstan was regarded as part of the native range (Loiseau et al. 2009; Lombaert et al. 2011, 2014). However, this is incorrect because H. axyridis was not recorded in the south-east of Kazakhstan in the nineteenth century or in the first half of the twentieth century. In 1968–1970 attempts to introduce H. axyridis from the Far East to the south-east of Kazakhstan for biological control of aphids (Savojskaja 1971) failed. Now H. axyridis is a common species in the south-east of Kazakhstan and was recently detected in the neighbouring region of Kyrgyzstan. Both morphological and genetic studies strongly indicate that the H. axyridis occurring in this region are not descendants of the released beetles, since current populations are similar to West-Siberian populations and differ from Far East populations. It is hypothesised that H. axyridis appeared in south-eastern Kazakhstan and Kyrgyzstan after the construction of the Turkestan-Siberian Railway, and that the beetles spread along this railway (Orlova-Bienkowskaja et al. 2015).
European part of Russia, Moldova, Ukraine and Belarus
Releases of H. axyridis for biological control of aphids in the Soviet Union began about 80 years ago. Attempts to introduce H. axyridis were made in Transcaucasia in the 1930s, in the Chernovtsy region (Ukraine) in 1964, in the Kiev region (Ukraine) in the 1960s, in Crimea in 1969, in the Tashkent region (Uzbekistan) in 1969, in Minsk (Belarus) in 1968–1970, in south-eastern Kazakhstan in 1968–1970 and in Adjaria and Mtsheta region (Georgia) in 1982–1988. It is thought that none of the intentionally released populations established (Izhevsky 1990; Verizhnikova and Shylova 2013). Established populations are thought to have originated from secondary spread eastward of H. axyridis from western Europe to European Russia and neighbouring countries. Established populations were detected in the Kaliningrad region (Russian enclave in the Baltic region; Zakharov et al. 2011). Recently, H. axyridis has been observed spreading rapidly in the Caucasus and the south of European Russia (Orlova-Bienkowskaja 2014). Established populations have been found in Adygea, Krasnodar region, Abkhazia, the Stavropol region, Georgia (Belyakova and Reznik 2013), Crimea (Rybalchenko, personal communication), Daghestan (Ilyina, personal communication) and Rostov region (Arzanov, personal communication). Individual specimens of H. axyridis have also been found in the central belt of European Russia (Belgorod and Lipetsk regions; Orlova-Bienkowskaja 2013; Ukrainsky and Orlova-Bienkowskaja 2014), but it is unknown if the species is established there.
In 2003, several specimens of H. axyridis f. spectabilis were found in the wild in Kiev (Ukraine) and since 2007 a stable population of H. axyridis has existed there (Verizhnikova and Shylova 2013). In 2009 H. axyridis was detected in several locations in western Ukraine, and there has been rapid expansion since, with the species now abundant all over the country (Nekrasova and Tytar 2014). The spread of H. axyridis has been so rapid that it has been observed to occur across a whole country before being detected: H. axyridis was not detected in Moldova until 2011, when it had already become common throughout the country (Timuş and Stahi 2013).
Harmonia axyridis has been recently detected in Belarus, in the Brest region. The first specimen was found in 2011, but 14 individuals were subsequently found in 2014 (Lukashuk and Ryndevich, unpublished).
Current research in Russia and the Ukraine relates to the development and effects of photoperiod on development and maturation (Belyakova and Reznik 2013; Reznik et al. 2015), genetics (Zakharov et al. 2011), morphological variability (Blekhman 2008), population dynamics (Nekrasova and Tytar 2014) and current expansion of the range in Russia and adjacent countries (Ukrainsky and Orlova-Bienkowskaja 2014). There is also considerable interest in elucidating the routes of invasion by genetic methods as has been done for many other regions (Lombaert et al. 2014). It is possible that the populations in the Caucasus and in the south of European Russia include hybrids between invasive European individuals and those introduced from the Far East for use in biological control. As the secondary range is expanding eastwards, it is predicted that it will soon reach the western border of the native range. Thus there is an opportunity to observe the consequences of interactions between native and invasive populations in West Siberia.
North America
Hamonia axyridis was repeatedly introduced throughout the twentieth century to the USA with the aim of establishing populations (Gordon 1985; Harmon et al. 2007; Koch et al. 2006b). Intentional releases of H. axyridis include a number of states but notably California in 1916, and multiple eastern states from 1978 to 1992 (Chapin and Brou 1991; Gordon 1985; McClure 1987; Tedders and Schaefer 1994). Harmonia axyridis was first detected beyond intentional release sites in the United States in 1988 in south-eastern Louisiana and eastern Mississippi (Chapin and Brou 1991). A second, independent set of releases led to establishment in the 1980s in the Pacific Northwest (LaMana and Miller 1996; Lombaert et al. 2010, 2014). It is unknown whether all established populations resulted from these releases or from additional, accidental introductions. Nonetheless by the mid-1990s, H. axyridis had been found across the country with detection reports from 45 of the 48 contiguous states by 2007 (Dreistadt et al. 1995; Hesler et al. 2001; Krafsur et al. 1997; LaMana and Miller 1996; Mizell III 2007) and most recently from Montana in 2009 (Foley et al. 2009) and Arizona in 2008 (Fothergill et al. 2010). Harmonia axyridis was first detected in Canada in 1994 (Coderre et al. 1995). Currently this species is found throughout most of North America north of Mexico with Labrador, Saskatchewan, Alaska and Wyoming the only areas where it has not yet been reported (Foley et al. 2009; Fothergill et al. 2010; Hicks et al. 2010; Koch et al. 2006b). Harmonia axyridis is also established and widespread in Mexico (Brown et al. 2011b).
Outside of the native range, North America has the longest history of experience with H. axyridis and may serve as a case study of potential impacts, positive or negative, that this species may cause in other invaded areas (Koch et al. 2006a; Koch 2003; Koch and Galvan 2008). Some recent lines of research in North America are reviewed elsewhere in this paper. Briefly, research continues on attaining benefits from H. axyridis as a biological control agent of pests, and on understanding and minimizing adverse impacts of the invasion (pest of fruit and wine production, nuisance household invader, and impacts on native fauna).
South America
Harmonia axyridis is reported in most countries in South America (Amat-García et al. 2011; González and Kondo 2012; Koch et al. 2011; Kondo and González 2013; Nedvěd and Krejčík 2010; Saini 2004; Solano and Arcaya 2014). Based on factors such as climate and habitat, it has been predicted that H. axyridis would become established across broad areas of South America (Koch et al. 2006b). The invasion by H. axyridis has been followed intensively in Chile.
Chile
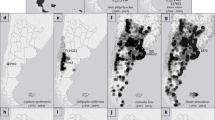
In 1998, a flightless strain of H. axyridis was introduced from France to central Chile for biological control in greenhouses, but those populations did not establish (Grez et al. 2010; Table 1). In 2003, large numbers of flying H. axyridis associated with aphids in poplar trees were reported near the release sites. In 2008, surveys of ladybird populations in alfalfa revealed only one individual H. axyridis (from a sample of approximately 90,000 ladybirds), but rapid population increase was observed over the next 2 years in alfalfa and other habitat or crop types (Grez et al. 2010). Harmonia axyridis is currently one of the two most abundant species in alfalfa fields in central Chile, representing 50–90 % of ladybirds (Grez et al. 2014a, b). It is expanding into other habitats, including native vegetation, such as sclerophyllous matorral, although at very low densities (Grez et al. 2013). Harmonia axyridis is spreading toward southern Chile, colonizing regions with colder climates. In contrast, few records are reported towards northern Chile, where the Atacama Desert is probably acting as a barrier for its invasion. From its original distribution in 2010, covering a range of 250 km (north to south) near Santiago, it has expanded its distribution to 2600 km (north to south), and from sea level to >3000 m a.s.l., with a north to south spread of approximately 160 km year−1. Genetic analyses suggest that current Chilean populations come from the East North America strain (Lombaert et al. 2014), representing an accidental introduction. Only f. succinea has been found in Chile.
Current research in Chile relates to the impacts of this species on the diversity of ladybird assemblages and abundance, including native species, in different habitats, as well as its potential damage to vineyards. There are also extensive field and laboratory experiments in progress to assess IGP and competition as mechanisms for understanding the dominance of H. axyridis over other ladybirds. Studies of physiological performance and life history traits under different temperature conditions in the laboratory have also been carried out (Barahona-Segovia et al. 2015). The invasion process is being systematically tracked through surveys coordinated by Grez and Zaviezo, including data from the Surveillance Department of the National Agrarian and Livestock Service (SAG), and from media and social networks specifically developed for this purpose (web page: http://www.chinita-arlequin.uchile.cl/; Facebook: https://www.facebook.com/chinita.arlequin; twitter: https://twitter.com/chinitaarlequin). The high interest and participation of citizens through these media suggest that the presence of H. axyridis is of growing concern to Chileans.
Spatial distribution models are also being developed to forecast the future distribution of H. axyridis in the country. Chile is a long (4329 km) and narrow (180 km on average) country, isolated by the Atacama Desert to the north, the Pacific Ocean to the west, and the Andes mountain range to the east. The Chilean environment has strong latitudinal gradients in abiotic conditions, from the most arid desert in the world in the North, followed by a semi-arid region, Mediterranean type ecosystems in central Chile, the temperate rainforests toward the South, and the southernmost sub-Antarctic ecosystems (Luebert and Pliscoff 2006). This gradient offers a unique opportunity to study how climate modulates the distribution and abundance of this invasive alien species. Also, along this gradient, a highly diverse native fauna of coccinellids (~115 species; González 2008) offers a unique opportunity to evaluate the possible changes of native communities after H. axyridis arrival.
The main gaps in knowledge in Chile relate to natural enemies and possible control or containing methods. The only observed natural enemy thus far is the parasitoid D. coccinellae. Also, there are many unanswered questions in relation to the ecology and biology of H. axyridis during summer time, when this species disappears from crops, apparently estivating or migrating to other, as yet undetermined, locations. Its role as a biological control agent of aphids and other pests is still unknown.
Europe
A network of scientists was established through a working group (Benefits and Risks of Exotic Biological Control Agents) of the Western-Palearctic Regional Section of the International Organisation of Biological Control in response to the rapid spread of H. axyridis throughout Europe. Consequently there has been ongoing collaborations to map the distribution throughout Europe (Brown et al. 2008a) and so here we present information reflecting this activity on a country by country basis. There is considerable scope to enlarge the network and research collaborations and we look forward to doing so in the coming years.
Austria
Harmonia axyridis was first recorded in eastern Austria in 2006 (Rabitsch and Schuh 2006) and soon reported from other provinces, where it may have been present but undetected for some time. It was never imported or sold as a biological control agent in the country and the arrival via natural spread from north-western Europe is most likely. After almost a decade, however, the species has been found in all federal provinces and there is no doubt that it has become the most abundant ladybird species in Austria.
Unfortunately, no systematic surveillance or monitoring data are available to trace the expansion within Austria or to document any impact on native biodiversity. Personal observations from entomologists, however, indicate that the species has strongly increased in abundance and range over the last decade. The establishment and use of citizen science initiatives in gathering information are currently under development.
Belgium
In Belgium, H. axyridis was first applied as a biological control agent in 1997. The species was first observed in the wild in 2001 (Adriaens et al. 2003) and this led to the end of its commercial use in Belgium. In less than 5 years the species invaded the entire country, its distribution covering all Belgian ecoregions (Adriaens et al. 2008). The area of occupancy showed an average rate of increase of 189 % (5000 km2) per year from 10,000 km2 in 2002 to 31,000 km2 in 2006. Harmonia axyridis has become the most abundant species in (semi-)natural systems and also dominates the aphidophagous guilds in certain agro-ecosystems (Vandereycken et al. 2013).
At the time of arrival and spread of H. axyridis in Belgium a country-wide validated citizen-science survey (Gardiner et al. 2012) focussing on the ecology and distribution of 40 native ladybird species was running. This allowed almost real-time monitoring of this insect invasion as well as detailed analysis of niche overlap with native species (Adriaens et al. 2008). Future research on the decline of native coccinellids should also consider factors such as land use change, climate change, habitat quality and effects of pesticides as potential contributors to this phenomenon.
Czech Republic
Although H. axyridis was released in the Czech Republic for protection of hops in the early 2000s, it did not establish. The first occurrence of the invasive population dates back to 2006. In 2007, it occurred near the western borders of the country and in a few cities, but in 2008 it was found in many cities and towns including the eastern-most (Špryňar 2008). In 2009, the species could be found in most anthropogenic-altered landscapes, but not in remote areas such as mountains over 1100 m, continuous forests and closed military zones. Mass infestation of houses during autumn migrations raised public awareness and a few cases of biting and allergic reactions were reported. Unintended transfers of single specimens over hundreds of kilometres were recorded (Nedvěd 2014).
Denmark
Harmonia axyridis was first recorded in Copenhagen 2006, and presumed to have arrived from Germany (Brown et al. 2008a; Pedersen et al. 2008). In the following years the beetle spread within the Greater Copenhagen area, becoming established across the island of Zealand. By 2007/8, its spread continued westwards to the islands of Lolland, Langeland, Ærø, and in the east to Bornholm (perhaps arriving from Poland or Germany) where populations are now established (Howe 2015; Howe et al. 2015; Steenberg and Harding 2009b; Steenberg et al. 2009). By 2008, H. axyridis had reached Jutland in the far west of Denmark (Steenberg et al. 2009). The most northern record to date (latitude 57°N) was from produce in a supermarket, highlighting the potential for spread through anthropogenic pathways. Data from Zealand indicates stable populations with H. axyridis dominating ladybird assemblages where established. Greatest densities occur within urban areas of central Copenhagen (Ravn and Howe, unpublished data).
It is interesting to note that the distribution of H. axyridis in Denmark is limited in the north of the country. Indeed, following 10 years’ of establishment within Denmark, aside from the aforementioned supermarket record there are no records of H. axyridis north of 56°N (Ravn and Howe, unpublished date), which corresponds with a lack of records from Scotland (UK). Whether this represents a true limit to the northern distribution attributable to present climatic conditions requires further research, particularly in relation to future predictions of spread based on expected climate warming.
France
In France, H. axyridis was first introduced for biological control in 1982 but very few records of feral populations exist until 2004 (Coutanceau 2006a), when the species started to spread across the country from the north, close to its probable introduction point in Belgium (Adriaens et al. 2003). Maps of first observations of H. axyridis at particular locations in France suggest a heterogeneous process of diffusion, with some regions rapidly colonized whereas in others there is a lag in invasion, or H. axyridis remains absent (see maps in http://vinc.ternois.pagesperso-orange.fr/cote_nature/Harmonia_axyridis). First observation records have been used in sophisticated modelling approaches to make inferences about the relative impact of various environmental and anthropogenic factors on that spread (Veran et al. 2015) and confirmed that the rate of colonization of H. axyridis in France was heterogeneous in time and space. Anthropogenic factors explained more variation of the diffusion process than environmental ones. The relative surface of urbanized area was the major anthropogenic factor increasing the probability of colonization. More specifically, low urban densities, corresponding to rural areas, represented unfavourable habitats. Finally, average summer temperature was the main environmental factor affecting colonization, with negative impact for both high and low values.
