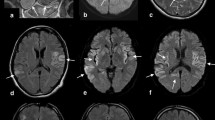
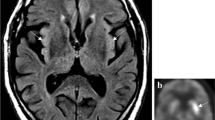
Abstract
Introduction
Anti-N-methyl-D-aspartate receptor (NMDAr) antibody encephalitis is an autoimmune disorder characterized by synaptic NMDAr current disruption and receptor hypofunction, often affecting women during pregnancy. Clinical manifestations associated with anti-NMDAr encephalitis can occur both in the mother and fetus.
Methods
We generated a systematic search of the literature to identify epidemiological, clinical, and serological data related to pregnant women with anti-NMDAr encephalitis and their children, analyzing the fetal outcomes. We examined the age and neurologic symptoms of the mothers, the presence of an underlying tumor, immunotherapies used during pregnancy, duration of the pregnancy, and type of delivery.
Results
Data from 41 patients were extrapolated from the included studies. Spontaneous interruption of pregnancy, premature birth, and cesarean section were reported in pregnant women with NMDAr encephalitis. Several fetal and neonatal symptoms (e.g., movement disorders, spina bifida, poor sucking, respiratory distress, cardiac arrhythmias, infections, icterus, hypoglycemia, and low birth weight) depending on the mother’s serum anti-NR1 concentration were also reported.
Conclusions
We characterized the outcomes of children born from mothers with anti-NMDAr encephalitis, analyzing the pivotal risk factors related to pregnancy and maternal disorder. Neuropsychiatric involvement seems strictly related to pathogenic NMDAr antibodies detected in maternal and/or neonatal serum.
These findings clarify a complex condition to manage, outlining the risks associated with pregnant women with anti-NMDAr encephalitis and also providing a concrete guide for therapeutic strategies to prevent potential harm to the fetus and the child’s neurodevelopment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anti-N-methyl-D-aspartate receptor (NMDAr) autoimmune encephalitis (AE) is one of the most common causes of noninfectious encephalitis during pregnancy [1,2,3]. It is characterized by an autoimmune response against the NR1 subunit of NMDAr, which causes a reversible internalization of the receptor into neurons, leading to a more extended NMDAr channel opening and excessive synaptic and extra-synaptic NMDAr activation [4,5,6].
From the clinical point of view, the subacute onset of several neurological (e.g., cognitive decline, speech impairment, seizures, central hypoventilation, and movement disorders) and psychiatric (e.g., psychosis, anxiety, and depression) symptoms is recognized as diagnostic hallmarks. Furthermore, according to Graus’ criteria [7], laboratory (i.e., cerebral spinal fluid/serum specific auto-antibodies positivity) and radiological (i.e., mesial-temporal signal abnormalities in MRI T2 fluid-attenuated inversion recovery (FLAIR) images of the brain) findings can help the diagnostic process. Anti-NMDAr AE is frequently associated with an underlying tumor pathology, mostly ovarian teratoma, which detection is fundamental for treatment purposes.
Experimental and clinical evidence support the risk of early postnatal mortality and the increased prevalence of neurologic and systemic abnormalities in newborns delivered by mothers affected by anti-NMDAr AE during pregnancy. This phenomenon is partially related to the specific treatment employed for AE management (i.e., antiseizure medications and immunomodulatory drugs) as well as diagnostic interventions (i.e., computer tomography (CT) or magnetic resonance image (MRI) scans with contrast agents) whose teratogenic potential is already well documented. On the other hand, animal models have shown that maternal-to-fetal anti-NR1 auto antibodies transfer can be associated with a dose-dependent altered fetal neurodevelopment which may lead to growth retardation and impaired cognitive functions. Anti-N1 antibodies are an IgG class of antibodies that can cross the placental barrier from the 13th week of gestational age onwards.
