Answer
362.1k+ views
Hint: Shape of orbitals are essentially the share of electron wave shapes and are named as s, p, d, f basis of the observations of line spectra. Also, rather than saying that the electron wave has an s-shape, we say electron is available in s-orbital
Complete step by step answer:
As we know that the electron wave shapes are chosen based on observations of line spectra. The words s, p, d, f were chosen based on the observations as certain lines were observed as “sharp”, “principal”, “diffuse’, or “fundamental” series. Since, electrons exhibit both particle and wave nature, instead of saying “the electron wave has a d shape” we say “the electron is in a d orbital”.
Orbital is essential as the wave function of an electron and is defined by the three quantum numbers energy (n), angular momentum (l), and Magnetic moment (ml) and it describes the region in space having a high probability of finding the electron.
In the case of d-orbital, l=2 and the first principal shell to have a d subshell corresponds to n=3. The ml values for five d orbitals are -2, -1, 0, +1, and +2 i.e., we can say d-subshell has five orientations. All these d-orbitals have the same energy and are called degenerate orbitals. The shape of the d-orbitals is given below:
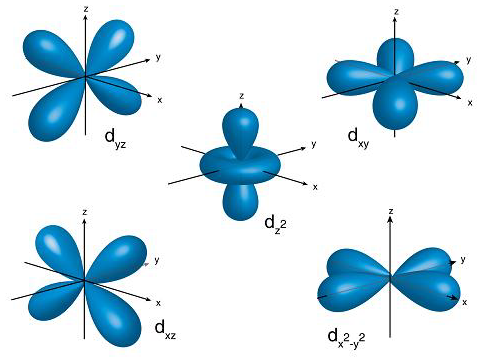
Hence, we can say d-orbitals have double dumbbell-shaped.
So, the correct answer is Option C.
Note: The maximum number of electrons for any subshell can be calculated using the azimuthal quantum number (l) is $2(2l + 1)$. So for the case of s-orbital l=0 and hence it can have only 2 electrons. For the case of l-orbital, l=1 and the maximum number of electrons is 6, similarly, for d-orbital, l=2 and maximum 10 electrons can be present, and p-orbital with l=3 can hold maximum 14 electrons
Complete step by step answer:
As we know that the electron wave shapes are chosen based on observations of line spectra. The words s, p, d, f were chosen based on the observations as certain lines were observed as “sharp”, “principal”, “diffuse’, or “fundamental” series. Since, electrons exhibit both particle and wave nature, instead of saying “the electron wave has a d shape” we say “the electron is in a d orbital”.
Orbital is essential as the wave function of an electron and is defined by the three quantum numbers energy (n), angular momentum (l), and Magnetic moment (ml) and it describes the region in space having a high probability of finding the electron.
In the case of d-orbital, l=2 and the first principal shell to have a d subshell corresponds to n=3. The ml values for five d orbitals are -2, -1, 0, +1, and +2 i.e., we can say d-subshell has five orientations. All these d-orbitals have the same energy and are called degenerate orbitals. The shape of the d-orbitals is given below:

Hence, we can say d-orbitals have double dumbbell-shaped.
So, the correct answer is Option C.
Note: The maximum number of electrons for any subshell can be calculated using the azimuthal quantum number (l) is $2(2l + 1)$. So for the case of s-orbital l=0 and hence it can have only 2 electrons. For the case of l-orbital, l=1 and the maximum number of electrons is 6, similarly, for d-orbital, l=2 and maximum 10 electrons can be present, and p-orbital with l=3 can hold maximum 14 electrons
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Three beakers labelled as A B and C each containing 25 mL of water were taken A small amount of NaOH anhydrous CuSO4 and NaCl were added to the beakers A B and C respectively It was observed that there was an increase in the temperature of the solutions contained in beakers A and B whereas in case of beaker C the temperature of the solution falls Which one of the following statements isarecorrect i In beakers A and B exothermic process has occurred ii In beakers A and B endothermic process has occurred iii In beaker C exothermic process has occurred iv In beaker C endothermic process has occurred

What is the stopping potential when the metal with class 12 physics JEE_Main

The momentum of a photon is 2 times 10 16gm cmsec Its class 12 physics JEE_Main

How do you arrange NH4 + BF3 H2O C2H2 in increasing class 11 chemistry CBSE

Is H mCT and q mCT the same thing If so which is more class 11 chemistry CBSE

Trending doubts
Difference between Prokaryotic cell and Eukaryotic class 11 biology CBSE

Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Give 10 examples for herbs , shrubs , climbers , creepers

Change the following sentences into negative and interrogative class 10 english CBSE

Fill the blanks with proper collective nouns 1 A of class 10 english CBSE

What is pollution? How many types of pollution? Define it

How do you solve x2 11x + 28 0 using the quadratic class 10 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE



