
The Latest Updates on COVID-19 Treatments and Preventative Medications in the Pipeline
Key takeaways:
Many medications are available to treat COVID-19, including oral treatments such as Paxlovid (nirmatrelvir / ritonavir) and Lagevrio (molnupiravir).
Epidemiologists from the University of Texas at Austin recently released findings that suggest more Paxlovid prescriptions could lead to better U.S. health outcomes. Using it to treat at least 20% of symptomatic COVID cases could lead to a substantial drop in hospitalizations and healthcare costs.
Pemgarda (pemivibart) is the newest medication for COVID. But it’s not designed to treat COVID. Pemgarda helps prevent COVID in certain adults and kids ages 12 years and older. It was authorized for emergency use in March 2024.
Access savings to related medications
Table of contents

During a public health emergency, such as COVID-19, the FDA can issue emergency use authorizations (EUAs) to make new medications and medical products available without full FDA approval. Today, many medications are available through an EUA to treat COVID, both inside and outside of the hospital. Four are even fully approved.
However, the FDA frequently revises how these treatments should be used. These updates are made as health experts learn more about their effectiveness, especially against new viral variants. These updates happen frequently, so it can be tricky to stay up to date with the latest news and recommendations.
Here, we’ll talk about the latest updates for medications that are authorized and approved to treat or prevent COVID.
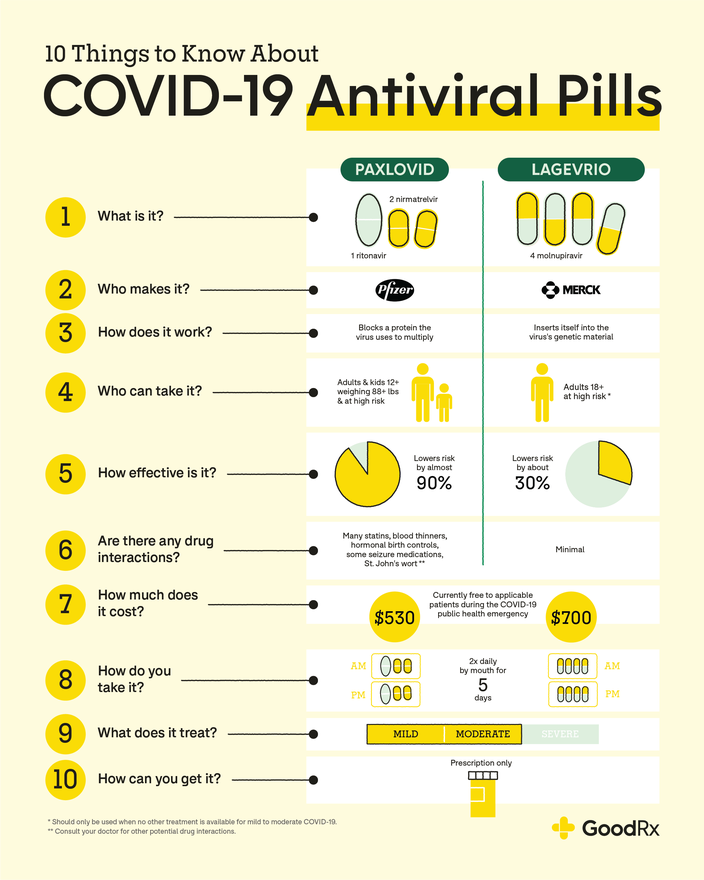
Paxlovid (nirmatrelvir / ritonavir)
What is it: Paxlovid is an oral treatment that contains two antiviral medications: nirmatrelvir and ritonavir. It comes as two different types of tablets that are packaged together. It works by blocking a protein the virus needs to make copies of itself.
Status: Paxlovid was first authorized for use in December 2021. It became fully FDA approved for adults in May 2023.
Recommended use: The National Institutes of Health (NIH) recommends Paxlovid as the first-choice treatment for mild-to-moderate COVID in people ages 12 and older who are at high risk for severe illness.