IAS expanding their range provide unique opportunities to explore the effect of spatial spread on life-history traits, making it possible to test for a spatial arrangement of dispersal abilities along the expanding range (Phillips et al. 2007; Purse et al. 2014). Moreover, the question of the evolution of dispersal capacity in invasive populations is highly relevant, because it may accelerate spread, with serious applied and theoretical consequences (Phillips et al. 2010; Travis and Dytham 2002). Using controlled experiments in laboratory conditions, clear evidence was found of a strong, rapid increase in flight speed with range expansion of H. axyridis from the core of the invaded area in Western Europe (Belgium) to the front of this invaded area in South and Western parts of France (Lombaert et al. 2014). This shift towards a higher flying speed at the invasion front was remarkably rapid, as it was demonstrated after only 8 years of expansion, corresponding to about 16 generations (Koch 2003).
Using population genetic approaches, it has been shown that the H. axyridis sampled across France (see below for exceptions) belonged to a single genetic unit that has invaded the Western part of Europe (Lombaert et al. 2010, 2011). This invasive population bears traces of genetic admixture between an eastern North American wild source, which was found to have served as a “bridgehead source” for many worldwide invasive outbreaks, and a biological control strain used in Europe (Lombaert et al. 2010). Experimental studies in the laboratory have investigated the phenotypic impacts of such genetic admixture (Facon et al. 2011; Tayeh et al. 2012, 2013, 2015; Turgeon et al. 2011). Biological control individuals were found to display classic r-selected traits with a shorter lifespan and an earlier egg production when compared to native and US invasive individuals. European invasive individuals have shown phenotypic traces of the genetic admixture between US invasive and biological control individuals. For most traits (such as age at the start of reproduction, total adult and reproductive lifespan), European invasive individuals displayed intermediate values between both parents. Thus genetic admixture has had a long-lasting effect in the wild by shaping the life-history strategy of the European invasive individuals of H. axyridis. In France, and more generally Western Europe, the exact role of admixture with the European biological control strain in the process of invasion remains poorly known. The single eastern North American origin of one South African and two South American outbreaks indicates that the genetic admixture observed in Western Europe is not required for an eastern North American propagule to establish and start an invasive population in diverse ecological contexts (Lombaert et al. 2014). It seems therefore unlikely that admixture in Western Europe has radically changed the outcome from failed to successful invasion. Such admixture has probably simply modulated the rate (or impact) of an invasion process that would have been successful anyway.
In addition to the single vast admixed population which invaded Western Europe, a genetically distinct population established in South East of France has been identified (Lombaert et al. 2014). This population was first observed in 2005, and it appeared to have originated exclusively from the European biological control strain introduced into Europe from 1982. This is surprising, at first sight, because European biological control individuals have long been thought to be unable to survive in the wild (Ferran et al. 1997). In support of this, it is apparent that this distinct population does not seem to have expanded spatially, unlike most of the other H. axyridis outbreaks known all over the world. This locally established population might attest, however, to the ability of the European biological control strain to found small overwintering populations in the wild, in areas with clement winters.
Germany
In 2000 the first German record of H. axyridis in the wild was reported from Frankfurt City in 2000. This was followed 2 years later by reports of H. axyridis, in higher abundance than the first records, from Frankfurt region and also Hamburg (Klausnitzer 2002). It has spread rapidly into other regions across Germany and is now considered established throughout Germany, although there has been no nation-wide monitoring to confirm this assumption. In south-west Germany near Frankfurt peak population densities were observed in early autumn until 2009 and also in 2012 but there seems to have been declines in numbers since then. However, this information is not derived from systematic monitoring.
Several German populations from 2008 to 2009 were examined for the presence of antagonistic microorganisms (bacteria, fungi, microsporidia) and invertebrate parasites (Herz and Kleespies 2012).
Italy
In Italy, H. axyridis was released in protected crops as a biological control agent from 1995 to 1999. However, the use of H. axyridis for biological control was suspended because concerns over adverse impacts became apparent. The first occurrence of establishment was in 2006 in Piedmont followed by subsequent observations in Emilia-Romagna in 2008 and rapid establishment in 16 out of 20 Italian regions with particularly widespread occurrences across northern and central Italy (Burgio et al. 2008b; Cornacchia and Nardi 2012). The origin of the populations is unknown; individuals could be either offspring of those released in situ or immigrants from other parts of Italy, France or Switzerland (Burgio et al. 2008b).
Research studies in Italy have focused on H. axyridis biology (Bazzocchi et al. 2004) and specifically life table parameters. Research on the occurrence of parasitoids attacking H. axyridis is ongoing (Francati 2015; Rondoni et al. 2013). Other studies are focusing on the development of a liver-based artificial diet, which could assist in maintenance of cultures of adult H. axyridis (Sighinolfi et al. 2008), and on the susceptibility of H. axyridis larvae to lambda-cyhalothrin insecticide (Benelli et al. 2015).
Norway
Harmonia axyridis was assessed as a potential biological control agent for use in Norwegian greenhouses in 2001 (Statens landbrukstilsyn 2001). It was concluded that H. axyridis might become established outdoors and thereby pose a risk to the environment. The assessment was therefore negative with respect to import and commercial use of H. axyridis in Norway.
The first record of H. axyridis in Norway was in Oslo in 2006, the adult female f. succinea arrived as a stowaway on horticultural plants, Thuja sp. (Cupressaceae) imported from the Netherlands to Norway (Staverløkk 2006). In late 2007 and throughout 2008 several adults were found indoors and outdoors at a number of locations in the urban and suburban areas of Oslo (Sæthre et al. 2010a, b).
Observations in areas some distance from Oslo, such as Tvedestrand in Aust-Agder County (2008), Våle in Vestfold County (2008) and Trondheim (2009; the latter about 600 km north of Oslo) revealed further spread or separate introduction to new areas (Sæthre et al. 2010a, b). Repeated introductions (probably on imported plants) are likely to be the most important factor for introduction of the species to new areas in Norway. Natural geographic barriers and long distances within Norway limit the species possibilities for rapid natural dispersal. However, anthropogenic spread facilitates dispersal and of particular note was the occurrence of between 2000 and 3000 adult H. axyridis in a cargo of timber imported to Åndalsnes (Møre og Romsdal County) in March 2008 from Pennsylvania, USA. Some specimens were also recorded at Snåsa (Nord-Trøndelag County) on the imported timber which was transported from Åndalsnes. According to the importing timber company, actions had been taken to eradicate the beetles.
In late 2008 a website was launched to engage the public in submitting observations on-line and this has made a major contribution to documenting the distribution of H. axyridis in Norway (Sæthre et al. 2010a, b). Records are available at http://www.artsportalen.artsdatabanken.no/#/Harmonia+axyridis/7468. In autumn 2015 high numbers of H. axyridis were reported from overwintering aggregations across the city of Oslo. However, so far, the distribution of H. axyridis in Norway appears to be limited to urban and suburban regions, and it has to date not been recorded or reported in commercial crops or in natural habitats. Further studies on the biology, ecology, cold tolerance and winter survival will contribute to better predictions of the dispersal and establishment potential of H. axyridis in Scandinavia.
Slovakia
Harmonia axyridis was first recorded in Slovakia in 2008 (Majzlan 2008). It arrived as an unintentional introduction by secondary spread, following the spread across Austria (Rabitsch and Schuh 2006), Poland (Przewozny et al. 2007) and the Czech Republic (Špryňar 2008). There was less than 1 year between the first record of establishment and widespread occurrence of H. axyridis. By the end of 2009, it was recorded across Slovakia, and by the end of 2012 it occurred in numerous habitats, particularly gardens, orchards and urban areas. The records from 2008 to 2012 document the invasion clearly (Panigaj et al. 2014). The distribution and time sequence of the records support the maximum rate of the spread of H. axyridis to be approximately 200 km year−1, the spread being accelerated by human movement (Panigaj et al. 2014). The local topography played a crucial role in the spread: 47 % of the records of the coccinellid were from lowlands (94–200 m), 36 % from low hilly areas (200–400 m), 11 % from moderate altitudes (400–600 m) and only 6 % from higher areas (600–1250 m a.s.l.; Panigaj et al. 2014). Despite great efforts in 2013 and 2014, only a single specimen of H. axyridis was recorded from altitudes above 1000 m.
Wildlife records in Slovakia are mostly shared through popular naturalist’s web pages (e.g. www.nahuby.sk, www.fotonet.sk) which also provide information about H. axyridis. The Facebook page Lienky Slovenska (Ladybirds of Slovakia) was launched in 2015 to encourage the public to take part in ladybird surveys focusing on H. axyridis. The international cooperation with scientists and collaboration with volunteers within the Slovakia is critical for progress with further research on H. axyridis.
Switzerland
The first H. axyridis adult was found in 2004 in Basel, but it was only in 2006 that establishment was confirmed in several locations (Eschen et al. 2007). From then, the ladybird rapidly invaded all areas of low and middle altitudes within 2 years. A long-term inventory was initiated in the northwest of Switzerland in 2006 to record the impact of H. axyridis on native ladybirds (Eschen et al. 2007). Ladybird populations were monitored using standardised sampling methods at 45 sites: 15 broadleaved hedgerows, 15 meadows and 15 conifer sites several times a year. The monitoring was interrupted only in 2014 but has since restarted. Other surveys were made in other habitats on an irregular basis, in particular on urban trees. Since 2008, H. axyridis has become by far the most abundant ladybird on broad-leaved shrubs and trees, accounting for 60–80 % of all ladybirds collected throughout the year. In contrast, in meadows and on conifers, H. axyridis still remains rather uncommon, except on some specific plants such as nettle (Kenis and Eschen, unpublished data).
Further studies in Switzerland have also focused on the impact of H. axyridis on native ladybird populations. The occurrence of intraguild predation in the field using polymerase chain reaction (PCR) to identify target prey DNA within a predator’s gut has been investigated (Aebi et al. 2011). IGP between H. axyridis and eleven native non-target European ladybirds in laboratory experiments has been studied (Katsanis 2011).
The Netherlands
Harmonia axyridis was first released as a biological control agent in greenhouses in 1995, on outdoor crops, arboriculture and in urban areas since 1996, largely for the control of aphid pests (Cuppen et al. 2004). Releases were stopped by the end of 2003. Harmonia axyridis has established at some sites very rapidly with the first report of H. axyridis in the wild in 2002 in Groesbeek, Gelderland (as a pupa on a leaf of Hedera helix L. (Araliaceae) (Cuppen et al. 2004) followed by a specimen in Rotterdam, South Holland in 2003, and around the same time another specimen was collected in Reimerswaal, Zeeland. In July 2003 the first adult H. axyridis was collected on a lighted white sheet at night at a nature reserve (De Kaaistoep, North Brabant; Cuppen et al. 2004; van Wielink and Spijkers 2013). In this locality, up to 2014, 6516 specimens have been collected using light, 71.9 % of which were males (yearly range between 65 and 80 %; van Wielink, unpublished data).
From 2004 onwards, numbers of H. axyridis started to rise dramatically and monitoring efforts were increased (via http://www.knnv.nl, http://www.stippen.nl and http://www.waarneming.nl). While up until 2004 (mainly) the south part of the country was invaded, by 2007 more than 2000 records had been received covering the entire country, including the Wadden Islands (Brown et al. 2008a). Harmonia axyridis is now widely distributed and the dominant ladybird species, predominantly on trees and shrubs but also on herbs in urban and anthropogenic habitats, it is less abundant in (semi-) natural areas such as heathland and grasslands. In agricultural areas H. axyridis is considered the dominant ladybird in corn, but not in cereals. During the early years of invasion, until 2010, local abundance was particularly high during early summer and autumn but this has not been so apparent in recent years.
Research in the Netherlands has focused on natural enemies (Haelewaters et al. 2012; Raak-van den Berg et al. 2014; Sloggett 2010) and the high overwintering survival of H. axyridis (70.8–88.2 %; Raak-van den Berg et al. 2012). The latter study found that overwintering survival was higher (1) at sheltered places compared to exposed sites and (2) when ladybirds were overwintering at south-western sides of buildings. As a comparison, winter survival of A. bipunctata in the Netherlands is 17–78 % (Brakefield 1985).
United Kingdom
The UK has a long history of involvement of volunteers in gathering information on wildlife through biological recording (Pocock et al. 2015; Roy et al. 2015a). The Coccinellidae Recording Scheme (hosted by the Biological Records Centre which is part of the Centre for Ecology & Hydrology) was established in 1971 (Roy et al. 2011a). The legacy of ladybird recording in the UK provided a unique dataset through which to explore the impacts of H. axyridis on other ladybirds. Harmonia axyridis arrived in Britain through dispersal and introduction events from regions (mainly Europe) in which it was deliberately released as a biological control agent (Brown et al. 2008b). Harmonia axyridis was first recorded in the UK in 2003 (Roy et al. 2012c) and was established by 2005. An on-line survey (www.ladybird-survey.org) was launched to monitor the spread of H. axyridis while promoting the continued recording of other ladybirds. Tens of thousands of people have provided records of H. axyridis and other species of ladybirds (Roy et al. 2015b), providing an invaluable large-scale and long-term dataset which has been used to explore the invasion process and concomitantly trends in the distribution of other ladybirds (Comont et al. 2012, 2014a; Purse et al. 2014; Roy et al. 2012b). For example, declines in the distribution of seven (of eight assessed) native species of ladybird have been demonstrated, and correlated with the arrival of H. axyridis, using the records collated through the UK Ladybird Survey (Roy et al. 2012b).
The rapid spread of H. axyridis—more than 100 km per year across the UK (Brown et al. 2008b)—has been attributed to its high natural dispersal capability through both flight (Jeffries et al. 2013; Lombaert et al. 2014; Maes et al. 2014) and anthropogenic transport (Brown et al. 2011b). A number of factors are considered to have contributed to the successful establishment and dominance of this polymorphic species within aphidophagous guilds across the UK, including high reproductive capacity, intra-guild predation, eurytopic nature and high resistance to natural enemies within the invaded range (Roy and Brown 2015).
Considerable attention has been given to experimental research (Comont et al. 2014b) and systematic field surveys (Brown et al. 2011a) to further understanding of the interactions between H. axyridis and other species. Future work will reflect the opportunities presented by H. axyridis to explore the complex and dynamic role of natural enemy interactions in the invasion process through community (network) approaches (Roy and Lawson Handley 2012). The role of citizen scientists in gathering information on species interactions is considered an important component of this research.
Africa
In North Africa, H. axyridis was introduced for biological control use in Tunisia around 1990 and in Egypt before 2000 (El-Arnaouty et al. 2000). It is thought to be established in limited areas of the latter, but not the former (Brown et al. 2011b).
According to unpublished records and misplaced voucher specimens discovered only in mid-2015, H. axyridis was intentionally released in South Africa around 1980 (Stals unpublished). This contradicts the previously published view that the species had never been intentionally introduced to this country (Stals and Prinsloo 2007; Stals 2010). The beetles were sourced from the USA, apparently originating from Japan. The release was made in an attempt to control the black pine aphid, Cinara cronartii Tissot & Pepper, in the Sabie area, in the present-day Mpumalanga Province. In the newly found records, a later, but undated, entry notes that the species had not established. No other information is presently available. It seems probable that this release failed, since no specimens of H. axyridis collected in southern Africa before 2001 are present in any public insect collection in South Africa.