This systematic review analyzed the available data on perinatal outcomes of newborns whose mothers have been affected by anti-NMDAr encephalitis during pregnancy. We also highlighted possible risk factors associated with increased newborns’ perinatal mortality and morbidity.
Methods
Searching strategy and review organization
We systematically reviewed the literature using the following search strategy: (“autoimmune encephalitis”/exp OR “autoimmune encephalitis”) AND (“fetal outcome”/exp OR “pregnancy”). The following electronic databases and data sources were systematically searched: MEDLINE (accessed through PubMed), Scopus, and Google Scholar. As per inclusion criteria, we evaluated all studies which (1) reported a confirmed diagnosis of anti-NMDAr encephalitis during pregnancy according to Graus’ criteria and (2) reported fetal and/or newborn outcomes. We included only papers written in English.
Results of this systematic review have been reported following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The quality of the included studies was assessed using the Newcastle–Ottawa Quality Assessment Scale (NOS). According to this scale, each study has been evaluated based on eight items, described as follows: (1) representativeness of the exposed cohort, (2) selection of the not exposed cohort, (3) ascertainment of exposure, (4) demonstration that outcome of interest was not present at the start of the study, (5) comparability of the cohorts included, (6) assessment of outcome, (7) adequate length of the follow-up, (8) adequacy of follow-up of cohorts. This score ranges from 0 to 9, and a quality score equal to or higher than three was considered acceptable.
Data collection
The following demographic and clinical information about the mother have been collected: age, gestational age, history of epilepsy, comorbidities, neurologic symptoms at AE onset, seizure characteristics (seizure type), status epilepticus (SE) characteristics, presence of an underlying tumor, EEG features, magnetic resonance image (MRI) findings, immunomodulatory therapy, ASM administered (number, and type), and surgery procedures performed.
Data on stillbirth, type of delivery (vaginal or cesarean), Apgar score 1 and 5 min after delivery, neonatal symptoms, and NMDAr antibodies dosage at birth were collected.
The data were recorded within a specialized Excel spreadsheet.
Statistics
Statistical analysis was performed on the final dataset containing all information pooled from the studies selected by our systematic review. Data were analyzed in IBM SPSS™; the normality of continuous data was checked via the Kolmogorov–Smirnov test. The Fisher chi-square test was employed to compare the perinatal outcome (born at term Vs preterm delivery; born at term Vs spontaneous abortion) according to maternal clinical and treatment features. Alpha level was set at 0.05 for statistical significance.
Results
Literature search
The literature search reported above yielded 156 articles. Seventy-five abstracts were excluded because they did not focus on anti-NMDAr AE during pregnancy or perinatal outcome or did not report individual patients’ data. Of the 138 records screened, the full texts of 63 articles were reviewed for eligibility (Fig. 1). Thirty-seven articles initially considered for possible inclusion were eventually excluded (excluded articles with reasons for exclusion are reported in Fig. 1), and twenty-six were finally included in our review [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33]. They included twenty-three case reports, and three case series (Table 1). According to the NOS evaluation, 13 articles were scored 5, 6 were scored 4, and 7 were scored 3 (Supp. Tab.1).
Maternal demographics and clinical features
A literature search showed thirthy-nine pregnant women with a median age of 25 years (range, 16–36 years). Twenty-one patients (21/39, 53.8%) presented anti-NMDAr encephalitis onset within the first trimester of pregnancy, whereas 17 patients (18/39, 46.2%) during the second one. The most common presenting symptoms included abnormal behaviors, movement disorders, autonomic disturbance, and seizures. According to seizure type, nineteen patients (19/39, 48.7%) had tonic–clonic generalized seizures, and six patients (4/39, 10.3%) had only focal seizures. Thirteen patients (13/39, 33.3%) presented status epilepticus and required intensive care management. The oncological evaluation revealed the presence of ovarian teratoma in seventeen patients (14/39, 35.9%). EEG analysis results were reported in 26 patients (26/39, 66.7%) and documented slow activity and interictal epileptic abnormalities in 23 cases (23/39, 59%), whereas ictal discharges and extreme delta brush in three patients (3/39, 7.7%). Brain MRI was normal in all cases except for 21 patients (21/39, 58.3%), who showed cerebellum, hippocampus, bilateral amygdala, basal ganglia, and insular cortex hyperintensity in T2-weighted MRI scans.