How it’s taken: You’ll take three tablets by mouth 2 times a day for 5 days. Paxlovid should be taken within 5 days of getting symptoms.
Effectiveness: When it was first authorized, Paxlovid was shown to lower the risk of hospitalization or death from COVID by about 90% when taken within 5 days of symptom onset. More recent studies have also found that it’s highly effective at reducing the risk of hospitalization and death — notably among older adults. It may also help reduce the risk of “long COVID,” but this isn’t fully proven. And it likely provides more protection than molnupiravir, too.
Availability: Paxlovid availability has improved over time. Your pharmacist or another member of your healthcare team are authorized to prescribe it to you.
Other considerations: Paxlovid can interact with many other medications. Check with your healthcare professional (HCP) to see if Paxlovid is safe for you to take.
Latest news on Paxlovid
More Paxlovid prescriptions could translate to fewer hospitalizations and less healthcare cost
March 7, 2024
Recent research from the University of Texas at Austin suggests that if more people took Paxlovid, it could substantially reduce hospitalizations and save a considerable amount of money for the healthcare system. Due to Paxlovid’s eligibility criteria, many more people could take Paxlovid compared to those who actually do.
This data suggests that if Paxlovid was used to treat at least 20% of symptomatic COVID cases in the U.S. over a 10-month period, the medication could prevent up to 850,000 hospitalizations and save over $170 billion in U.S. healthcare costs.
To read more about this data, click or tap here.
Molnupiravir (Lagevrio)
What is it: Like Paxlovid, molnupiravir (Lagevrio) is an antiviral medication. It’s available as an oral capsule. It works by interfering with the process the virus uses to make copies of itself.
Status: Molnupiravir was first authorized in December 2021. It’s still available for use.
Recommended use: Molnupiravir is considered to be an alternative option for treating mild-to-moderate COVID in adults at high risk of severe illness. It appears to be less effective than Paxlovid and remdesivir.
How it’s taken: You’ll take four capsules by mouth 2 times a day for 5 days. Molnupiravir should be taken within 5 days of getting symptoms.
Effectiveness: Initial studies found that molnupiravir helped lower the risk of hospitalization or death from COVID by about 30% when taken within 5 days of symptom onset. More recent data from lab studies and small clinical studies suggest it works against the Omicron variant. A May 2023 meta analysis also found it can help reduce the risk of death compared to placebo.
Availability: You can use the GoodRx Health COVID Pills Tracker to find molnupiravir available near you.
Other considerations: Molnupiravir should only be used by adults and shouldn’t be taken if you’re pregnant. It can be harmful to an unborn baby and may also affect sperm, so reliable birth control is recommended if you’re having sex.
Latest news on molnupiravir
Lagevrio and Paxlovid to transition to the commercial market
November 1, 2023
The U.S. government recently announced that Lagevrio and Paxlovid — also known as molnupiravir and nirmatrelvir / ritonavir, respectively — are transitioning to the commercial market. This is similar to how most other medications are sold, distributed, and dispensed. Prior to this, the U.S. government was distributing free doses of these medications to those who qualify.
To read more about this process change, click or tap here.
Remdesivir (Veklury)
What is it: Remdesivir (Veklury) is an intravenous (IV) antiviral medication. It works by blocking an enzyme (protein) needed for the virus to replicate.
Status: Remdesivir was first authorized for use in May 2020 for inpatient (hospital) use. It became fully FDA approved in October 2020 for people ages 12 and older. In January 2022, it was expanded for outpatient use for all ages. And in April 2022, it became fully approved for certain kids who are ages 28 days and older.
Recommended use: The NIH recommends remdesivir as a second-choice option for certain non-hospitalized people at least 12 years old with mild-to-moderate COVID at high risk for severe illness. This means that Paxlovid is preferred, but remdesivir is an option if you can’t access or receive Paxlovid. It’s also approved for this purpose in children who are 28 days or older and weigh at least 3 kg (about 7 pounds). Remdesivir is an available option for people who are in the hospital, too.