Unaware of the above, Stals and Prinsloo (2007) announced that H. axyridis was first recorded in South Africa in 2004, in the Western Cape Province. However, museum records later revealed that adults and immature stages had been found in the Cape Town area as early as 2001 (Stals 2010). This remains the earliest known date and location of establishment in southern Africa. Sabie, the 1980s release point, is c. 1800 km away from Cape Town. Harmonia axyridis specimens from the Sabie area were only recorded in the austral summer of 2008 (Stals unpublished), when the contemporary spread of the invader into Mpumalanga was already well underway (Stals 2010).
The introduction pathway of the contemporary invaders in South Africa is unknown, but all analysed South African populations originated from eastern North America (Lombaert et al. 2010, 2014). Introduction was likely unintentional, but it is unknown whether there was more than one introduction to South Africa, disregarding the almost certainly failed introduction of the 1980s.
The only coordinated data-gathering initiatives for insects in South Africa are for Lepidoptera and Neuroptera. Nonetheless, recording of the range expansion through southern Africa mainly depends upon volunteer contributions. Until 2010, contributions were encouraged through sustained calls in popular media (Stals 2008, 2010). Since 2011, the citizen science web application iSpot (Silvertown et al. 2015) has been exploited as a recording platform for southern Africa and became the source of many high-quality observations of H. axyridis. An expert Coleoptera taxonomist assesses all contributions, querying contributors where necessary.
Harmonia axyridis rapidly spread widely through much of South Africa (Stals 2010; unpublished). It seems established largely in cooler and more mesic parts of the country, viz. the south-western, southern and interior-eastern Cape regions, and the more northern eastern and east-central areas, with few records from the semi-arid western and west-central reaches or the hot northern regions. No records have as yet come from the subtropical eastern coastal belt. The invader is well established and commonly encountered in the Fynbos and Grassland Biomes; established and not infrequently encountered in the Savanna Biome; but infrequently reported from other biomes. In South Africa, the majority of records come from urban and rural gardens or dwellings, and from agricultural land. Other southern African countries with records of H. axyridis are Lesotho (first record June 2008, Stals 2010) and Swaziland (first record November 2013, Stals unpublished), and the ladybird has likely established in both these countries.
In the rest of sub-Saharan Africa, H. axyridis has only been recorded in Kenya (Nedvěd et al. 2011) and Tanzania (Nedvěd and Háva 2016). In Kenya, a population was discovered in December 2010 at a coastal holiday centre and may represent an established population (Nedvěd et al. 2011). In Tanzania, only two H. axyridis individuals were found at a beach resort in Zanzibar in April 2014 and may represent a transient introduction with no establishment. These occurrences suggest that the invasion of tropical Africa is possible. The origin of the beetles in East Africa has not yet been investigated and all examined specimens were of the f. succinea.
Research on H. axyridis in South Africa is scarce but it is recognised that there is a need to assess its biology and ecology in order to evaluate its impact on native communities and agroecosystems. Researchers from the Centre for Invasion Biology, Stellenbosch University, are focusing on the thermal biology and life history of H. axyridis in South Africa and compare this species’ traits to those of native aphidophagous ladybirds. This information can highlight characteristics that promote the invasiveness of H. axyridis in southern Africa and provide data for modelling its potential spread within and beyond borders (Shinner 2014). The study of its behavioural responses and adaptation to climate variation will remain a focus in the years to come as well as modes and mechanisms of introduction and range expansion investigated using molecular techniques.
However, many research aspects that are key for evaluating the establishment, spread and impact of H. axyridis are lacking. No systematic field surveys are taking place and therefore the invader’s abundance across habitat types is also unknown. More importantly, data on the native ladybird communities (species richness and abundance) occurring in areas with and without H. axyridis are not being collected and the impacts on these communities are thus unknown. Of overriding importance may be the complete lack of baseline data prior to this invasion; it is possible that no readily comparable and uninvaded habitats may remain for urgent collection of baseline information. In addition, the taxonomy and phylogeny of the native Coccinellini need to be examined, in particular those African species currently placed in the genus Harmonia.
Under South African national legislation, H. axyridis is a Category 1b Listed Invasive Species (Department of Environmental Affairs South Africa 2014a), which in terms of the National Environmental Management: Biodiversity Act, 2004, legally is a “species which must be controlled” (Department of Environmental Affairs South Africa 2014b). How such control is to be achieved is unclear at present.
Failure of Harmonia axyridis to establish in some regions
Harmonia axyridis is much more commonly and widely distributed in the northern than in the southern hemisphere, perhaps unsurprising given its Asian origin. While H. axyridis distribution extends to boreal regions in its native range, its invasive distribution in the north and south of Europe, and also in northern Canada and Alaska, is more limited and suggests that climatic factors may be important in limiting the spread of this species. Globally, there are very few records from tropical regions (23.4°N–23.4°S); where it has been reported from the tropics it is not widely spread (e.g., Colombia, Venezuela, Kenya, Tanzania). In both South America and Africa, limits to its distribution apparently include warm tropical but also arid environments.
In Europe there are some habitats and regions that appear to be resistant to the establishment of H. axyridis. Some examples are the limited evidence of establishment in Greece (Kontodimas et al. 2008), Turkey (Bukejs and Telnov 2015), Spain, including the Canary Islands (Goldarazena and Calvo 2007; Jacas et al. 2006; Pons et al. 2015), Bosnia and Herzegovina (Kulijer 2010), Portugal, including the Madeira and Azores archipelagos (Garcia 1986; Soares et al. 2008), Northern Ireland (Murchie et al. 2008), the Republic of Ireland (http://www.invasivespeciesireland.com), and Réunion (Quilici, personal communication). It is as important to consider the regions in which H. axyridis has failed to establish as those in which it has succeeded. Only recently has consideration been given to understanding invasion failures within the context of invasion processes (Zenni and Nuñez 2013). The factors limiting invasion by H. axyridis in some geographic areas are worthy of exploration and could potentially provide insights into whether the southern and northern European ecosystems are more resilient to invasion than other parts of Europe and beyond. Investigation of equivalent patterns on the southern African subcontinent would also be informative.
Records of successful breeding by H. axyridis are very limited in Scotland (Roy and Brown 2015) and although climatic conditions are not thought to have been a barrier to the colonization and spread of H. axyridis in southern Britain, it is possible that climate has limited its abundance not only in northern England and Scotland (Brown et al. 2008b; Roy and Brown 2015), but also Denmark (Steenberg and Harding 2009b) and further north throughout Scandinavia. The combination of lower temperatures and higher precipitation in Scotland compared to England could be limiting the distribution of H. axyridis within Scotland. The Orkney and Shetland Islands are considered climatically unsuitable for H. axyridis (Poutsma et al. 2008), and in support of this there have only been occasional records of adults, arriving on produce imported from the mainland, from these northern islands (Ribbands et al. 2009). There are no records of immature stages of H. axyridis, or other ladybird species, on these islands.
Although thousands of individuals of both f. succinea and f. conspicua have been released in the Azores (Garcia 1986; Schanderl et al. 1992), H. axyridis has not become established there (Evans et al. 2011; Soares et al. 2008). It has been hypothesised that prey features related to local plant habitats and landscape structure, together with the ladybird characteristics in terms of body size, might explain why the invader is absent (Hemptinne et al. 2012). The coastal terrestrial habitats that form 9 % of the landscape are the richest in terms of food resources for aphidophagous ladybirds. It is predicted that other large and medium-sized species of ladybirds (Coccinella undecimpunctata L.), which have requirement for high prey consumption, will also decline because of shortage of prey (Borges et al. 2006; Cabral et al. 2006; Sebastião et al. 2015; Soares et al. 2001) for example C. septempunctata is considered extinct from the Azores. The Azorean communities are nowadays dominated by minute Scymnini species and larger species are no longer recorded. Despite the competitive advantage of H. axyridis against the native C. undecimpunctata (Felix and Soares 2004; Nóia et al. 2008), it is apparent that Azorean habitat characteristics and the high feeding rate of H. axyridis are hampering its invasion.
Comparison of traits
There have been a number of studies examining the influence of life-history traits on invasion and H. axyridis is no exception (Comont et al. 2012, 2014a). The traits databases compiled for these studies provide a rare opportunity to explore variation in life-history traits between localities (native and invaded) around the world. Extending and combining traits databases to a global scale will provide intriguing insights. The invasion process and ecological attributes (Table 1) and the compilation of life history traits of H. axyridis (Table 2) represent the start of this process and highlight gaps in understanding. However, qualitative comparisons of the traits of H. axyridis across the invaded range and with the native range reveal patterns that are worthy of further investigation.
Habitat
The habitat of H. axyridis is wide and although poorly documented in many regions the exploitative and opportunistic nature of this species is evident (Table 1). During early stages of invasion it has been noted that H. axyridis is more prevalent within urban and agricultural landscapes than in semi-natural landscapes (Brown et al. 2008b; Grez et al. 2014a, b). Harmonia axyridis is common within gardens and parks throughout the year and its presence in agricultural fields, orchards or vineyards has been documented globally and local damage due to quality loss of fruits is known (Koch et al. 2004a). Across Europe and Japan H. axyridis is commonly associated with trees and shrubs. For example in urban areas in Europe it is the most abundant ladybird on lime (Tilia spp.) and maple trees (Acer spp.) but is also frequently found on Scots pine, Pinus sylvestris L. (Pinaceae). The habitats of H. axyridis in Japan are disturbed areas such as agricultural fields, orchards, parks, residential yards and gardens (Osawa 2011) and H. axyridis is generally uncommon in natural forests (Osawa 2011).
In winter H. axyridis has a propensity to aggregate in buildings. Across Europe adults usually start aggregating in October and leave overwintering sites in April. The aggregating behaviour has been shown to depend on two blends of long chain hydrocarbon molecules, one leading conspecifics towards aggregation sites and the other ensuring cohesion of the aggregations (Durieux et al. 2012). These findings, with the identification of a volatile sex pheromone in female H. axyridis (Verheggen et al. 2007), might offer some potential in the development of specific control methods for H. axyridis.
It is critical that we have a better understanding of the habitat preference and suitability of H. axyridis; this is especially relevant when facing current global environmental change, especially urbanization, agricultural intensification and climate change which will undoubtedly affect the spread and distribution of H. axyridis. Habitat suitability could be estimated using Species Distribution Models based on fine-scale records on the presence/absence or densities of H. axyridis populations. Such results would have wide relevance to invasion biology.
Diet breadth
The wide diet breadth of H. axyridis is evident from Table 1 and, as already stated, includes pest and non-pest insects but also fruits. Perhaps of most interest and relevance to understanding the threat posed by this species to biodiversity, is the range of non-pest insects consumed and associated population-level effects. Much research has focussed on interactions between H. axyridis and other coccinellids (Pell et al. 2008) but there is a need for further work on other taxonomic groups for example expanding the research on butterflies such as the studies on the monarch butterfly Danaus plexippus (L.) (Koch et al. 2003, 2006a). Furthermore it is critical that future research considers the population-level effects of H. axyridis on different species and implications for ecosystem function. Current studies have not clearly documented the diet preference, adaptability and feeding efficiency of H. axyridis, which are essential for assessing its potential impact in recipient ecosystems.
Thermal tolerance
Only a few studies, representing populations of H. axyridis from a few regions, have considered thermal tolerance (Table 2; Koch et al. 2004b). Of particular importance would be to measure lower and upper temperature tolerance of several life stages using ecologically relevant conditions for the population examined. Indeed to build Species Distribution Models, thermal tolerance needs to be carefully measured in the laboratory so that the reaction norm of physiological/behavioural performance as a function of ambient temperature can be established. However, H. axyridis usually overwinters in shelters and aggregations and cold tolerance measurements should be interpreted in regards to ecologically relevant conditions. Indeed H. axyridis can be found at a range of altitudes: in Chile individuals have been found at >3000 m a.s.l. while in central Europe it is regularly present from the eastern lowlands (114 m a.s.l.) to montane/subalpine forests (approx. 1600 m a.s.l., unpubl. data) in the Alps, with one isolated record of a probable wind-drifted individual at 2280 m a.s.l. in Carinthia.
Although laboratory studies show that invasive populations of H. axyridis do not survive at 34 °C (Benelli et al. 2015), the species has been documented in Kenya (Nedvěd et al. 2011) and Tanzania (Nedvěd and Háva 2016) but there is no evidence that this species is abundant in the tropics. In addition, the wide spread and occurrence of H. axyridis in mesic cool climates or pockets of urban gardens within drier environments in its novel range suggests that climatic factors other than temperature also play a role in shaping its distribution.
Desiccation resistance (Nedved and Kalushkov 2011) and the potential cross-effects of temperature and humidity on temperature limits may provide useful information for modelling its future distribution in face of climate change (Hoffmann et al. 2013). Perhaps more importantly, the study of the plasticity and evolutionary adaptation of tolerance limits in H. axyridis using both native and invasive populations would provide insights into its adaptive capacity to future climatic challenges. The effect of different climate regimes on the body weight and fat body content of H. axyridis and C. septempunctata has been the focus of laboratory studies in Germany (Krengel et al. 2012). This study concluded that that C. septempunctata has life history adaptations that would confer an advantage over H. axyridis at elevated temperatures.
Reproductive potential
A number of traits (voltinism, fecundity and egg hatching success) reveal the high reproductive potential of H. axyridis (Table 2). In the native range, H. axyridis is generally considered to have two generations per year, although it can have three and occasionally up to eight generations in some years and localities (Osawa 2011). In Kyoto, in the centre of Japan, the overwintering adults mate and lay eggs in spring (Osawa 2000). The adults of the first generation emerge in mid May to June (Osawa 2000). In mid summer, the beetles aestivate in small groups in leaf-shelters on trees and the behaviour is regarded to be an adaptation to high temperature (Toda and Sakuratani 2006). In autumn, H. axyridis adults fly towards overwintering sites, white or pale objects on hilltops or valleys, where they aggregate from early November (Obata 1986).
Within much of the invaded range, H. axyridis only achieves two generations per year, whereas in the native range up to eight generations have been observed. Increases in global temperatures could facilitate an increase in voltinism, and corresponding increase in abundance across the invaded range in the future. On the contrary, increase in temperatures may limit its reproduction due to the high egg mortality observed at temperatures above 30 °C (Table 2).
We suggest that the three traits related to population demography and thus invasiveness, namely voltinism, fecundity and hatching success, need to be recorded in a standardised unit so that population dynamics can be estimated from matrix population models. Furthermore, the trade-offs between, and rates of adaptation in, life-history traits are of intrinsic theoretical value (e.g. the trade-off between these three demographic traits among populations in different environments) and colour polymorphism of H. axyridis (Table 2) is also an interesting model system in population genetics. Overall, we suggest that the strength of biotic interactions centralised around H. axyridis (i.e. diet preference and foraging efficiency) and the three demographic traits are key variables for monitoring/assessing its performance and impact in invaded ecosystems.