Immunotherapy was administered to thirty-five patients (36/39, 92.3.%). Thirty-five patients (35/39, 89.7%) received high oral or EV corticosteroid therapy, 26 mothers (26/39, 66.7%) were treated with IGEV, 6 with third-line treatments (i.e. RTX, cyclophosphamide, and azathioprine) (6/39, 15.4%), and 14 with plasmapheresis (14/39, 35.9%). ASM was administered in 25 (25/39, 64.1%) patients, with levetiracetam (LEV) and phenytoin (PHT) being the most used.
Extensive demographics and clinical information are listed in Table 1.
Perinatal outcomes
Data from 41 subjects in the perinatal period were evaluated. In two cases, the mothers suffered from a first episode of AE during a first pregnancy and a relapse during a second one. In 7 cases (7/41, 17.1%), a spontaneous interruption of the pregnancy was reported, whereas a voluntary interruption was reported in 3 (3/41, 7.3%) Of the 31 remaining alive subjects, 19 (19/31, 61.3%) were born from a cesarean section, and 10 (10/31, 32.3%) had a vaginal delivery. Nineteen subjects (18/31, 58.1%) experienced premature birth.
The APGAR score was available in 14 infants, showing a 5-min score within the normal range in 10. Serum anti-NMDAr antibody levels were tested in 7 cases (7/41, 17.1%) and found positive in 4. These patients showed perinatal complications, which mostly included neuromuscular and respiratory symptoms.
Above all the neurological manifestations reported, impaired neonatal reflexes (i.e., Moro, sucking, and grasping), cervical dystonia, strabismus, movement disorders, spina bifida, and seizures were the most reported. On the other hand, non-neurological symptoms mostly included respiratory depression, low birth weight, and supraventricular tachycardia 13.
Extensive information about perinatal outcomes is listed in Table 1.
Statistical analysis of the pooled data
According to the data analysis of single patients, no differences were observed between mothers who complete their pregnancies and those who experienced a spontaneous interruption (Table 2). However, a trend towards a reduced risk of abortion was also observed in women treated with IVIg (p = 0.06). In addition, a trend towards an increased risk of pre-term born was observed in mothers who underwent surgery procedures for teratoma removal (p=0.06) (Table 3).
Discussion
Anti-NMDAr encephalitis is the most frequent autoimmune encephalitis during pregnancy [34]. According to our data, in the perinatal period, newborns delivered by mothers suffering from anti-NMDAr AE may show neurological (i.e., non-finalistic limb movements, cervical dystonia, strabismus, spina bifida, impaired Moro reflexes, poor sucking and grasping) as well as non-neurological (i.e., respiratory distress, neonatal infection, icterus, hypoglycemia, low birth weight, and supraventricular tachycardia) sequelae (Fig. 2). All individuals developing perinatal symptoms presented positive serum NMDAr antibodies [12, 13, 22, 28]. This evidence supports the notion of a harmful maternal-to-fetal NR1 autoantibody transfer extensively described in preclinical models. However, further concurrent factors should be explored as a putative cause of newborns’ perinatal symptoms onset. In fact, several therapeutic interventions largely employed in AE management such as ASM and immunomodulant therapies (IMT) are associated with a great risk of perinatal complications.