How it’s given: Remdesivir is given as an IV infusion into your vein. Nonhospitalized people receive daily infusions for 3 days. Treatment should be started as soon as possible after diagnosis and within 7 days of symptom onset. People who’ve been hospitalized typically receive daily infusions for 5 to 10 days.
Effectiveness: In certain nonhospitalized people who are at high risk for severe COVID, remdesivir can help lower the risk of hospitalization or death by about 87% compared to placebo. If you’re in the hospital, its effectiveness is more variable.
Availability: If you’re in the hospital, your care team may give remdesivir to you if it’s available at their location and if you’re eligible. If you’re not in the hospital, your HCP can let you know if it’s available at an infusion clinic near you.
Other considerations: In an outpatient setting, remdesivir requires travel to and from an infusion location. And it’s recommended to get an infusion on 3 back-to-back days.
Latest news on remdesivir
Remdesivir can now treat COVID regardless of your liver health
September 8, 2023
The FDA recently announced that remdesivir is now approved to treat COVID in people with all stages of liver disease. It's the first and only COVID antiviral that can be used with mild, moderate, or severe liver impairment. You likely won’t need any dose adjustments either.
This approval is based on a phase 1 study that analyzed remdesivir’s safety for this purpose. There wasn’t much data available about this prior to this study.
To read more about this update, click or tap here.
Tocilizumab (Actemra)
What is it: Tocilizumab (Actemra) is an IV biologic medication. It works by lowering an inflammation-causing chemical in the body that can be elevated in the lungs from COVID.
Status: Tocilizumab was first authorized for use in June 2021. It became fully approved in December 2022.
Recommended use: Tocilizumab should only be used to treat people with severe COVID who are in the hospital. If you’re hospitalized due to COVID, your care team may choose to give it to you in a variety of situations — especially if you need respiratory support.
How it’s given: Tocilizumab is given as a single-dose IV infusion over 1 hour. The specific dose depends on someone’s body weight. An additional dose can be given if more of a response is needed. The medication also comes as an under-the-skin injection, but this version isn’t authorized to treat COVID.
Effectiveness: Before being authorized, four clinical trials showed that tocilizumab helps lower the risk of death due to COVID. Newer studies suggest a similar outcome, but it seems to be most effective when used within 10 days of developing symptoms.
Other considerations: Separate from COVID, tocilizumab is also FDA approved to treat autoimmune disorders such as rheumatoid arthritis (RA), giant cell arteritis, and juvenile idiopathic arthritis. This is an example of a medication that was repurposed to treat COVID.
Anakinra (Kineret)
What is it: Anakinra (Kineret) is an under-the-skin injection. This medication helps manage lung inflammation by blocking a similar chemical to tocilizumab. Called IL-1, it’s thought to be part of an overactive immune response in COVID.
Status: Anakinra was authorized for emergency use in early November 2022.
Recommended use: Anakinra is authorized to treat hospitalized adults with pneumonia caused by COVID. But it should only be given to adults who need supplemental oxygen, are at risk for severe respiratory failure, and likely have a high amount of a specific inflammatory protein.
How it’s taken: Your HCP may inject one dose under your skin every day for up to 10 days. However, it’s not officially incorporated into NIH treatment guidelines.
Effectiveness: In a study called SAVE-MORE, people receiving anakinra were less likely to develop severe COVID after 28 days compared to placebo. But a different study found that it didn’t reduce the need for mechanical ventilation.
Other considerations: Outside of COVID, anakinra is FDA approved to treat RA and two other health conditions. This is another repurposed medication for COVID.
Baricitinib (Olumiant)
What is it: Baricitinib (Olumiant) is an oral medication. It’s classified as a Janus kinase (JAK) inhibitor, and it works by lowering inflammation in the body.
Status: Baricitinib was first authorized for use in November 2020. It became fully FDA approved for COVID in May 2022.
Recommendation: Baricitinib is only meant to treat certain people with severe COVID who are in the hospital. Your care team may choose to give baricitinib to you in a few situations. If it’s not available, they may recommend taking a similar medication called tofacitinib (Xeljanz) instead.