The reproduction success of individual females has been measured in variable ways in H. axyridis according to regions (Table 2). Usually reproduction is measured as lifetime fecundity (total number of eggs laid by female), but this requires very long breeding and observation, since many females of H. axyridis live and reproduce for over 4 months (Awad et al. 2013). Alternatively, fecundity during the first month of reproduction (beginning by the day of the first egg laying, not including pre-oviposition period) is used. Both measures require feeding the females ad libitum with a suitable prey. Widely used types of prey that may become a standard are the pea aphid Acyrthosiphon pisum (Harris) (Hemiptera: Aphididae) and frozen eggs of the flour moth Ephestia kuehniella (Zeller) (Berkvens et al. 2010a; Kögel et al. 2012). Other measures of reproductive performance could include daily fecundity (after a standard time period for example 2 weeks after the start of egg laying) and cluster size (again after a standard time period and perhaps an average over 2 weeks).
Fertility is defined as the product of fecundity and hatching rate. Hatching rate (percentage of eggs that hatch to the first larval instar) should be calculated over the first month of reproduction, since it declines strongly later (Awad et al. 2013). Hatching rate in H. axyridis in different countries varied from 15 % in Belgium (Berkvens et al. 2008) to 100 % in Italy (unpublished data), although it has not been measured in most countries (Table 2). Hatching success may depend on many factors for example food source, colour morph, endosymbionts and multiple fertilisation. Thus permanent presence of one or more males with each female is recommended.
Colour forms
More than 200 colour patterns of H. axyridis elytra have been observed in the native range (China), but most of them can be categorized into four major colour forms (Du and Chen 2010; Tang et al. 2012). Controlled crossing experiments have shown that the majority of color patterns are controlled by 15 alleles at one locus with multiple alleles (Komai 1956). However, all but four of these alleles are rare in natural populations, with a combined frequency of less than one per cent. The four major alleles are succinea, axyridis, conspicua, spectabilis, all but the first of these being melanic forms (Michie et al. 2010): (1) yellow to red background colour of elytra with the number of black dots ranging from 0 to 19 (f. succinea); (2) black background colour of elytra with many orange-red dots (of varying size and position; f. axyridis); (3) black background of elytra with one large orange-red dot in the top-centre of each elytron (f. conspicua); (4) black background colour of elytra with two orange-red dots on each elytron and the top one larger than the bottom one (f. spectabilis). The impressive diversity of colour patterns documented in China has been attributed to the diverse environmental conditions in the region as well as to complex interaction between rare allelic forms (Tan 1946). A hierarchical dominance with respect to phenotype expression was demonstrated between the four major colour alleles: conspicua > spectabilis > axyridis > succinea (Michie et al. 2010; Tan and Li 1934; Tan 1946). More specifically, the expression of the colour pattern in the heterozygous individuals (i.e. individuals bearing two different allelic forms) conforms to the rule of mosaic dominance heredity, which states that any portion of the elytra which has black pigment in the homozygote for a given allele will have black pigment also in the heterozygotes in which that allele is present (Tan 1946). Interestingly, Michie et al. (2010) reported that the non-melanic morph succinea dramatically increases its degree of melanization at cold temperatures, and that there is genetic variation in reaction norms, with different families responding to temperature in different ways. The exact genomic architecture and gene content of the multiallelic locus controlling color morph variation and phenotypic plasticity of the non-melanic morph remains unknown.
In some of the earliest research in population genetics, the frequencies of these alleles were found to vary hugely across the native range of H. axyridis (Dobzhansky 1933). The geographical variation appeared to be linked to climate, with the non-melanic form succinea being found most often in hot, arid regions and melanic forms being more frequent in cooler, more humid ones (Dobzhansky 1933). This might be considered as a consequence of climatic adaptation. However, a different pattern was found in Japan, where succinea decreases in frequency from northeast to south-west without any significant correlation to temperature or other climatic factors (Komai 1956). Moreover, in north China f. succinea dominates, whereas in south China the melanic colour forms dominate (Du and Chen 2010; Tang et al. 2012). In addition to this geographic variation, small to large seasonal changes in allele frequencies have been reported in some native populations (Jiang et al. 2007; Osawa and Nishida 1992; Tang et al. 2012). For example, in Beijing over half the population is melanic in the spring, but this drops to less than one-fifth by the autumn. Therefore, the melanic individuals presumably have a large fitness advantage in the winter and a disadvantage in the summer. This has been attributed to the effects of thermal melanism, with melanism being costly in summer and beneficial in winter, possibly mediated by mate choice (Wang et al. 2009).
The range of colour forms is limited within the invaded range. Indeed in most invaded regions only the non-melanic morph f. succinea is present, except in Europe where the melanic morphs f. conspicua and spectabilis are also present (and rarely f. axyridis), probably due to the genetic admixture with European biological control strains which occurred in this region (Lombaert et al. 2010, 2014). So far, it is not possible to say whether the predominance of f. succinea observed in the invaded range is due to historical and/or demographical contingencies (i.e. simple random effects) or to any selective process that would have favoured the establishment and the spatial spreading of f. succinea, for example the phenotypic plasticity of this morph (Michie et al. 2010). Geographical and seasonal variation of melanic and non-melanic morphs remains rather sparsely studied in Europe (Purse et al. 2014). In Belgium, the initial point of introduction of the species, the most commonly encountered morph is f. succinea, and the proportion of melanics (f. spectabilis, f. conspicua) is about 25 % (Adriaens et al. 2008). In this particular region of Europe, strong cold hardiness and differences in several fitness parameters between melanics and non-melanics from field populations have been demonstrated (Berkvens et al. 2008, 2010a).
Natural enemies
Many regional reports have been published about one or several natural enemies of H. axyridis both within the native and invaded range but much could be revealed from a systematic approach comparing the native and invaded range (Table 1). There are a number of theories relating to IAS and their interactions with natural enemies but perhaps the most widely known is the Enemy Release Hypothesis (ERH; Jeffries and Lawton 1984; Roy et al. 2011b; Roy and Lawson Handley 2012). The ERH predicts that an alien species introduced to a new region will increase in distribution and abundance because of reduced impacts from natural enemies. Adopting a ‘biogeographical’ (compare richness and impacts of enemies in native and introduced populations of an alien host) or ‘community’ (compare native and alien species occurring within the same community) approach for H. axyridis would be informative (Colautti et al. 2004). With both ‘biogeographical’ and ‘community’ approaches it is essential to consider how the loss of enemy diversity translates into population regulation. A small number of enemies may have large effects and so functional diversity of enemies may be a better predictor of impacts upon hosts than overall diversity. There is considerable scope for global collaboration in exploring H. axyridis within the context of ERH.
Generalist predators do attack ladybirds, for example sparrows have been confirmed as predators of H. axyridis in Slovakia (Veselý et al. in press), but parasites are the dominant natural enemies of ladybirds. Ladybirds are attacked by over 100 species of hymenopteran and dipteran parasitoids and several fungi (Ceryngier et al. 2012; Herz and Kleespies 2012; Riddick et al. 2009). Of these, the majority of research globally has focused on the parasitoid Dinocampus coccinellae (Schrank; Hymenoptera: Braconidae), which attacks both native and alien ladybirds (Ceryngier et al. 2012). Dinocampus coccinellae is a parasitoid of ladybirds that has a global distribution (Ceryngier et al. 2012) but currently the influence of alien ladybirds on the abundance and distribution of this parasitoid is unknown. Ladybirds vary in their quality as a host (Comont et al. 2014b; Koyama and Majerus 2008), but D. coccinellae does not discriminate between suitable and unsuitable ladybirds and will attack individuals differentially based on colour and movement (Cartwright et al. 1982; Obrycki et al. 1998; Richardson and Deloach 1972). Thus, the presence of unsuitable alien ladybird hosts such as H. axyridis may function as an ecological trap for the parasitoid, with positive effects on native ladybirds, or alternatively increase the abundance of the parasitoid, with adverse consequences for native ladybirds. It is apparent that H. axyridis is less susceptible to D. coccinellae than are other ladybirds (Berkvens et al. 2010b; Comont et al. 2014b) but it seems to be the most abundant and frequent H. axyridis parasitoid in many countries (Table 1; Francati 2015). Ongoing work is exploring the differences in immune response between H. axyridis and other ladybirds (Murray et al. 2015).
A semi-field study from the UK confirmed low rates of parasitism of H. axyridis by parasitoids, particularly in comparison to the native C. septempunctata (Comont et al. 2014b). Pupae of H. axyridis were parasitized, primarily by Phalacrotophora fasciata (Fuller) and Phalacrotophora berolinensis Schmitz (Diptera: Phoridae), at an exceptionally low level (1.73 %) and adults were not found to be parasitized at all in this study; parasitism of the co-occurring C. septempunctata was high (20.91 % pupae, 5.67 % adults). Surveys in the Netherlands from 2003 revealed a number of natural enemies (H. virescens, Parasitylenchus bifurcatus Poinar & Steenberg (Nematoda: Allantonematidae), Coccipolipus hippodamiae and Dinocampus coccinellae) attacking H. axyridis but only from 2008 onwards (Raak-van den Berg et al. 2014; Table 1). The suggestion is made that after a time lag of 6 years these natural enemies are starting to use H. axyridis as a novel host, following host shift from native ladybirds and adaptation to H. axyridis.
Recent research has demonstrated that H. axyridis individuals contain high numbers of obligate parasitic microsporidia (Vilcinskas et al. 2013). It appears that these microsporidia do not adversely affect H. axyridis but cause high mortality when artificially injected into C. septempunctata (Vilcinskas et al. 2013). Artificial injection, as used within this study to transmit the microsporidia from H. axyridis to C. septempunctata, is far removed from the natural mechanisms involved in microsporidia transmission (Solter et al. 2013), so further studies are required to explore the ecological relevance. Indeed further research is needed to understand how the presence of all established alien ladybirds influence parasitism and pathogen infection of declining native ladybirds.
The fungal pathogen Beauveria bassiana (Bals.-Criv.) Vuill. (Ascomycota: Cordycipitaceae) commonly infects native species of ladybird (such as C. septempunctata), but again, H. axyridis seems highly resistant (Roy et al. 2008b). However, in Denmark several entomopathogenic fungi were isolated from overwintering and early season H. axyridis (larvae, pupae, adults) including B. bassiana, Isaria farinosa (Holmsk.) Fr. (Ascomycota: Cordycipitaceae), Lecanicillium lecanii (Zimm.) Zare & W. Gams (Ascomycota: Cordycipitaceae), and L. muscarium (Petch) Zare & W. Gams (Steenberg and Harding 2009a; Table 1). Similarly, mid-summer surveys in Denmark of all H. axyridis life stages revealed infections from the same fungal assemblage with I. farinosa most prevalent, followed by Lecanicillium spp. (Howe, Ravn, Jensen, Meyling, unpublished data).
Additional work has assessed the sexually-transmitted ectoparasitic mite Coccipolipus hippodamiae (McDaniel & Morrill) (Acari: Podapolipidae) as a biological control candidate against H. axyridis (Roy et al. 2011c). This mite, which occurs naturally in Europe and North America (Table 1), causes sterility in female H. axyridis, but some native ladybird species are also susceptible to the mite, and thus it does not represent a suitable control strategy for H. axyridis (Rhule et al. 2010).
The obligate ectoparasitic fungus Hesperomyces virescens Thaxt. (Ascomycota: Laboulbeniaceae) has been capturing the imagination of a number of scientists working on H. axyridis. This species has historically received very little attention and so is of particular note. While most Laboulbeniales exhibit a high degree of host specificity, H. virescens has been reported from over 30 ladybird species in 15 genera (Bernardi et al. 2014; Ceryngier et al. 2012; Ceryngier and Twardowska 2013; Haelewaters et al. 2016). Transmission of H. virescens occurs mainly during sexual contact, as exemplified by the non-random distribution of thalli on the body of males and females (Riddick et al. 2009; Welch et al. 2001); infection can be considered as a sexually transmitted disease (Welch et al. 2001). In H. axyridis, however, H. virescens is also socially transmitted; in overwintering aggregations, transmission of H. virescens through direct physical contact is the most important mechanism of spread (Nalepa and Weir 2007; Riddick 2006). Infection is caused by grooming, resulting in high thallus densities on older hosts (Haelewaters et al. 2012). Hesperomyces virescens was reported for the first time on H. axyridis in Ohio in summer 2002 (Garcés and Williams 2004). Harmonia axyridis is multivoltine, promiscuous, and overwinters in aggregations, all of which contribute to the rapid spread of H. virescens and higher infection prevalence on this host, compared to other ladybird hosts (De Kesel 2011). Interestingly, the parasite prevalence of H. virescens on H. axyridis varies between locations and between years (Haelewaters et al. 2012; Raak-van den Berg et al. 2014). In Belgium, for example, an increase from 0.5 to 96.5 % of parasite prevalence was noted after only 4 years (De Kesel 2011). Currently, H. virescens infection of H. axyridis is widespread in Western Europe, the eastern United States and to a lesser extent in South Africa (Haelewaters et al. 2016). Hesperomyces virescens has also been reported on H. axyridis in its native range with one record from China (Haelewaters et al. 2014). Ongoing work is assessing the influence of (dual) fungal infections on H. axyridis and Olla v-nigrum (Mulsant), a North American native ladybird species (Haelewaters et al. 2015a).
There is clearly much to uncover about the interactions between H. axyridis and natural enemies; global collaborations will provide unique opportunities for exploring these on biogeographic scales.
Conclusions and future directions
Harmonia axyridis has inspired global collaborations and has also been the impetus for understanding biological invasions within and between countries. Many countries have documented the distribution and noted the rapid spread of H. axyridis following establishment. Further research is required to improve our understanding of the factors involved in determining the global patterns of invasion by H. axyridis which will have wide relevance for invasion biology. Many countries have engaged members of the public in monitoring the distribution of H. axyridis and the lessons learnt from such initiatives have been shared and proved informative for developing approaches to citizen science (Gardiner et al. 2012; Pocock et al. 2015; Roy et al. 2012a) and inspiring new projects both within and between countries. Indeed the role of volunteers in monitoring IAS is recognised (Roy et al. 2015b) and there is considerable scope to share resources and technology (August et al. 2015) to increase involvement in monitoring ladybirds and other IAS around the world.
The interactions between H. axyridis and other species have fascinated ecologists both in the native and invaded ranges of this species. There has been considerable focus on the potential impacts of H. axyridis on biodiversity, particularly intra-guild interactions and specifically IGP. Competitive interactions have received less attention but are worthy of future research emphasis. There is still much to unravel about the interactions between H. axyridis and its natural enemies. Comparisons between the native and invaded range will be particularly fruitful. Molecular studies have provided insights into genetic aspects of invasion and there is certainly more that can be revealed from detailed studies at a global scale. It is exciting to consider the potential of ongoing research to sequence the genome of H. axyridis and consider the opportunities that this research might present for future studies (Chown et al. 2015; http://www.agence-nationale-recherche.fr/?Project=ANR-13-EBID-0001).
Many of the studies examining the interactions between H. axyridis and other species have involved mesocosm experiments under laboratory conditions. There is a need to increase the scale of such studies to consider the negative and positive ecosystem-level impacts of H. axyridis. Harmonia axyridis contributes to pest control services in a number of crop systems but there is a lack of evidence in relation to ecosystem function and resilience of invaded systems. New molecular methods provide opportunities for detailed studies on the interactions between H. axyridis and the diverse range of species with which it interacts. Ecological network analysis represents an appealing and exciting way to explore these complex communities (Roy and Lawson Handley 2012). The coupling of citizen science approaches with global collaborations between researchers will provide the scale of information required to address some of the complex ecological questions that remain unanswered.