According to the literature, ASM exposure during pregnancy may increase the rate of preterm birth, intrauterine growth restriction, low Apgar score, neonatal hypoglycemia and sepsis, respiratory distress, major congenital malformations (MCMs), and/or cognitive-behavioral impairment [35]. Specifically, some ASM like valproate acid (VPA), phenobarbital (PB), phenytoin (PHT), carbamazepine (CBZ), and topiramate (TPM) have been labeled as the most dangerous in terms of fetal harm. Thus, their use during pregnancy should be avoided. On the other hand, lamotrigine (LMT) and LEV seems to be associated with a very low rate of major congenital malformations (MCMs) and perinatal distress [36, 37]. Surprisingly, according to our results, newborns exposed to VPA during pregnancy mostly presented normal Apgar score, a low rate of miscarriage (3/9, 33.3%), and prematurity with complications (1/9, 11%). However, this data should be interpreted with caution in light of publication and reporting biases.
A solid set of evidence indicates that IMT may increase perinatal disorders in newborns [34]. Even though first-line IMT (i.e., corticosteroids, plasma exchange, and intravenous immunoglobulin) seem to be safe, second-line IMT (i.e., azathioprine, mycophenolate mofetil, cyclophosphamide, and rituximab) should be used with caution given the potential harmful profile towards fetal and newborns’ health. In line with this evidence, our study did not revealed a significantly increased risk of spontaneous pregnancy interruption in mothers who received treatment with RTX. RTX is a chimeric anti-CD20 monoclonal antibody that leads to depletion of B cells in humans, with consequent hypogammaglobulinemia. RTX can cross the placental barrier, and its use during pregnancy has been associated with neonatal transient lymphopenia and decreased gamma globulin levels.
A potential increase of pre-term birth was also described for women with ovarian teratoma who underwent subsequent surgical treatment. According to the literature, pregnant women suffering from cancer generally show an increased risk of abortion (i.e., 10% higher) than the general population. Furthermore, large epidemiological studies have shown that non-obstetric surgery in pregnant patients is associated with small, but real, increases in the risks of stillbirth, preterm delivery, and the need for cesarean section. This is mainly related to the anesthesia risk, the pre-operatory imaging, the development of changes in fetal hemodynamics, and the fetal surgical stress, still largely unknown [38,39,40,41,42]. However, a fetal monitoring during surgery, anesthesia between 4 and 20 gestational weeks, a regular patient follow-up with high-resolution ultrasonography, and attention to clinical symptoms and other signs were associated with a relatively safe non-obstetric surgery [38,39,40,41,42].
Conclusions
The management of pregnancy in women with anti-NMDAr encephalitis remains challenging. Our study depicted the potential outcomes of children born from mothers suffering from anti-NMDAr encephalitis and analyzed risk factors related to pregnancy and maternal disorders. To prevent complications that could harm the mother and the child, a personalized management should be enforced, targeting potential fetal risks related to anti-NMDAr encephalitis, autoantibodies, and therapy administered during pregnancy.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Huang Q, Xie Y, Hu Z, Tang X (2020) Anti-N-methyl-D-aspartate receptor encephalitis: A review of pathogenic mechanisms, treatment, prognosis. Brain Res 1727:146549. https://doi.org/10.1016/j.brainres.2019.146549