How it’s taken: You’ll take 1 tablet daily for 14 days or until you’re discharged from the hospital, whichever comes first.
Effectiveness: Clinical trials (ACTT-2 and COV-BARRIER) and real world studies both show that baricitinib can help lower the risk of death due to COVID when used alongside other treatments in the hospital.
Other conditions: Outside of COVID, baricitinib is FDA approved to treat RA and alopecia areata. This is another example of a medication that was repurposed to treat COVID.
Latest news on baricitinib
New research pinpoints who may benefit most from baricitinib
March 1, 2024
A new analysis of a clinical trial has identified who may benefit most from baricitinib: hospitalized adults with COVID who have low lymphocyte levels, high neutrophil counts, and low platelets. These are all different types of blood cells.
When baricitinib is combined with remdesivir, people who fit into this “high-risk” category are less likely to die or eventually need invasive mechanical ventilation. This is compared to people who receive remdesivir by itself.
To read more about this research, click or tap here.
Vilobelimab (Gohibic)
What it is: Vilobelimab (Gohibic) is an injectable medication that’s infused into a vein. Like tocilizumab, it’s a type of biologic medication. But it targets a different part of the immune system to help lessen inflammation.
Status: Vilobelimab was authorized for emergency use in April 2023.
Recommendation: Vilobelimab can treat hospitalized adults with severe COVID. You might receive it if you end up needing mechanical ventilation or ECMO for life support.
How it’s taken: Your healthcare team may decide to administer vilobelimab if it’s been 48 hours (2 days) or less since you were started on mechanical ventilation or ECMO. You may receive it up to 6 times over a 22-day period.
Effectiveness: Vilobelimab’s effectiveness is primarily measured by how well it can prevent death. In initial studies, it helped lower the risk of death by about 24% compared to placebo after 28 days.
Other considerations: This is the first time that vilobelimab has been authorized or approved for use. It hasn’t been incorporated into the NIH’s COVID treatment guidelines. But in late January 2024, its manufacturer announced a program that’s aimed at helping broaden access to the treatment.
Convalescent plasma
What is it: Convalescent plasma is the liquid part of the blood that’s been collected from people who’ve recovered from COVID. It contains antibodies that can help fight COVID in someone with an active infection.
Status: Convalescent plasma was first authorized for use in August 2020.
Recommended use: Some data shows that convalescent plasma should only be used in very select situations, if recommended by your HCP.
How it’s given: Convalescent plasma is infused into a vein over the course of about 1 to 2 hours. Additional infusions may be given if needed.
Effectiveness: There’s conflicting evidence. Some studies show it may help lower the likelihood of death in certain people hospitalized with severe COVID. But others say it doesn’t have any added value. The NIH says certain people who have a weakened immune system may benefit from it, but there’s weak supporting evidence.
Availability: Convalescent plasma is still authorized in the U.S. to treat certain hospitalized people with COVID.
Pemgarda (pemivibart)
What is it: Pemgarda (pemivibart) is a medication that’s infused into a vein. It’s designed to prevent COVID in certain adults and kids ages 12 years and older that are considered moderately to severely immunocompromised.
Status: Pemgarda was authorized for emergency use in late March 2024. It’s not fully approved yet.
Recommended use: Pemgarda is currently the only pre-exposure prophylaxis (PrEP) medication for COVID. If you’re not expected to respond well to COVID vaccines, your healthcare team may recommend receiving Pemgarda infusions for added protection. To be eligible, you shouldn’t have COVID or known recent exposure to someone with COVID.
How it’s given: Pemgarda infusions can be given once every 3 months. Each infusion takes about 1 hour to receive. A HCP will administer each dose to you, as you won’t be able to receive it at home.
Effectiveness: So far, it appears that Pemgarda may reduce the risk of developing symptomatic COVID by about 70% compared to placebo.
Availability: Pemgarda’s manufacturer stated that the medication would be shipped to healthcare facilities very soon. Your HCP can tell you when and where you can receive it. It’s expected to be covered by Medicare and most commercial insurance plans.