References
Adriaens T, Branquart E, Maes D (2003) The multicoloured Asian ladybird Harmonia axyridis Pallas (Coleoptera: Coccinellidae), a threat for native aphid predators in Belgium? Belg J Zool 133:195–196
Adriaens T, Gomez GMY, Maes D (2008) Invasion history, habitat preferences and phenology of the invasive ladybird Harmonia axyridis in Belgium. Biocontrol 53:69–88
Adriaens T, San Martin y Gomez G, Bogaert J et al (2015) Testing the applicability of regional IUCN Red List criteria on ladybirds (Coleoptera, Coccinellidae) in Flanders (north Belgium): opportunities for conservation. Insect Conserv Divers 8:404–417
Aebi A, Brown PMJ, De Clercq P et al (2011) Detecting arthropod intraguild predation in the field. Biocontrol 56:429–440
Alyokhin A, Sewell G (2004) Changes in a lady beetle community following the establishment of three alien species. Biol Invasions 6:463–471
Amat-García G, Amat-García E, Ariza-Marín E (2011) Insectos invasores en los tiempos de cambio climático. Innovación y Ciencia 18:45–53
August T, Harvey M, Lightfoot P et al (2015) Emerging technologies for biological recording. Biol J Linn Soc 115:731–749
Awad M, Kalushkov P, Nedvědová T et al (2013) Fecundity and fertility of ladybird beetle Harmonia axyridis after prolonged cold storage. Biocontrol 58:657–666
Bahlai C, Sears M (2009) Population dynamics of Harmonia axyridis and Aphis glycines in Niagara Peninsula soybean fields and vineyards. J Entomol Soc Ont 140:27–39
Bahlai CA, Colunga-Garcia M, Gage SH et al (2014) The role of exotic ladybeetles in the decline of native ladybeetle populations: evidence from long-term monitoring. Biol Invasions 17:1005–1024
Bahlai CA, vander Werf W, O’Neal M et al (2015) Shifts in dynamic regime of an invasive lady beetle are linked to the invasion and insecticidal management of its prey. Ecol Appl
Barahona-Segovia R, Grez AA, Bozinovic F (2015) Testing the hypothesis of greater eurythermality in invasive than in native ladybird species: from physiological performance to life-history strategies. Ecol Entomol. doi:10.1111/een.12287
Bazzocchi GG, Lanzoni A, Accinelli G et al (2004) Overwintering, phenology and fecundity of Harmonia axyridis in comparison with native coccinellid species in Italy. Biocontrol 49:245–260
Belyakova NA, Reznik SY (2013) First record of the harlequin ladybird, Harmonia axyridis (Coleoptera: Coccinellidae) in the Caucasus. Eur J Entomol 110:699–702
Benelli M, Simon R, Francati S et al (2015) Effect of two temperatures on biological traits and susceptibility to a pyrethroid insecticide in an exotic and native coccinellid species. Bull Insectol 68:23–29
Berkvens N, Bonte J, Berkvens D et al (2008) Influence of diet and photoperiod on development and reproduction of European populations of Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae). Biocontrol 53:211–221
Berkvens N, Landuyt C, Deforce K et al (2010a) Alternative foods for the multicoloured Asian lady beetle Harmonia axyridis (Coleoptera: Coccinellidae). Eur J Entomol 107:189–195
Berkvens N, Moens J, Berkvens D et al (2010b) Dinocampus coccinellae as a parasitoid of the invasive ladybird Harmonia axyridis in Europe. Biol Control 53:92–99
Bernardi M, Barragán A, Rossi W (2014) New records of Laboulbeniales (Fungi: Ascomycota) from Ecuador and other countries. Webbia 69:281–289
Blekhman A (2008) Population variation of elytral ridge occurrence in ladybirds Harmonia axyridis Pallas. Russ J Genet 44:1351–1354
Borges I, Soares A, Hemptinne JL (2006) Abundance and spatial distribution of aphids and scales select for different life histories in their ladybird beetle predators. J Appl Entomol 130:461–464
Botezatu AI, Kotseridis Y, Inglis D et al (2013) Occurrence and contribution of alkyl methoxypyrazines in wine tainted by Harmonia axyridis and Coccinella septempunctata. J Sci Food Agric 93:803–810
Brakefield PM (1985) Differential winter mortality and seasonal selection in the polymorphic ladybird Adalia bipunctata (L) in the Netherlands. Biol J Linn Soc 24:189–206
Brown MW, Miller SS (1998) Coccinellidae (Coleoptera) in apple orchards of eastern West Virginia and the impact of invasion by Harmonia axyridis. Entomol News 109:143–151
Brown PMJ, Roy HE (2015) Reflections on the long-term assessment of ladybird (Coleoptera: Coccinellidae) populations in the Czech Republic and the United Kingdom. Soc Zool Bohem 79:19–27
Brown PMJ, Adriaens T, Bathon H et al (2008a) Harmonia axyridis in Europe: spread and distribution of a non-native coccinellid. Biocontrol 53:5–21
Brown PMJ, Roy HE, Rothery P et al (2008b) Harmonia axyridis in Great Britain: analysis of the spread and distribution of a non-native coccinellid. Biocontrol 53:55–67
Brown PMJ, Frost R, Doberski J et al (2011a) Decline in native ladybirds in response to the arrival of Harmonia axyridis: early evidence from England. Ecol Entomol 36:231–240
Brown PMJ, Thomas CE, Lombaert E et al (2011b) The global spread of Harmonia axyridis (Coleoptera: Coccinellidae): distribution, dispersal and routes of invasion. Biocontrol 56:623–641
Brown PMJ, Ingels B, Wheatley A et al (2015) Intraguild predation by Harmonia axyridis (Coleoptera: Coccinellidae) on native insects in Europe: molecular detection from field samples. Entomol Sci 18:130–133
Bukejs A, Telnov D (2015) The first record of the invasive lady beetle Harmonia axyridis (Pallas, 1773)(Coleoptera: Coccinellidae) in Turkey. Zool Ecol 25:59–62
Burgio G, Lanzoni A, Accinelli G et al (2008a) Estimation of mortality by entomophages on exotic Harmonia axyridis versus native Adalia bipunctata in semi-field conditions in northern Italy. Biocontrol 53:277–287
Burgio G, Santi F, Lanzoni A et al (2008b) Harmonia axyridis recordings in northern Italy. Bull Insectol 61:361–364
Cabral S, Soares AO, Moura R et al (2006) Suitability of Aphis fabae, Myzus persicae (Homoptera : Aphididae) and Aleyrodes proletella (Homoptera: Aleyrodidae) as prey for Coccinella undecimpunctata (Coleoptera: Coccinellidae). Biol Control 39:434–440
Caltagirone L, Doutt R (1989) The history of the vedalia beetle importation to California and its impact on the development of biological control. Annu Rev Entomol 34:1–16
Cartwright B, Eikenbary R, Angalet G (1982) Parasitism by Perilitus coccinellae [Hym.: Braconidae] of indigenous coccinellid hosts and the introduced Coccinella septempunctata [Col.: Coccinellidae], with notes on winter mortality. Entomophaga 27:237–243
Ceryngier P, Twardowska K (2013) Harmonia axyridis (Coleoptera: Coccinellidae) as a host of the parasitic fungus Hesperomyces virescens (Ascomycota: Laboulbeniales, Laboulbeniaceae): a case report and short review. Eur J Entomol 110:549–557
Ceryngier P, Roy HE, Poland RL (2012) Natural enemies of ladybird beetles. In: Hodek I, van Emden HF, Honek A (eds) Ecology and behaviour of the ladybird beetles (Coccinellidae). Wiley, London
Chacón J, Landis D, Heimpel G (2008) Potential for biotic interference of a classical biological control agent of the soybean aphid. Biol Control 46:216–225
Chapin JB, Brou VA (1991) Harmonia axyridis (Pallas), the 3rd species of the genus to be found in the United States (Coleoptera, Coccinellidae). Proc Entomol Soc Wash 93:630–635
Chown SL, Hodgins KA, Griffin PC et al (2015) Biological invasions, climate change and genomics. Evol Appl 8:23–46
Coderre D, Lucas É, Gagné I (1995) The occurrence of Harmonia axyridis (Pallas)(Coleoptera: Coccinellidae) in Canada. Can Entomol 127:609–611
Colautti RI, Ricciardi A, Grigorovich IA et al (2004) Is invasion success explained by the enemy release hypothesis? Ecol Lett 7:721–733
Colunga-Garcia M, Gage SH (1998) Arrival, establishment, and habitat use of the multicolored Asian lady beetle (Coleoptera: Coccinellidae) in a Michigan landscape. Environ Entomol 27:1574–1580
Comont RF, Roy HE, Lewis OT et al (2012) Using biological traits to explain ladybird distribution patterns. J Biogeogr 39:1772–1781
Comont R, Roy H, Harrington R et al (2014a) Ecological correlates of local extinction and colonisation in the British ladybird beetles (Coleoptera: Coccinellidae). Biol Invasions 16:1805–1817
Comont RF, Purse BV, Phillips W et al (2014b) Escape from parasitism by the invasive alien ladybird, Harmonia axyridis. Insect Conserv Divers 7:334–342
Cornacchia P, Nardi G (2012) Nuovi dati su Harmonia axyridis in Italia (Coleoptera, Coccinellidae). Boll Assoc Rom Entomol 67:51–68
Costamagna AC, Landis DA (2007) Quantifying predation on soybean aphid through direct field observations. Biol Control 42:16–24
Costamagna AC, Landis DA, Brewer MJ (2008) The role of natural enemy guilds in Aphis glycines suppression. Biol Control 45:368–379
Cottrell TE (2004) Suitability of exotic and native lady beetle eggs (Coleoptera: Coccinellidae) for development of lady beetle larvae. Biol Control 31:362–371
Coutanceau J-P (2006a) Harmonia axyridis (Pallas, 1773): une coccinelle asiatique introduite, acclimatée et en extension en France. Bulletin de la Société entomologique de France 111:395–401
Coutanceau JP (2006b) Harmonia axyridis (Pallas, 1773): une coccinelle asiatique introduite, acclimatée et en extension en Fr. Bulletin de la Societe Entomologique de France 111:395–401
Cuppen J, Heijerman T, van Wielink P et al (2004) Het lieveheersbeestje Harmonia axyridis in Nederland: Een aanwinst voor onze fauna of een ongewenste indringer (Coleoptera: Coccinellidae)? Nederlandse Faunistische Mededelingen 20:1–12
Davidson LN, Evans EW (2010) Frass analysis of diets of aphidophagous lady beetles (Coleoptera: Coccinellidae) in Utah alfalfa fields. Environ Entomol 39:576–582
De Clercq P, Bale JS (2011) Risks of invertebrate biological control agents—Harmonia axyridis as a case study. Regulation of biological control agents. Springer, New York, pp 243–255
De Kesel A (2011) Hesperomyces (Laboulbeniales) and coccinellid hosts. Sterbeeckia 30:32–37
Department of Environmental Affairs South Africa (2014a) National Environmental Management: Biodiversity Act 2004 (Act No. 10 of 2004). Alien and Invasive Species Lists, 2014. Gov Gaz 37886:3–74
Department of Environmental Affairs South Africa (2014b) National Environmental Management: Biodiversity Act 2004 (Act No. 10 of 2004). Alien and Invasive Species Regulations, 2014. Gov Gaz 37885:3–27
Dobzhansky T (1924) Die geographische und individuelle Variabilität von Harmonia axyridis Pall. in ihren Wechselbeziehungen. Biologisches Zentralblatt 44:401–421
Dobzhansky T (1933) Geographical variation in ladybeetles. Am Nat 67:97–126
Dreistadt S, Hagen K, Bezark L (1995) Harmonia axyridis (Pallas)(Coleoptera: Coccinellidae), first western United States record for this Asiatic lady beetle. Pan-Pac Entomol 71:135–136
Du W, Chen I (2010) Investigations of the color variations of Harmonia axyridis (Pallas) in Changchun. Chin Agric Sci Bull 4:057
Dudek K, Dudek M, Tryjanowski P (2015) Wind turbines as overwintering sites attractive to an invasive lady beetle, Harmonia axyridis Pallas (Coleoptera: Coccinellidae). Coleopt Bull 69:1–5
Durieux D, Fischer C, Brostaux Y et al (2012) Role of long-chain hydrocarbons in the aggregation behaviour of Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae). J Insect Physiol 58:801–807
El-Arnaouty S, Beyssat-Arnaouty V, Ferran A et al (2000) Introduction and release of the coccinellid Harmonia axyridis Pallas for controlling Aphis craccivora Koch on faba beans in Egypt. Egypt J Biol Pest Control 10:129–136
Eschen R, Babendreier D, Nauer S et al (2007) Surveys for ladybirds (Coleoptera: Coccinellidae) in Switzerland and confirmation of the presence of the invasive alien ladybird species, Harmonia axyridis (Pallas). Mitteilungen der Schweizerischen Entomologischen Gesellschaft 80:7–14
Evans EW (2004) Habitat displacement of North American ladybirds by an introduced species. Ecology 85:637–647
Evans EW, Soares AO, Yasuda H (2011) Invasions by ladybugs, ladybirds, and other predatory beetles. Biocontrol 56:597–611
Facon B, Hufbauer RA, Tayeh A et al (2011) Inbreeding depression is purged in the invasive insect Harmonia axyridis. Curr Biol 21:424–427
Felix S, Soares AO (2004) Intraguild predation between the aphidophagous ladybird beetles Harmonia axyridis and Coccinella undecimpunctata (Coleoptera: Coccinellidae): the role of body weight. Eur J Entomol 101:237–242
Ferran A, Giuge L, Brun J et al (1997) Coccinelle Harmonia axyridis Pallas: mise au point sur son introduction et son utilisation en lutte biologique. Adalia 36:21–24
Foley IA, Ivie MA, Denke PM (2009) The first state record for the multicolored Asian lady beetle, Harmonia axyridis (Pallas)(Coleoptera: Coccinellidae), from Montana. Coleopt Bull 63:351–352
Fothergill K, Moore W, Losey J et al (2010) First Arizona records of the multicolored Asian lady beetle, Harmonia axyridis (Pallas)(Coleoptera: Coccinellidae). Coleopt Bull 64:51–52
Francati S (2015) Native preimaginal parasitism of Harmonia axyridis: new record of association with Phalacotrophora fasciata in Italy. Bull Insectol 68:3–6
Gagnon AÈ, Doyon J, Heimpel G et al (2011) Prey DNA detection success following digestion by intraguild predators: influence of prey and predator species. Mol Ecol Resour 11:1022–1032
Galvan TL, Burkness EC, Hutchison WD (2006) Influence of berry injury on infestations of the multicolored Asian lady beetle in wine grapes. Plant Health Prog
Galvan TL, Koch RL, Hutchison WD (2008) Impact of fruit feeding on overwintering survival of the multicolored Asian lady beetle, and the ability of this insect and paper wasps to injure wine grape berries. Entomol Exp Appl 128:429–436
Garcés S, Williams R (2004) First record of Hesperomyces virescens Thaxter (Laboulbeniales: Ascomycetes) on Harmonia axyridis (Pallas)(Coleoptera: Coccinellidae). J Kans Entomol Soc 77:156–158
Garcia V (1986) Approaches to integrated control of some citrus pests in the Azores and Algarve (Portugal). In: Integrated pest control in citrus-groves: proceedings of the experts’ meeting, Acireale (Italy), 26–29 March 1985. AA Balkema