Kalam S, Baheerathan A, McNamara C, Singh-Curry V (2019) Anti–NMDAR encephalitis complicating pregnancy. Pract Neurol 19:131–135. https://doi.org/10.1136/practneurol-2018-002042
Liu H, Chen X (2021) Recurrent anti-NMDAR encephalitis during pregnancy combined with two antibodies positive. Arch Womens Ment Health 24:1045–1050. https://doi.org/10.1007/s00737-021-01124-5
Jurek B, Chayka M, Kreye J, Lang K, Kraus L, Fidzinski P et al (2019) Human gestational N -methyl- D -aspartate receptor autoantibodies impair neonatal murine brain function. Ann Neurol 86:656–670. https://doi.org/10.1002/ana.25552
Lamale-Smith LM, Moore GS, Guntupalli SR, Scott JB (2015) Maternal-fetal transfer of anti–N-methyl-D-aspartate receptor antibodies. Obstet Gynecol 125:1056–1058. https://doi.org/10.1097/AOG.0000000000000548
Jagota P, Vincent A, Bhidayasiri R (2014) Transplacental transfer of NMDA receptor antibodies in an infant with cortical dysplasia. Neurology 82:1662–1663. https://doi.org/10.1212/WNL.0000000000000384
Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T et al (2016) A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 15:391–404. https://doi.org/10.1016/S1474-4422(15)00401-9
Kalam S, Baheerathan A, McNamara C, Singh-Curry V (2019) Anti-NMDAR encephalitis complicating pregnancy. Pract Neurol 19(2):131–135. https://doi.org/10.1136/practneurol-2018-002042
Kim J, Park SH, Jung YR, Park SW, Jung DS (2015) Anti-NMDA receptor encephalitis in a pregnant woman. J Epilepsy Res 5(1):29–32. https://doi.org/10.14581/jer.15008
Mathis S, Pin JC, Pierre F, Ciron J, Iljicsov A, Lamy M, Neau JP (2015) Anti-NMDA receptor encephalitis during pregnancy: a case report. Medicine 94(26):e1034. https://doi.org/10.1097/MD.0000000000001034
Chan LW, Nilsson C, Schepel J, Lynch C (2015) A rare case of anti-N-methyl-D-aspartate receptor encephalitis during pregnancy. N Z Med J 128(1411):89–91
Lamale-Smith LM, Moore GS, Guntupalli SR, Scott JB (2015) Maternal-fetal transfer of anti-N-methyl-D-aspartate receptor antibodies. Obstet Gynecol 125(5):1056–1058. https://doi.org/10.1097/AOG.0000000000000548
Jagota P, Vincent A, Bhidayasiri R (2014) Transplacental transfer of NMDA receptor antibodies in an infant with cortical dysplasia. Neurology 82(18):1662–1663. https://doi.org/10.1212/WNL.0000000000000384
Lu J, Samson S, Kass J, Ram N (2015) Acute psychosis in a pregnant patient with Graves' hyperthyroidism and anti-NMDA receptor encephalitis. BMJ Case Rep 2015:bcr2014208052. Published 2015 Apr 22. https://doi.org/10.1136/bcr-2014-208052
Magley J, Towner D, Taché V, Apperson ML (2012) Pregnancy outcome in anti-N-methyl-D-aspartate receptor encephalitis. Obstet Gynecol 120(2 Pt 2):480–483. https://doi.org/10.1097/AOG.0b013e31825935d4
Kumar MA, Jain A, Dechant VE et al (2010) Anti-N-methyl-D-aspartate receptor encephalitis during pregnancy. Arch Neurol 67(7):884–887. https://doi.org/10.1001/archneurol.2010.133
Shahani L (2015) Steroid unresponsive anti-NMDA receptor encephalitis during pregnancy successfully treated with plasmapheresis. BMJ Case Rep 2015:bcr2014208823. Published 2015 Apr 29. https://doi.org/10.1136/bcr-2014-208823
McCarthy A, Dineen J, McKenna P et al (2012) Anti-NMDA receptor encephalitis with associated catatonia during pregnancy. J Neurol 259(12):2632–2635. https://doi.org/10.1007/s00415-012-6561-z