Latest news on Pemgarda
COVID PrEP is available once again
April 1, 2024
Pemgarda’s authorization for COVID highlights the return of COVID PrEP medications. Other preventative COVID medications, such as Evusheld (tixagevimab / cilgavimab), were available in the past. But they were pulled from shelves due to effectiveness concerns against newer viral variants.
To read more about this medication, click or tap here.
Bebtelovimab
What is it: Bebtelovimab is a monoclonal antibody medication that’s injected into your vein. Monoclonal antibodies are human-made antibodies that help fight illnesses like COVID.
Status: Bebtelovimab was originally authorized for emergency use in February 2022, but it’s no longer authorized for use.
Recommended use: Bebtelovimab currently isn’t recommended or authorized for use in the U.S.
How it’s taken: Bebtelovimab is injected into your vein as a single dose. It takes about 30 seconds to infuse it into your body. If your HCP recommends it for you, you should get it within 7 days of getting symptoms.
Effectiveness: Official product labeling states that bebtelovimab “may be effective” at treating mild to moderate COVID. And while studies initially suggested it works against the Omicron variant, more recent data shows it’s not effective against the Omicron BQ.1 and BQ.1.1. subvariants.
Availability: As of late November 2022, bebtelovimab isn’t available in the U.S.
Latest news on bebtelovimab
Bebtelovimab is no longer authorized in any U.S. regions due to effectiveness concerns against two Omicron subvariants
December 2, 2022
This week, the FDA announced that bebtelovimab is no longer authorized in any U.S. regions. This is because it’s not considered effective against two fast-spreading Omicron subvariants — Omicron BQ.1 and BQ.1.1. At the time of the announcement, these subvariants made up more than 57% of COVID cases nationwide.
Eli Lilly, the manufacturer of bebtelovimab, has stopped distributing the medication. They may resume distribution in the future if bebtelovimab is deemed effective against new subvariants.
To read more about this update, click or tap here.
Sotrovimab
What is it: Sotrovimab is a monoclonal antibody medication.
Status: Sotrovimab was first authorized for use in May 2021, but it currently isn’t authorized in any U.S. region.
Recommended use: Sotrovimab currently isn’t recommended or authorized for use in the U.S.
How it’s taken: Sotrovimab is infused into your vein as a single dose. If your HCP recommends it for you, you should get it within 10 days of getting symptoms.
Effectiveness: In a clinical trial of nonhospitalized adults with COVID, sotrovimab was found to lower the risk of hospitalization or death by about 85%. But this was before the Omicron BA.2 subvariant became predominant.
Availability: Sotrovimab currently isn’t available in the U.S.
Latest news on sotrovimab
Sotrovimab is no longer authorized in any U.S. regions due to effectiveness concerns against certain viral variants
April 19, 2022
The FDA recently announced that sotrovimab is no longer authorized in any U.S. state or territory. Medication effectiveness concerns drove this decision, as sotrovimab isn’t thought to work well against certain viral variants, including the Omicron BA.2 subvariant.
Bamlanivimab and etesevimab
What is it: Bamlanivimab and etesevimab are two monoclonal antibody medications. They work by binding to different parts of the virus’ spike protein, preventing it from entering and infecting your cells.
Status: Bamlanivimab was initially authorized for emergency use in November 2020. The FDA revised bamlanivimab’s EUA in February 2021 to be combined with etesevimab. As of January 2022, the combination is no longer authorized for use in the U.S.
Recommended use: Bamlanivimab and etesevimab aren’t recommended due to resistance concerns with the Omicron variant. They were previously available to treat mild-to-moderate COVID in nonhospitalized people. They were also available for post-exposure prophylaxis (PEP) in certain situations.
How it’s given: Both medications are infused into your vein at the same time as a single infusion.
Effectiveness: Data suggests that bamlanivimab and etesevimab are highly unlikely to be effective against the Omicron variant, which is the predominant variant in the U.S.
Availability: Bamlanivimab and etesevimab aren’t currently available in the U.S.