Gardiner M, Landis D (2007) Impact of intraguild predation by adult Harmonia axyridis (Coleoptera: Coccinellidae) on Aphis glycines (Hemiptera: Aphididae) biological control in cage studies. Biol Control 40:386–395
Gardiner M, Landis D, Gratton C et al (2009a) Landscape diversity enhances biological control of an introduced crop pest in the north-central USA. Ecol Appl 19:143–154
Gardiner MM, Landis DA, Gratton C et al (2009b) Landscape composition influences patterns of native and exotic lady beetle abundance. Divers Distrib 15:554–564
Gardiner MM, Allee LL, Brown PM et al (2012) Lessons from lady beetles: accuracy of monitoring data from US and UK citizen-science programs. Front Ecol Environ 10:471–476
Glemser EJ, Dowling L, Inglis D et al (2012) A novel method for controlling multicolored Asian lady beetle (Coleoptera: Coccinellidae) in vineyards. Environ Entomol 41:1169–1176
Goetz DW (2008) Harmonia axyridis ladybug invasion and allergy. Allergy Asthma Proc 29:123–129
Goldarazena A, Calvo D (2007) First record of Harmonia axyridis (Coleoptera: Coccinellidae) from the lberian Peninsula. Boletin Sociedad Entomol Aragonesa 41:437–439
González G (2008) Lista y distribución geográfica de especies de Coccinellidae (Insecta: Coleoptera) presentes en Chile. Boletín del Museo Nacional de Historia Natural (Chile) 57:77–107
González GF, Kondo T (2012) Primer registro de la especie invasora Harmonia axyridis (Pallas)(Coleoptera: Coccinellidae) en Ecuador. Boletín de la Sociedad Entomológica Aragonesa 51:310
Gordon RD (1985) The Coleoptera (Coccinellidae) of America north of Mexico. J NY Entomol Soc 93:1–912
Grez A, Zaviezo T, González G et al (2010) Harmonia axyridis en Chile: una nueva amenaza. Cienc Investig Agrar 37:145–149
Grez AA, Rand TA, Zaviezo T et al (2013) Land use intensification differentially benefits alien over native predators in agricultural landscape mosaics. Divers Distrib 19:749–759
Grez AA, Zaviezo T, Gardiner MM (2014a) Local predator composition and landscape affects biological control of aphids in alfalfa fields. Biol Control 76:1–9
Grez AA, Zaviezo T, Hernández J et al (2014b) The heterogeneity and composition of agricultural landscapes influence native and exotic coccinellids in alfalfa fields. Agric For Entomol 16:382–390
Guo JY, Wan FH (2001) Effect of three diets on development and fecundity of the ladybeetles Harmonia axyridis and Propylaea japonica. Chin J Biol Control 17:116–120
Haelewaters D, van Wielink P, van Zuijlen JW et al (2012) New records of Laboulbeniales (Fungi, Ascomycota) for the Netherlands. Entomol Ber 72:26–33
Haelewaters D, Comont RF, Zhao SY et al (2014) Hesperomyces virescens (Fungi, Ascomycota, Laboulbeniales) attacking Harmonia axyridis (Coleoptera, Coccinellidae) in its native range. Chin Sci Bull 59:528–532
Haelewaters D, Shapiro-Ilan DI, Cottrell TE (2015a) Will dual fungal infections increase Harmonia axyridis mortality in natural populations? In: 3rd meeting of IOBC-WPRS study group “Benefits and risks of exotic biological control agents” 13–15 May 2015, Bornholm, Denmark
Haelewaters D, Zhao SY, Royer IR et al (2015b) Laboulbeniales (Ascomycota) of the Boston Harbor Islands I: species parasitizing Coccinellidae and Staphylinidae. Northeast Nat 22:459–477
Haelewaters D, Minnaar IA, Clusella-Trullas S (2016) First finding of the parasitic fungus Hesperomyces virescens (Laboulbeniales) on native and invasive ladybirds (Coleoptera, Coccinellidae) in South Africa. Parasite 23:5
Harmon JP, Stephens E, Losey J (2007) The decline of native coccinellids (Coleoptera: Coccinellidae) in the United States and Canada. J Insect Conserv 11:85–94
Hautier L, Gregoire JC, de Schauwers J et al (2008) Intraguild predation by Harmonia axyridis on coccinellids revealed by exogenous alkaloid sequestration. Chemoecology 18:191–196
Hautier L, San Martin G, Callier P et al (2011) Alkaloids provide evidence of intraguild predation on native coccinellids by Harmonia axyridis in the field. Biol Invasions 13:1805–1814
Heimpel GE, Frelich LE, Landis DA et al (2010) European buckthorn and Asian soybean aphid as components of an extensive invasional meltdown in North America. Biol Invasions 12:2913–2931
Hemptinne J-L, Magro A, Evans E et al (2012) Body size and the rate of spread of invasive ladybird beetles in North America. Biol Invasions 14:595–605
Herz A, Kleespies RG (2012) Occurrence of natural enemies in different populations of the invasive ladybird Harmonia axyridis (Pallas, 1771) (Coleoptera, Coccinellidae) in Germany. Mitt Dtsch Ges Allg Angew Entomol 18:201–206
Hesler LS (2014) Inventory and assessment of foliar natural enemies of the soybean aphid (Hemiptera: Aphididae) in South Dakota. Environ Entomol 43:577–588
Hesler LS, Kieckhefer RW (2008) Status of exotic and previously common native coccinellids (Coleoptera) in South Dakota landscapes. J Kans Entomol Soc 81:29–49
Hesler L, Kieckhefer R, Beck D (2001) First record of Harmonia axyridis (Coleoptera: Coccinellidae) in South Dakota and notes on its activity there and in Minnesota. Entomol News 112:264–270
Hicks B, Majka CG, Moores SP (2010) Harmonia axyridis (Pallas)(Coleoptera: Coccinellidae) found in Newfoundland. Coleopt Bull 64:50
Hodek I, van Emden HF, Honek A (2012) Ecology and behaviour of the ladybird beetles (Coccinellidae). Wiley-Blackwell, Chichester
Hoffmann AA, Chown SL, Clusella-Trullas S (2013) Upper thermal limits in terrestrial ectotherms: how constrained are they? Funct Ecol 27:934–949
Honek A, Martinkova Z, Kindlmann P et al (2014) Long-term trends in the composition of aphidophagous coccinellid communities in Central Europe. Insect Conserv Divers 7:55–63
Honek A, Martinkova Z, Dixon AFG et al. (2016) Long-term changes in communities of native coccinellids: population fluctuations and the effect of competition from an invasive non-native species. Insect Conserv Divers. doi:10.1111/icad.12158
Hoogendoorn A, Heimpel G (2002) Indirect interactions between an introduced and a native ladybird beetle species mediated by a shared parasitoid. Biol Control 25:224–230
Hoogendoorn M, Heimpel GE (2004) Competitive interactions between an exotic and a native ladybeetle: a field cage study. Entomol Exp Appl 111:19–28
Howe AG (2015) Harlekinmariehønen-en bornholmsk status. Natur på Bornholm 13:48–52
Howe AG, Ransijn J, Ravn HP (2015) A sublethal effect on native Anthocoris nemoralis through competitive interactions with invasive Harmonia axyridis. Ecol Entomol 40: 639–649
Howe AG, Ravn HP, Pipper CB, Aebi A (2016) Potential for exploitative competition, not intraguild predation, between invasive harlequin ladybirds and flowerbugs in urban parks. Biol Invasions 18:517–532
Iazloveţchii I, Sumencova V (2013) New invasive species in the Republic of Moldova: multicolored Asian ladybird Harmonia axyridis Pallas (Coleoptera: Coccinellidae). In: VIII international conference of zoologists. Actual problems of protection and sustainable use of the animal world diversity (Chisinau, 10–12.10. 2013). Book of abstract. Academy of Sciences of Moldova, Chisinau, pp 136–137
Ingels B, De Clercq P (2011) Effect of size, extraguild prey and habitat complexity on intraguild interactions: a case study with the invasive ladybird Harmonia axyridis and the hoverfly Episyrphus balteatus. Biocontrol 56:871–882
Ingels B, Van Hassel P, Van Leeuwen T et al (2015) Feeding history affects intraguild interactions between Harmonia axyridis (Coleoptera: Coccinellidae) and Episyrphus balteatus (Diptera: Syrphidae). PLoS ONE 10:e0128518
Izhevsky S (1990) Introduction and applications of entomophags. Moscow, VO “Agropromizdat
Jacas J-A, Urbaneja A, Viñuela E (2006) History and future of introduction of exotic arthropod biological control agents in Spain: a dilemma? Biocontrol 51:1–30
Jansen JP, Hautier L (2008) Ladybird population dynamics in potato: comparison of native species with an invasive species, Harmonia axyridis. Biocontrol 53:223–233
Jeffries MJ, Lawton JH (1984) Enemy free space and the structure of ecological communities. Biol J Linn Soc 23:269–286
Jeffries DL, Chapman J, Roy HE et al (2013) Characteristics and drivers of high-altitude ladybird flight: insights from vertical-looking entomological radar. PLoS ONE 8(12):e82278
Jiang W-H, Pan X-H, Liu J-X et al (2007) Studies on the type of spot in the wings of Harmonia axyridis (Pallas) in Baoding. Hebei J For Orchard Res 2:022
Katsanis A (2011) Multitrophic interactions between the invasive coccinellid, Harmonia axyridis, and non-target insects. Bern University, Bern
Katsanis A, Babendreier D, Nentwig W et al (2013) Intraguild predation between the invasive ladybird Harmonia axyridis and non-target European coccinellid species. BioControl 1–11
Kemp E, Cottrell T (2015) Effect of lures and colors on capture of lady beetles (Coleoptera: Coccinellidae) in tedders pyramidal traps. Environ Entomol nvv108
Kenis M, Roy HE, Zindel R et al (2008) Current and potential management strategies against Harmonia axyridis. Biocontrol 53:235–252
Kenis M, Adriaens T, Brown P et al (2010) Impact of Harmonia axyridis on European ladybirds: which species are most at risk? IOBC/wprs Bull 58:57–59
Klausnitzer B (2002) Harmonia axyridis (Pallas, 1773) in Germany (Col., Coccinellidae). Entomologische Nachrichten und Berichte 46:177–183
Koch RL (2003) The multicolored Asian lady beetle, Harmonia axyridis: a review of its biology, uses in biological control, and non-target impacts. J Insect Sci 3
Koch RL, Galvan TL (2008) Bad side of a good beetle: the North American experience with Harmonia axyridis. Biocontrol 53:23–35
Koch RL, Hutchison WD, Venette RC et al (2003) Susceptibility of immature monarch butterfly, Danaus plexippus (Lepidoptera: Nymphalidae: Danainae), to predation by Harmonia axyridis (Coleoptera: Coccinellidae). Biol Control 28:265–270
Koch R, Burkness E, Burkness SJW et al (2004a) Phytophagous preferences of the multicolored Asian lady beetle (Coleoptera: Coccinellidae) for autumn-ripening fruit. J Econ Entomol 97:539–544
Koch RL, Carrillo MA, Venette RC et al (2004b) Cold hardiness of the multicolored Asian lady beetle (Coleoptera: Coccinellidae). Environ Entomol 33:815–822
Koch R, Venette R, Hutchison W (2006a) Predicted impact of an exotic generalist predator on monarch butterfly (Lepidoptera: Nymphalidae) populations: a quantitative risk assessment. Biol Invasions 8:1179–1193
Koch RL, Venette RC, Hutchison WD (2006b) Invasions by Harmonia axyridis (Pallas)(Coleoptera: Coccinellidae) in the Western Hemisphere: implications for South America. Neotrop Entomol 35:421–434
Koch RL, Fernandes MG, Dutra CC (2011) First confirmed record of Harmonia axyridis (Pallas, 1773)(Coleoptera: Coccinellidae) in the state of Mato Grosso do Sul, Brazil. Check List 7:476–477
Kögel S, Eben A, Hoffmann C et al (2012) Influence of diet on fecundity, immune defense and content of 2-isopropyl-3-methoxypyrazine in Harmonia axyridis Pallas. J Chem Ecol 38:854–864
Komai T (1956) Genetics of ladybirds. Adv Genet 8:155–188
Kondo T, González GF (2013) The multicolored Asian lady beetle, Harmonia axyridis (Pallas, 1773)(Coleoptera: Coccinellidae), a not so new invasive insect in Colombia and South America. Insecta Mundi 1–7
Kontodimas DC, Stathas GJ, Martinou AF (2008) The aphidophagous predator Harmonia axyridis (Coleoptera: Coccinellidae) in Greece, 1994–1999. Eur J Entomol 105:541–544
Koyama S, Majerus MEN (2008) Interactions between the parasitoid wasp Dinocampus coccinellae and two species of coccinellid from Japan and Britain. Biocontrol 53:253–264
Krafsur E, Kring T, Miller J et al (1997) Gene flow in the exotic colonizing ladybeetle Harmonia axyridis in North America. Biol Control 8:207–214
Krengel S, Stangl GI, Brandsch C et al (2012) A comparative study on effects of normal versus elevated temperatures during preimaginal and young adult period on body weight and fat body content of mature Coccinella septempunctata and Harmonia axyridis (Coleoptera: Coccinellidae). Environ Entomol 41:676–687
Kulijer D (2010) First record of invasive species Harmonia axyridis (Pallas, 1773)(Coleoptera: Coccinellidae) in Bosnia and Herzegovina. Acta entomologica serbica 15:141–143
Kurosawa Y, Hisamatsu S, Sasaji H (1985) The Coleoptera of Japan in color, vol III. Hoikusha Publishing Co., Osaka (in Japanese)
LaMana ML, Miller JC (1996) Field observations on Harmonia axyridis Pallas (Coleoptera: Coccinellidae) in Oregon. Biol Control 6:232–237
LaRock DR, Mirdad Z, Ellington JJ et al (2003) Control of green peach aphids Myzus persicae (Homoptera: Aphididae) with lady beetles Harmonia axyridis on Chile Capsicum annum (Coleoptera: Coccinellidae) in the greenhouse. Southwest Entomol 28:249–253
Liere H, Kim TN, Werling BP et al (2014) Trophic cascades in agricultural landscapes: indirect effects of landscape composition on crop yield. Ecol Appl
Lindborg R, Eriksson O (2004) Historical landscape connectivity affects present plant species diversity. Ecology 85:1840–1845
Loiseau A, Malausa T, Lombaert E et al (2009) Isolation and characterization of microsatellites in the harlequin ladybird, Harmonia axyridis (Coleoptera, Coccinellidae), and cross-species amplification within the family Coccinellidae. Mol Ecol Resour 9:934–937
Lombaert E, Malausa T, Devred R et al (2008) Phenotypic variation in invasive and biocontrol populations of the harlequin ladybird, Harmonia axyridis. Biocontrol 53:89–102
Lombaert E, Guillemaud T, Cornuet J-M et al (2010) Bridgehead effect in the worldwide invasion of the biocontrol harlequin ladybird. PLoS ONE 5:e9743
Lombaert E, Guillemaud T, Thomas CE et al (2011) Inferring the origin of populations introduced from a genetically structured native range by approximate Bayesian computation: case study of the invasive ladybird Harmonia axyridis. Mol Ecol 20:4654–4670