Ito Y, Abe T, Tomioka R, Komori T, Araki N (2010) Rinsho Shinkeigaku 50(2):103–107. https://doi.org/10.5692/clinicalneurol.50.103
Xiao X, Gui S, Bai P et al (2017) Anti-NMDA-receptor encephalitis during pregnancy: A case report and literature review. J Obstet Gynaecol Res 43(4):768–774. https://doi.org/10.1111/jog.13262
Liu H, Chen X (2021) Recurrent anti-NMDAR encephalitis during pregnancy combined with two antibodies positive. Arch Womens Ment Health 24(6):1045–1050. https://doi.org/10.1007/s00737-021-01124-5
Joubert B, García-Serra A, Planagumà J, Martínez-Hernandez E, Kraft A, Palm F, Iizuka T, Honnorat J, Leypoldt F, Graus F, Dalmau J (2020) Pregnancy outcomes in anti-NMDA receptor encephalitis: Case series. Neurol Neuroimmunol Neuroinflamm 7(3):e668. https://doi.org/10.1212/NXI.0000000000000668
Jung KO, Moon HJ (2020 Jun) A case of NMDAR encephalitis treated in the third trimester - novel arterial spin labeling findings and a review of literature. J Neuroimmunol 15(343):577235. https://doi.org/10.1016/j.jneuroim.2020.577235
Scorrano G, Dono F, Evangelista G, Chiarelli F, Anzellotti F (2023 Oct) Fetal outcome in anti-NMDAR encephalitis during pregnancy: a case report. Acta Neurol Belg 123(5):1989–1991. https://doi.org/10.1007/s13760-022-02020-0
Demma L, Norris S, Dolak J (2017) Neuraxial anesthesia in a patient with anti-N-methyl-D-aspartate receptor encephalitis in preg- nancy: management for cesarean delivery and oophorectomy. Int J Obstet Anesth 31:104–107. https://doi.org/10.1016/j.ijoa.2017.05.006
Tailland M, Le Verger L, Honnorat J, Biquard F, Codron P, Cassereau J (2020) Post-herpetic anti-N-methyl-d-aspartate receptor encephalitis in a pregnant woman. Rev Neurol (Paris) 176(1–2):129–131. https://doi.org/10.1016/j.neurol.2019.01.402
Lu YT, Hsu CW, Tsai WC, Cheng MY, Shih FY, Fu TY et al (2016) Status epilepticus associated with pregnancy: a cohort study. Epilepsy Behav 59:92–97. https://doi.org/10.1016/j.yebeh.2016.03.034
Chourasia N, Watkins MW, Lankford JE, Kass JS, Kamdar A (2018) An infant born to a mother with anti-N-methyl-d-aspartate re- ceptor encephalitis. Pediatr Neurol 79:65–68. https://doi.org/10.1016/j.pediatrneurol.2017.11.010
Ueda A, Nagao R, Maeda T, Kikuchi K, Murate K, Niimi Y, Shima S, Mutoh T (2017) Absence of serum anti-NMDAR antibodies in anti-NMDAR encephalitis mother predicts having healthy newborn. Clin Neurol Neurosurg 161:14–16. https://doi.org/10.1016/j.clineuro.2017.07.012
Zhang S, Yang Y, Long T, Li Z (2021) Systemic lupus erythematosus associated with recurrent anti-NMDA receptor encephalitis during pregnancy. Arch Womens Ment Health 24(3):525–528. https://doi.org/10.1007/s00737-020-01088-y
Liao Z, Jiang X, Ni J (2017) Anesthesia management of cesarean sec- tion in parturient with anti-N-methyl-D-aspartate receptor encephalitis: a case report. J Anesth 31(2):282–285. https://doi.org/10.1007/s00540-016-2304-0
Kokubun N, Komagamine T, Hirata K (2016) Pregnancy and delivery in anti-NMDA receptor encephalitis survivors. Neurol Clin Pract 6(5):e40–e43. https://doi.org/10.1212/CPJ.0000000000000229
Mizutamari E, Matsuo Y, Namimoto T, Ohba T, Yamashita Y, Katabuchi H (2016) Successful outcome following detection and removal of a very small ovarian teratoma associated with anti-NMDA receptor encephalitis during pregnancy. Clin Case Rep 4(3):223–225. https://doi.org/10.1002/ccr3.475