Latest news on bamlanivimab and etesevimab
Bamlanivimab and etesevimab not authorized to treat COVID caused by the Omicron variant
January 31, 2022
The FDA recently announced new data suggesting that bamlanivimab and etesevimab are “highly unlikely” to be effective against the Omicron variant. Because the Omicron variant is the predominant variant in the U.S., the FDA is currently stopping the use of this treatment in all U.S. states and territories.
Bamlanivimab and etesevimab may be available again in the future if they’re found to be effective against other variants.
To read more about this update, click or tap here.
REGEN-COV (casirivimab and imdevimab)
What is it: REGEN-COV is an IV treatment that includes two monoclonal antibody medications: casirivimab and imdevimab. It works by targeting the virus’ spike protein, preventing it from entering and infecting your cells.
Status: REGEN-COV was first authorized for use in November 2020. As of January 2022, it’s no longer authorized for use in the U.S.
Recommended use: REGEN-COV isn’t currently recommended due to resistance concerns with the Omicron variant. It was previously available to treat mild-to-moderate COVID and for PEP in people ages 12 and older who were at high risk of severe illness.
How it’s given: REGEN-COV can be infused into your vein outside of the hospital, but it can also be injected under your skin.
Effectiveness: Updated data has found that REGEN-COV is highly unlikely to be effective against the Omicron variant, which is the predominant variant in the U.S.
Availability: REGEN-COV isn’t currently available in the U.S.
Latest news on REGEN-COV
REGEN-COV not authorized to treat COVID caused by the Omicron variant
January 31, 2022
Similar to their findings for bamlanivimab and etesevimab, the FDA has found that REGEN-COV is “highly unlikely” to be effective against the Omicron variant. And because Omicron is now the main variant in the U.S., the FDA is currently stopping the use of this treatment in all U.S. states and territories.
REGEN-COV may be available again in the future if it’s found to be effective against other variants.
To read more about this update, click or tap here.
Therapies being studied to treat COVID
As long as COVID is around, researchers and health experts will keep trying to find innovative new ways to treat and prevent COVID. A few medications are being studied in clinical trials, and it’s possible that they could be authorized at some point in the future.
Simnotrelvir
Simnotrelvir is an oral antiviral that’s being studied in clinical trials for mild-to-moderate COVID. So far, data shows that it may help adults with COVID recover faster from their symptoms. It’s being studied as a 5-day treatment course that’s given twice a day in combination with ritonavir — very similar in style to Paxlovid.
Latest news on simnotrelvir
Simnotrelvir-based therapy may lead to faster resolution of symptoms
March 1, 2024
A recent phase 2/3 study published in The New England Journal of Medicine found that simnotrelvir can help adults with mild-to-moderate COVID achieve a speedier recovery. When taken within 3 days of developing symptoms, combination treatment with simnotrelvir and ritonavir led to a significantly faster resolution of symptoms compared to placebo. People taking simnotrelvir also had lower viral levels after 5 days of treatment.
What’s more, simnotrelvir may be effective for people who are considered to be “standard risk.” By comparison, medications like Paxlovid are meant for people who are at high risk of progressing to severe illness.
This study was based out of China, where the medication has been authorized since late 2023. To read more about it, click or tap here.
Ensitrelvir
Ensitrelvir is an oral antiviral medication that’s also in clinical development. It’s taken once daily for 5 days, and it’s thought to help people with mild-to-moderate COVID recover faster from their symptoms. It may also help ease taste and smell changes associated with the illness. This medication has been approved in Japan under the name Xocova since 2022.
Latest news on ensitrelvir
Ensitrelvir may help improve COVID-related symptoms faster than placebo pills
March 1, 2024
Shinogi — the manufacturer of the ensitrelvir — recently released phase 3 study data that suggests ensitrelvir can improve COVID-related symptoms by about 1 day faster than placebo. This includes symptoms such as runny or stuffy nose, fever, and sore throat. Fatigue and cough symptoms also improved. People who took ensitrelvir felt better within about 7 days; those who took placebo pills felt better in about 8 days.