Lombaert E, Estoup A, Facon B et al (2014) Rapid increase in dispersal during range expansion in the invasive ladybird Harmonia axyridis. J Evol Biol 27:508–517
Loreau M, Mouquet N, Gonzalez A (2003) Biodiversity as spatial insurance in heterogeneous landscapes. Proc Natl Acad Sci 100:12765–12770
Losey JE, Perlman JE, Hoebeke ER (2007) Citizen scientist rediscovers rare nine-spotted lady beetle, Coccinella novemnotata, in eastern North America. J Insect Conserv 11:415–417
Losey JE, Allee LL, Stephens E et al (2014) Lady beetles in New York: insidious invasions, erstwhile extirpations, and recent rediscoveries. Northeast Nat 21:271–284
Lucas E, Vincent C, Labrie G et al (2007) The multicolored Asian ladybeetle Harmonia axyridis (Coleoptera: Coccinellidae) in Quebec agroecosystems ten years after its arrival. Eur J Entomol 104:737–743
Luebert F, Pliscoff P (2006) Sinopsis bioclimática y vegetacional de Chile. Editorial Universitaria
Maes S, Massart X, Grégoire J-C et al (2014) Dispersal potential of native and exotic predatory ladybirds as measured by a computer-monitored flight mill. Biocontrol 59:415–425
Majerus M, Strawson V, Roy H (2006) The potential impacts of the arrival of the harlequin ladybird, Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae), in Britain. Ecol Entomol 31:207–215
Majzlan O (2008) Faunistical contributions from Slovakia, Coleoptera, 4. Naturae Tutela 12:207–210
McClure MS (1987) Potential of the Asian predator, Harmonia axyridis Pallas (Coleoptera: Coccinellidae), to control Matsucoccus resinosae bean and godwin (Homoptera: Margarodidae) in the United States. Environ Entomol 16:224–230
Michaud JP (2002) Invasion of the Florida citrus ecosystem by Harmonia axyridis (Coleoptera: Coccinellidae) and asymmetric competition with a native species, Cycloneda sanguinea. Environ Entomol 31:827–835
Michie LJ, Mallard F, Majerus MEN et al (2010) Melanic through nature or nurture: genetic polymorphism and phenotypic plasticity in Harmonia axyridis. J Evol Biol 23:1699–1707
Mizell RF III (2007) Impact of Harmonia axyridis (Coleoptera: Coccinellidae) on native arthropod predators in pecan and crape myrtle. Fla Entomol 90:524–536
Murchie AK, Moore JP, Moore GA et al (2008) The harlequin ladybird (Harmonia axyridis (Pallas)) (Coleoptera: Coccinellidae), found in Ireland. Ir Nat J 29:25–26
Murray K, Roy HE, Tinsley M (2015) Assessing the role of host immunity in harlequin ladybird enemy release. In: Brown PMJ, Roy HE, Howe A, De Clercq P (eds) 3rd meeting of IOBC-WPRS study group “Benefits and risks of exotic biological control agents” 13–15 May 2015. Bornholm, Denmark
Nalepa CA (2007) Harmonia axyridis (Coleoptera: Coccinellidae) in buildings: relationship between body height and crevice size allowing entry. J Econ Entomol 100:1633–1636
Nalepa CA, Weir A (2007) Infection of Harmonia axyridis (Coleoptera: Coccinellidae) by Hesperomyces virescens (Ascomycetes: Laboulbeniales): role of mating status and aggregation behavior. J Invertebr Pathol 94:196–203
Nalepa CA, Kidd KA, Hopkins DI (2000) The multicolored Asian lady beetle (Coleoptera: Coccinellidae): orientation to aggregation sites. J Entomol Sci 35:150–157
Nalepa CA, Kennedy GG, Brownie C (2005) Role of visual contrast in the alighting behavior of Harmonia axyridis (Coleoptera: Coccinellidae) at overwintering sites. Environ Entomol 34:425–431
Nedvěd O (2014) Slunéčko východní (Harmonia axyridis) – pomocník v biologické ochraně nebo ohrožení biodiverzity? Jihočeská univerzita, České Budějovice, 65 pp
Nedvěd O, Háva J (2016) New record of the invasive lady beetle Harmonia axyridis in Afrotropical Region: Tanzania, Zanzibar. Afr Entomol 24:247–249
Nedved O, Kalushkov P (2011) Effect of air humidity on sex ratio and development of ladybird Harmonia axyridis (Coleoptera: Coccinellidae). Psyche 2012:173482
Nedvěd O, Krejčík S (2010) Record of the ladybird Harmonia axyridis (Coleoptera: Coccinellidae) from Uruguay. Klapalekiana 46:203–204
Nedvěd O, Háva J, Kulíková D (2011) Record of the invasive alien ladybird Harmonia axyridis (Coleoptera, Coccinellidae) from Kenya. ZooKeys 106:77–81
Nekrasova OD, Tytar VM (2014) Longstanding and seasonal population dynamics of the invasive species Harmonia axyridis (Coleoptera, Coccinellidae) in Ukraine. J VN Karazin Kharkiv Natl Univ Ser Biol 20:159–162
Nóia M, Borges I, Soares AO (2008) Intraguild predation between the aphidophagous ladybird beetles Harmonia axyridis and Coccinella undecimpunctata (Coleoptera: Coccinellidae): the role of intra and extraguild prey densities. Biol Control 46:140–146
Obata S (1986) Determination of hibernation site in the ladybird beetle, Harmonia axyridis Pallas (Coleoptera: Coccinellidae). Kontyû 54:218–233
Obrycki JJ, Giles KL, Ormord AM (1998) Experimental assessment of interactions between larval Coleomegilla maculata and Coccinella septempunctata (Coleoptera: Coccinellidae) in field cages. Environ Entomol 27:1280–1288
Obrycki JJ, Harwood JD, Kring TJ et al (2009) Aphidophagy by Coccinellidae: application of biological control in agroecosystems. Biol Control 51:244–254
Ohgushi T, Sawada H (1998) What changed the demography of an introduced population of an herbivorous lady beetle? J Anim Ecol 67:679–688
Orlova-Bienkowskaja MJ (2013) Dangerous invasive harlequin ladybird Harmonia axyridis (Pallas, 1773)(Coleoptera, Coccinellidae) in the European Russia. Russ J Biol Invasions 4:190–193
Orlova-Bienkowskaja MJ (2014) The outbreak of harlequin ladybird Harmonia axyridis (Pallas, 1773)(Coleoptera, Coccinellidae) in the Caucasus and possible sources of invasion. Russ J Biol Invasions 5:275–281
Orlova-Bienkowskaja MJ, Ukrainsky AS, Brown PM (2015) Harmonia axyridis (Coleoptera: Coccinellidae) in Asia: a re-examination of the native range and invasion to southeastern Kazakhstan and Kyrgyzstan. Biol Invasions 1–8
Osawa N (2000) Population field studies on the aphidophagous ladybird beetle Harmonia axyridis (Coleoptera: Coccinellidae): resource tracking and population characteristics. Popul Ecol 42:115–127
Osawa N (2011) Ecology of Harmonia axyridis in natural habitats within its native range. Biocontrol 56:613–621
Osawa N, Nishida T (1992) Seasonal variation in elytral color polymorphism in Harmonia axyridis (the ladybird beetle)—the role of nonrandom mating. Heredity 69:297–307
Pallas PS (1771) Reise durch verschiedene Provinzen des russischen Reichs. gedruckt bey der Kayserlichen Academie der Wissenschaften
Panigaj L, Zach P, Honěk A et al (2014) The invasion history, distribution and colour pattern forms of the harlequin ladybird beetle Harmonia axyridis (Pall.)(Coleoptera, Coccinellidae) in Slovakia, Central Europe. ZooKeys 412:89–102
Pedersen J, Runge JB, Jonsén BP (2008) Fund af biller i Danmark, 2006 og 2007 (Coleoptera). Entomologiske Meddelelser 76:105–144
Pell JK, Baverstock J, Roy HE et al (2008) Intraguild predation involving Harmonia axyridis: a review of current knowledge and future perspectives. Biocontrol 53:147–168
Pervez A, Omkar (2006) Ecology and biological control application of multicoloured Asian ladybird, Harmonia axyridis: a review. Biocontrol Sci Technol 16:111–128
Phillips BL, Brown GP, Greenlees M et al (2007) Rapid expansion of the cane toad (Bufo marinus) invasion front in tropical Australia. Austral Ecol 32:169–176
Phillips BL, Brown GP, Shine R (2010) Life-history evolution in range-shifting populations. Ecology 91:1617–1627
Pickering GJ, Blake AJ, Soleas GJ et al (2010) Remediation of wine with elevated concentrations of 3-alkyl-2-methoxypyrazines using cork and synthetic closures. J Food Agric Environ 8:97–101
Pocock MJO, Roy HE, Preston CD et al (2015) The Biological Records Centre: a pioneer of citizen science. Biol J Linn Soc 115:475–493
Pons X, Roca M, Lumbierres B et al (2015) Characterization of a newly established aggregation of the invasive ladybeetle Harmonia axyridis and current status of the invader in Spain. Span J Agric Res 13:1006
Poutsma J, Loomans AJM, Aukema B et al (2008) Predicting the potential geographical distribution of the harlequin ladybird, Harmonia axyridis, using the CLIMEX model. Biocontrol 53:103–125
Przewozny M, Barlozek T, Bunalski M (2007) Harmonia axyridis (Pallas, 1773) (Coleoptera: Coccinellidae) new species of ladybird beetle for Polish fauna. Pol J Entomol 76:177–182
Purse BV, Comont R, Butler A et al (2014) Landscape and climate determine patterns of spread for all colour morphs of the alien ladybird Harmonia axyridis. J Biogeogr 42:575–588
Raak-van den Berg C, Hemerik L, de Jong PW et al (2012) Mode of overwintering of invasive Harmonia axyridis in the Netherlands. Biocontrol 57:71–84
Raak-van den Berg C, van Wielink PS, de Jong PW et al (2014) Invasive alien species under attack: natural enemies of Harmonia axyridis in the Netherlands. Biocontrol 59:229–240
Rabitsch W, Schuh R (2006) First record of the multicoloured Asian ladybird Harmonia axyridis. Pallas (Pallas, 1773) in Austria. Beiträge zur Entomofaunistik 7:161–164
Ragsdale DW, Landis DA, Brodeur J et al (2011) Ecology and management of the soybean aphid in North America. Annu Rev Entomol 56:375–399
Ramsey S, Losey JE (2012) Why is Harmonia axyridis the culprit in coccinellid biting incidents? An analysis of means, motive, and opportunity. Am Entomol 58:166–170
Reznik SY, Dolgovskaya MY, Ovchinnikov A et al (2015) Weak photoperiodic response facilitates the biological invasion of the harlequin ladybird Harmonia axyridis (Pallas)(Coleoptera: Coccinellidae). J Appl Entomol 139:241–249
Rhule EL, Majerus ME, Jiggins FM et al (2010) Potential role of the sexually transmitted mite Coccipolipus hippodamiae in controlling populations of the invasive ladybird Harmonia axyridis. Biol Control 53:243–247
Ribbands B, Brown PMJ, Roy HE et al (2009) The most northerly record of the Harlequin ladybird (Col., Coccinellidae) in the British Isles. Entomol Mon Mag 145:43–44
Richardson JV, Deloach CJ (1972) Some aspects of host selection by Perilitus coccinellae Hymenoptera: Braconidae. Ann Entomol Soc Am 65:834
Riddick EW (2006) Influence of host gender on infection rate, density and distribution of the parasitic fungus, Hesperomyces virescens, on the multicolored Asian lady beetle, Harmonia axyridis. J Insect Sci 6:15
Riddick EW, Cottrell TE, Kidd KA (2009) Natural enemies of the Coccinellidae: parasites, pathogens, and parasitoids. Biol Control 51:306–312
Rondoni G, Comont RF, Ricci C et al (2013) Parasitisation of coccinellid species in Oxfordshire and London and first record of an Harmonia axyridis—phorid association from Italy. In: International symposium “Ecology of Aphidophaga 12″, Belgrade, Serbia, p 121
Rondoni G, Athey KJ, Harwood JD et al (2015) Development and application of molecular gut-content analysis to detect aphid and coccinellid predation by Harmonia axyridis (Coleoptera: Coccinellidae) in Italy. Insect Sci 22:719–730
Roy HE, Brown PMJ (2015) Ten years of invasion: Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) in Britain. Ecol Entomol 40:336–348
Roy HE, Lawson Handley L-J (2012) Networking: a community approach to invaders and their parasites. Funct Ecol 26:1238–1248
Roy HE, Wajnberg E (2008) From biological control to invasion: the ladybird Harmonia axyridis as a model species. Biocontrol 53:1–4
Roy HE, Pell JK, Clark S et al (1998) Implications of predator foraging on aphid pathogen dynamics. J Invertebr Pathol 71:236–247
Roy HE, Baverstock J, Ware RL et al (2008a) Intraguild predation of the aphid pathogenic fungus Pandora neoaphidis by the invasive coccinellid Harmonia axyridis. Ecol Entomol 33:175–182
Roy HE, Brown PMJ, Rothery P et al (2008b) Interactions between the fungal pathogen Beauveria bassiana and three species of coccinellid: Harmonia axyridis, Coccinella septempunctata and Adalia bipunctata. Biocontrol 53:265–276
Roy HE, Brown PMJ, Frost R et al (2011a) Atlas of the ladybirds (Coccinellidae) of Britain and Ireland. Biological Records Centre, Wallingford
Roy HE, Handley LJL, Schoenrogge K et al (2011b) Can the enemy release hypothesis explain the success of invasive alien predators and parasitoids? Biocontrol 56:451–468
Roy HE, Rhule E, Harding S et al (2011c) Living with the enemy: parasites and pathogens of the ladybird Harmonia axyridis. Biocontrol 56:663–679
Roy HE, Pocock MJO, Preston CD et al (2012a) Understanding citizen science and environmental monitoring: final report on behalf of UK Environmental Observation Framework
Roy HE, Adriaens T, Isaac NJB et al (2012b) Invasive alien predator causes rapid declines of native European ladybirds. Divers Distrib 18:717–725
Roy HE, Bacon J, Beckmann B et al (2012c) Non-native species in Great Britain: establishment, detection and reporting to inform effective decision making. Defra, London
Roy HE, Preston CD, Roy DB (2015a) Foreword: fifty years of the Biological Records Centre. Biol J Linn Soc 115:469–474
Roy HE, Rorke SL, Beckmann B et al (2015b) The contribution of volunteer recorders to our understanding of biological invasions. Biol J Linn Soc 115:678–689
Sæthre M-G, Staverløkk A, Hofsvang T (2010a) The history of Harmonia axyridis (Pallas 1773) in Norway. IOBC/wprs Bull 58:97–104
Saini ED (2004) Presencia de Harmonia axyridis (Pallas)(Coleoptera: Coccinelidae) en la provincia de Buenos Aires. Aspectos biológicos y morfológicos. Instituto Nacional de Tecnología Agropecuaria
Savojskaja GI (1971) On the acclimatization of Leis axyridis Pallas (Coleoptera, Coccinellidae) in Ile-Alatau. Trudy XIII Mezhdunarodnogo entomologicheskogo kongressa 2:181–182
Schanderl H, Ferran A, Coderre D et al (1992) Capacidade de dispersão de Harmonia axyridis PALLAS (Col., Coccinellidae) após largada inundativa para controlo de afídeos do milho, Rhopalosiphum padi L. e Sitobion avenae (F.)(Hom., Aphididae)
Sebastião D, Borges I, Soares AO (2015) Effect of temperature and prey in the biology of Scymnus subvillosus. Biocontrol 60:241–249