Dono F, Consoli S, Tappatà M, Evangelista G, Russo M, Lanzone J, Pozzilli V, Nucera B, Rinaldi F, Di Pietro M, Tinti L, Troisi S, Calisi D, D'Apolito M, Narducci F, Assenza G, Anzellotti F, Brigo F, Vollono C et al (2023 Dec) Autoimmune encephalitis during pregnancy: A diagnostic and therapeutic challenge-A systematic review with individual patients' analysis and clinical recommendations. Epilepsia Open 8(4):1221–1240. https://doi.org/10.1002/epi4.12806
Li Y, Meador KJ (2022) Epilepsy and pregnancy. CONTINUUM: Lifelong learning. Neurology 28:34–54. https://doi.org/10.1212/CON.0000000000001056
Tomson T, Battino D, Bromley R, Kochen S, Meador K, Pennell P et al (2019) Management of epilepsy in pregnancy: a report from the international league against epilepsy task force on women and pregnancy. Epileptic Disord 21:497–517. https://doi.org/10.1684/epd.2019.1105
Moise AC, Gerard EE (2023) Antiseizure medications in pregnancy. Obstet Gynecol Clin North Am 50:251–261. https://doi.org/10.1016/j.ogc.2022.10.014
Mizutamari E, Matsuo Y, Namimoto T, Ohba T, Yamashita Y, Katabuchi H (2016) Successful outcome following detection and removal of a very small ovarian teratoma associated with anti- NMDA receptor encephalitis during pregnancy. Clin Case Rep 4:223–225. https://doi.org/10.1002/ccr3.475
Koo F-H, Wang K-C, Chen C-Y, Chang W-H, Yeh C-C, Yang M-J et al (2013) An 11-year experience with ovarian surgery during pregnancy. J Chin Med Assoc 76:452–457. https://doi.org/10.1016/j.jcma.2013.04.008
Demma L, Norris S, Dolak J (2017) Neuraxial anesthesia in a patient with anti-N-methyl-D-aspartate receptor encephalitis in pregnancy: management for cesarean delivery and oophorectomy. Int J Obstet Anesth 31:104–107. https://doi.org/10.1016/j.ijoa.2017.05.006
Reedy MB, Källén B, Kuehl TJ (1997) Laparoscopy during pregnancy: A study of five fetal outcome parameters with use of the Swedish Health Registry. Ame J Obstet Gynecol 177:673–679. https://doi.org/10.1016/S0002-9378(97)70163-7
Liao Z, Jiang X, Ni J (2017) Anesthesia management of cesarean section in parturient with anti-N-methyl-d-aspartate receptor encephalitis: a case report. J Anesth 31:282–285. https://doi.org/10.1007/s00540-016-2304-0
Funding
Open access funding provided by Università degli Studi G. D'Annunzio Chieti Pescara within the CRUI-CARE Agreement. This work has been supported by non-profit agencies, the Italian Department of Health (RF-2013–02358785 and NET-2011–02346784-1), the AIRAlzh Onlus (ANCC-COOP), European Union’s Horizon 2020 Research and Innovation Programme under the Marie Skłodowska-Curie grant agreement iMIND (grant no. 84166), the Alzheimer’s Association—Part the Cloud: Translational Research Funding for Alzheimer’s Disease (18PTC-19–602325), and the Alzheimer’s Association—GAAIN Exploration to Evaluate Novel Alzheimer’s Queries (GEENA-Q-19–596282).
Author information
Authors and Affiliations
Contributions
FD and GS contributed to the conception and design of the study. GS, GE, SC, and CC organized the database. ADI performed the statistical analysis. FD, GS, and SLS wrote the manuscript and supervised all the data. All authors contributed to manuscript revisions and read and approved the submitted version.
Corresponding authors
Ethics declarations
Ethics approval
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Scorrano, G., Dono, F., Corniello, C. et al. Perinatal outcome in anti-NMDAr encephalitis during pregnancy—a systematic review with individual patients’ data analysis. Neurol Sci (2024). https://doi.org/10.1007/s10072-024-07448-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10072-024-07448-1