To read more about this data, click or tap here.
Mindeudesivir
Mindeudesivir, also known as VV116, is an oral medication in development that’s chemically similar to remdesivir. It’s taken twice daily for 5 days, similar to Paxlovid. It’s conditionally approved for adults with mild-to-moderate COVID in China.
Latest news on mindeudesivir
Mindeudesivir, a cousin to remdesivir, helps accelerate time to symptom resolution
March 1, 2024
Phase 3 study data published in Lancet Infectious Diseases also found that mindeudesivir is superior to placebo at reducing COVID-related symptoms in a timely manner. Those who took mindeudesivir felt better after around 11 days, while those who took placebo felt better after around 13 days.
What’s more, a previous study found that mindeudesivir is comparable (non-inferior) to Paxlovid at helping people recover from their illness faster. But this was among adults with mild-to-moderate COVID who were at high risk of progression to severe illness.
To read more about the current study, click or tap here.
IBIO123
IBIO123 is an inhaled immunotherapy medication that’s being studied in clinical trials. It’s designed to help manage respiratory symptoms associated with COVID in nonhospitalized adults. It combines three different monoclonal antibody medications — called IBIO-1, IBIO-2, and IBIO-3 — into one treatment. You can breathe it in through a nebulizer.
Latest news on IBIO123
Phase 1/2 clinical trial suggests IBIO123 helps resolve respiratory problems from symptomatic COVID
September 8, 2023
According to a recent phase 1/2 study published in The Lancet Infectious Diseases, IBIO123 can significantly help reduce respiratory (breathing) symptoms associated with mild to moderate COVID in nonhospitalized adults. About 41% of people who received one dose of IBIO123 saw their respiratory symptoms go away within 8 days. This was compared to 17% of people who received a placebo.
However, the study missed out on one of its primary goals. IBIO123 didn’t lead to a significant drop in viral load within 5 days of use. Regardless, more studies are on deck. And IBIO123’s manufacturer is currently looking for a partner to help take the medication through more advanced stages of development.
The study took place between December 2021 and January 2023. Omicron was the predominant variant during this time. To read more about this study, click or tap here.
Interferon lambda
Interferon lambda is an under-the-skin (subcutaneous) injection that’s being studied in clinical trials as an early treatment for COVID. It’s being developed to help non-hospitalized adults avoid needing to go to the hospital or emergency room (ER) for COVID. Interferon lambda is given as a single-dose injection to help prevent severe illness.
Latest news on interferon lambda
Clinical trial suggests interferon lambda injections could help prevent severe COVID
March 1, 2023
According to phase 3 study data published in The New England Journal of Medicine, one dose of interferon lambda can help reduce the risk of hospitalizations or ER visits related to COVID.
Compared to participants who received a placebo, those receiving interferon lambda had a 51% lower risk of hospitalizations or ER visits within a 28-day time period. Side effects were similar between the two groups. More than 80% of adults included in the study were previously vaccinated.
If it’s eventually authorized, some experts believe interferon lambda could be an alternative to medications like Paxlovid for people with mild to moderate COVID.
To read more about this update, click or tap here.
Sabizabulin
Sabizabulin is a microtubule inhibiting-medication that’s being studied in clinical trials. It’s an oral pill that’s taken once daily, and it’s thought to have both antiviral and antiinflammatory effects. It’s being studied in hospitalized people with moderate-to-severe COVID who are at high risk for acute respiratory distress syndrome (ARDS).
Latest news on sabizabulin
Sabizabulin is set for new phase 3 trial
September 8, 2023
Veru, the company that’s developing sabizabulin, announced that they’re set to start a new phase 3 trial for sabizabulin. This study will include over 400 people with moderate to severe COVID. Its purpose is to assess the medication’s ability to reduce the risk of death from any cause. Interim results are expected to be released during the second half of 2024.
This is a positive step forward for sabizabulin. In March 2023, the FDA declined a request to authorize sabizabulin for emergency use.
To read more about this update, click or tap here.