Seko T, Sumi A, Nakano A et al (2014) Suppression of aphids by augmentative release of larvae of flightless Harmonia axyridis. J Appl Entomol 138:326–337
Shinner R (2014) Thermal biology of the invasive ladybird Harmonia axyridis: physiological plasticity, thermoregulatory behaviour and fitness. M.Sc. thesis, Stellenbosch University, South Africa
Sighinolfi L, Febvay G, Dindo ML et al (2008) Biological and biochemical characteristics for quality control of Harmonia axyridis (Pallas)(Coleoptera, Coccinellidae) reared on a liver-based diet. Arch Insect Biochem Physiol 68:26–39
Silvertown J, Harvey M, Greenwood R et al (2015) Crowdsourcing the identification of organisms: a case-study of iSpot. ZooKeys 480:125–146
Sloggett JJ (2010) Predation of ladybird beetles by the orb-web spider Araneus diadematus. Biocontrol 55:631–638
Sloggett JJ (2012) Harmonia axyridis invasions: deducing evolutionary causes and consequences. Entomol Sci 15:261–273
Sloggett JJ, Obrycki JJ, Haynes KF (2009) Identification and quantification of predation: novel use of gas chromatography–mass spectrometric analysis of prey alkaloid markers. Funct Ecol 23:416–426
Smith CA, Gardiner MM (2013) Oviposition habitat influences egg predation of native and exotic coccinellids by generalist predators. Biol Control 67:235–245
Snyder WE (2009) Coccinellids in diverse communities: which niche fits? Biol Control 51:323–335
Snyder WE, Clevenger GM, Eigenbrode SD (2004) Intraguild predation and successful invasion by introduced ladybird beetles. Oecologia 140:559–565
Soares AO, Serpa A (2007) Interference competition between ladybird beetle adults (Coleoptera: Coccinellidae): effects on growth and reproductive capacity. Popul Ecol 49:37–43
Soares AO, Coderre D, Schanderl H (2001) Fitness of two phenotypes of Harmonia axyridis (Coleoptera : Coccinellidae). Eur J Entomol 98:287–293
Soares AO, Coderre D, Schanderl H (2003) Effect of temperature and intraspecific allometry on predation by two phenotypes of Harmonia axyridis Pallas (Coleoptera: Coccinellidae). Environ Entomol 32:939–944
Soares AO, Coderre D, Schanderl H (2004) Dietary self-selection behaviour by the adults of the aphidophagous ladybeetle Harmonia axyridis (Coleoptera: Coccinellidae). J Anim Ecol 73:478–486
Soares AO, Borges I, Borges PAV et al (2008) Harmonia axyridis: what will stop the invader? Biocontrol 53:127–145
Solano Y, Arcaya E (2014) Primer registro de Harmonia axyridis (Pallas, 1773) (Coleoptera: Coccinellidae) en Venezuela. Entomotropica 29:57–61
Solter LF, Kyei-Poku GK, Johny S (2013) Comment on “Invasive harlequin ladybird carries biological weapons against native competitors”. Science 341:1342
Špryňar P (2008) Faunistic records from the Czech Republic—252. Klapalekiana 44:77–79
Stals R (2008) Invasive ladybird spreading in South Africa. Biocontrol News Inf 29:24N–26N
Stals R (2010) The establishment and rapid spread of an alien invasive lady beetle: Harmonia axyridis (Coleoptera: Coccinellidae) in southern Africa. IOBC/wprs Bull 58:125–132
Stals R, Prinsloo G (2007) Discovery of an alien invasive, predatory insect in South Africa: the multicoloured Asian ladybird beetle, Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae). S Afr J Sci 103:123–126
Statens landbrukstilsyn (2001) Assessment of Harmonia axyridis. Intern Doc
Staverløkk A (2006) Fremmede arter og andre uønskede blindpassasjerer i import av grøntanleggsplanter. (Occurrence of alien species and other unwanted stowaways in imported horticultural plants). 111 pp. M.Sc. thesis, University of Life Sciences (UMB), Ås, Norway
Steenberg T, Harding S (2009a) Entomopathogenic fungi recorded from the harlequin ladybird, Harmonia axyridis. J Invertebr Pathol 102:88–89
Steenberg T, Harding S (2009b) The harlequin ladybird (Harmonia axyridis Pallas) in Denmark: spread and phenology during the initial phase of invasion. Entomologiske Meddelelser 77:27–39
Steenberg T, Harding S, Bønløkke J et al (2009) En ny art invaderer. Aktuel Naturvidenskab 3:4–7
Tan CC (1946) Mosaic dominance in the inheritance of color patterns in the lady-bird beetle, Harmonia axyridis. Genetics 31:195–210
Tan C-C, Li J-C (1934) Inheritance of the elytral color patterns of the lady-bird beetle, Harmonia axyridis Pallas. Am Nat 68:252–265
Tang B, Zhu J, Guo HS et al (2012) Studies of the diversity of multiple elytral color morphs of Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae). J Hangzhou Norm Univ 11:132–136
Tang DL, Qiu BL, Ren SX (2014) A review of insecticide resistance in the natural enemies of pest insects. Chin J Appl Entomol 51:13–25
Tayeh A, Estoup A, Laugier G et al (2012) Evolution in biocontrol strains: insight from the harlequin ladybird Harmonia axyridis. Evol Appl 5:481–488
Tayeh A, Estoup A, Hufbauer R et al (2013) Investigating the genetic load of an emblematic invasive species: the case of the invasive harlequin ladybird Harmonia axyridis. Ecol Evol 3:864–871
Tayeh A, Hufbauer RA, Estoup A, Ravigné V, Frachon L, Facon B (2015) Biological invasion and biological control select for different life histories. Nat Commun 6:7268. doi:10.1038/ncomms8268
Tedders WL, Schaefer PW (1994) Release and establishment of Harmonia axyridis (Coleoptera, Coccinellidae) in the southeastern United States. Entomol News 105:228–243
Thomas AP, Trotman J, Wheatley A et al (2013) Predation of native coccinellids by the invasive alien Harmonia axyridis (Coleoptera: Coccinellidae): detection in Britain by PCR-based gut analysis. Insect Conserv Divers 6:20–27
Timuş A, Stahi N (2013) The acclimatization and trophic spectrum of Harmonia axyridis in the Republic of Moldova. In: VIII international conference of zoologists. Actual problems of protection and sustainable use of the animal world diversity (Chisinau, 10–12.10. 2013). Book of abstract. Academy of Sciences of Moldova, Chisinau
Toda Y, Sakuratani Y (2006) Expansion of the geographical distribution of an exotic ladybird beetle, Adalia bipunctata (Coleoptera: Coccinellidae), and its interspecific relationships with native ladybird beetles in Japan. Ecol Res 21:292–300
Tourniaire R, Ferran A, Giuge L et al (2000) A natural flightless mutation in the ladybird, Harmonia axyridis. Entomol Exp Appl 96:33–38
Travis JM, Dytham C (2002) Dispersal evolution during invasions. Evol Ecol Res 4:1119–1129
Turgeon J, Tayeh A, Facon B et al (2011) Experimental evidence for the phenotypic impact of admixture between wild and biocontrol Asian ladybird (Harmonia axyridis) involved in the European invasion. J Evol Biol 24:1044–1052
Ukrainsky AS, Orlova-Bienkowskaja MJ (2014) Expansion of Harmonia axyridis Pallas (Coleoptera: Coccinellidae) to European Russia and adjacent regions. Biol Invasions 16:1003–1008
van Wielink P, Spijkers H (2013) Insects nightly attracted to light at a single site in De Kaaistoep, The Netherlands. Orders, families and species identified in 1995–2011. Entomol Ber (Amst) 73:200–214
Vandereycken A, Brostaux Y, Joie E et al (2013) Occurrence of Harmonia axyridis (Coleoptera: Coccinellidae) in field crops. Eur J Entomol 110:285–292
Varenhorst AJ, O’Neal ME (2012) The response of natural enemies to selective insecticides applied to soybean. Environ Entomol 41:1565–1574
Veran S, Piry S, Ternois V et al (2015) Modeling spatial expansion of invasive alien species: relative contributions of environmental and anthropogenic factors to the spreading of the harlequin ladybird in France. Ecography
Verheggen FJ, Fagel Q, Heuskin S et al (2007) Electrophysiological and behavioral responses of the Multicolored Asian Lady Beetle, Harmonia axyridis Pallas, to sesquiterpene semiochemicals. J Chem Ecol 33:2148–2155
Verizhnikova I (2011) Invasion by Harmonia axyridis (Coleoptera: Coccinellidae): population growth in Kyiv region. Bull Kharkiv Nat Agric Univ (Phytopathol Entomol) 9:23–26
Verizhnikova I, Shylova K (2013) Consequences of entomophage Harmonia axyridis Pall. (Coleoptera, Coccinellidae) introduction and its predicted area of acclimatization. Urgent Ecol Environ Manag Probl PFUR Mosc 15:65–68
Veselý P, Ernestová B, Nedvěd O et al (in press) Aposematic harlequin ladybirds (Harmonia axyridis) are not protected against tree sparrows (Passer montanus). Curr Zool
Vilcinskas A, Stoecker K, Schmidtberg H et al (2013) Invasive harlequin ladybird carries biological weapons against native competitors. Science 340:862-863
Vorontsov N, Blekhman A (2001) Geographical range and intraspecific structure of the ladybird Harmonia axyridis Pall., 1773 (Coleoptera, Coccinellidae). Evolution, ecology and biodiversity. In: Proceedings of the conference in memory of Nikolai Nikolaevich Vorontsov (1934–2000). Uchebno-nauchnyj tsent dovuzovskogo obrazovaniya, Moscow, pp 150–156
Wang S, Zhang RZ, Zhang F (2007) Research progress of biology and ecology of Harmonia axyridis (Pallas) (Coleopteta: Coccinellidae). Chin J Appl Ecol 18:2117–2126
Wang S, Michaud JP, Zhang RZ et al (2009) Seasonal cycles of assortative mating and reproductive behaviour in polymorphic populations of Harmonia axyridis in China. Ecol Entomol 34:483–494
Wang S, Michaud J, Tan X et al (2011) The aggregation behavior of Harmonia axyridis in its native range in Northeast China. Biocontrol 56:193–206
Ware RL, Majerus MEN (2008) Intraguild predation of immature stages of British and Japanese coccinellids by the invasive ladybird Harmonia axyridis. Biocontrol 53:169–188
Ware RL, Yguel B, Majerus MEN (2009) Effects of competition, cannibalism and intra-guild predation on larval development of the European coccinellid Adalia bipunctata and the invasive species Harmonia axyridis. Ecol Entomol 34:12–19
Weissenberger A, Brun J, Piotte C et al (1999) Comparison between the wild type and flightless type of the coccinellid Harmonia axyridis (Pallas) in control of the damson hop aphid. In: Fifth international conference on pests in agriculture. Association Natioale pour la Protection des Plantes (ANPP), pp 727–734
Welch VL, Sloggett JJ, Webberley KM et al (2001) Short-range clinal variation in the prevalence of a sexually transmitted fungus associated with urbanisation. Ecol Entomol 26:547–550
Wells PM (2011) Intraguild predation by Harmonia axyridis: effects on native enemies and aphid suppression. Department of Zoology, University of Cambridge
Wheeler A Jr, Hoebeke E (1995) Coccinella novemnotata in northeastern North America: historical occurrence and current status (Coleoptera: Coccinellidae). Entomol Soc Wash, USA
Woltz J, Landis D (2014) Comparison of sampling methods of Aphis glycines predators across the diel cycle. J Appl Entomol 138:475–484
Yang YW, Zhang QM, Du L et al (2006) Study on selective preferences of Harmonia axyridis (Pallas) collected from different habitats to predating aphids. China Plant Prot 26:5–7
Yasuda H, Evans EW, Kajita Y et al (2004) Asymmetric larval interactions between introduced and indigenous ladybirds in North America. Oecologia 141:722–731
Zakharov IA, Goryacheva II, Suvorov A (2011) Mitochondrial DNA polymorphism in invasive and native populations of Harmonia axyridis. Eur J Environ Sci 1:15–18
Zenni RD, Nuñez MA (2013) The elephant in the room: the role of failed invasions in understanding invasion biology. Oikos 122:801–815
Acknowledgments
The paper had its origin at a workshop on “Drivers, impacts, mechanisms and adaptation in insect invasions” hosted by the DST-NRF Centre of Excellence for Invasion Biology in Stellenbosch, South Africa, in November 2014. Additional financial support was provided by HortGro, the National Research Foundation of South Africa, Stellenbosch University, and SubTrop. We thank all our collaborators, and particularly the volunteer community, who have contributed to research around the world on H. axyridis. The number of references included reflects the range of inspiring studies on H. axyridis from so many people—we look forward to new and continued collaborations in the future. We are grateful to the editors of this special issue for inviting this review and providing an opportunity to explore ideas through the “Invasive Insects Workshop funding (NRF South Africa, CIB)”. We also thank the anonymous reviewers for all their useful comments and reflections. The UK Ladybird Survey and associated co-authors are supported by the Biological Records Centre (part of the Centre for Ecology & Hydrology), which receives support from both the Natural Environment Research Council and the Joint Nature Conservation Committee. The IOBC WPRS and Global Working Groups “Benefits and Risks of Exotic Biological Control Agents” and the COST Action TD1209 “Alien Challenge” have facilitated discussions and collaborations on H. axyridis. This study was supported by the French Agropolis Fondation (Labex Agro-Montpellier, BIOFIS Project Number 1001-001) and by a grant from the ERA-Net BiodivERsA, with the national funders ANR (France), DFG (Germany) and BELSPO (Belgium), as part of the 2012–2013 BiodivERsA call for research proposals. Support has been also received from FONDECYT 1140662 (Chile). The study of M.J. Orlova-Bienkowskaja and I. A. Zakharov was supported by Russian Science Foundation, Project No. 16-16- 00079. Gabriele Rondoni acknowledges financial support from Fondazione Cassa di Risparmio di Perugia. Riaan Stals acknowledges funding from the Department of Science and Technology, South Africa. The research of Peter Zach and colleagues was funded by the project VEGA 2/0035/13 and VEGA 2/0052/15. A. Honěk and Z. Martinkova were supported by grants GACR 14-26561S and COST CZ LD14084. Research in Switzerland is funded by the Swiss Federal Office for the Environment. Hans Peter Ravn was supported by the Villum Foundation. Danny Haelewaters acknowledges funding from the David Rockefeller Center for Latin American Studies at Harvard University and from the Mycological Society of America.