Abstract
Root-knot nematodes (RKN), Meloidogyne spp. are the most agricultural pest causing quality, yield crop losses, and increased host sensitivity to abiotic and biotic stress worldwide. For decades, the control of these parasitic nematodes was largely based on the application of nematicides. Recently, many restrictions were set on the use of nematicides due to environmental and human health concerns. Therefore, developing alternative control strategies is becoming increasingly needful to manage root-knot nematodes. Recently, biocontrol is attracting attention as a safer alternative to chemical products. It involves a wide range of control approaches including cultural practices, stimulation or introduction of antagonistic organisms, their metabolites, or their bioactive compounds. The present review aims to point out the principal biological approaches to deal with root-knot nematodes. Firstly, an informative overview of these microscopic plant-parasitic nematodes (PPN) will be provided. Then, a comparison of the main cultural practices with their potential uses, their advantages, and limitations in the management of Meloidogyne spp. will be discussed. In addition, the antagonistic activity of plant essential oils as well as that of nematophagous bacteria and fungi against Meloidogyne spp. will be described. Their nematicidal effect is discussed as they have been proven as an efficient biological control agent. The present review is a benefit to all persons interested in alternative methods for Meloidogyne spp. management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The world agricultural production intended for human consumption is widely at risk due to the incidence of crop pathogens and pests. These harmful organisms reduce food production and affect plants either by direct or indirect way and cause huge losses (Sikora et al. 2021). Roughly, they are responsible for serious losses estimated at about 20 and 40% of global agricultural productivity (Oerke 2006; Savary et al. 2012). Plant-parasitic nematodes (PPN) are the most damaging plant pathogens all over the world (Abd-elgawad and Askary 2015). PPN affect plant growth and cause yield losses ranging between 8.8 to 14.6% of the crop productivity corresponding to 100 to 157 billion US dollars annually (Ali et al. 2014). Meloidogyne spp. are the most important PPN in terms of economic cost and scientific focus due to their considerable economic damages on a large host plant species in different environments (Perry et al. 2010). RKN have been classified in the top ten of the list-emerging PPN as reviewed by Jones et al. (2013). RKN belong to Meloidogyne genus, with more than 98 species (Dong et al. 2012; Jones et al. 2013). Among them, Meloidogyne incognita, Meloidogyne javanica, Meloidogyne arenaria, and Meloidogyne hapla represent 95% of all RKN populations (Dong et al. 2012). In Africa, 22 Meloidogyne species were identified in which M. javanica and M. incognita and M. arenaria are regarded as the dominant species. These three species are attacking the most economically important crops like tomato, maize, cowpea, banana, potato, and sweet potato (Onkendi et al. 2014). These nematodes have been named “root-knot nematodes” for the swellings (galls or knots) that are the characteristic symptoms on the infected roots (Subedi et al. 2020). As obligate plant parasites, RKN cause also atypical symptoms which are similar to those resulting from many other biotic and abiotic stresses such as swollen roots and reduced root systems. Moreover, their damages involve also delayed plant growth, leaves chlorosis, yellowing, wilting, and eventual loss of foliage (Caillaud et al. 2008; Onkendi et al. 2014; Favery et al. 2016; Liu and Park 2018; Verdejo-Lucas and Talavera 2019). Consequently, plant susceptibility towards other pathogens and stresses (Kyndt et al. 2013).
Indeed, RKN control is more challenging than other plant pathogens owing to their obligately endoparasitic nature, their ability to attack the underground parts of the plants by feeding only from living plant root cells, their broad host range, their high reproductive rate, and their short generation time (Affokpon et al. 2011; Quentin et al. 2013; Palomares-Rius et al. 2017). Their management is mainly achieved by the use of chemical nematicides. However, these last present a serious risk for both environment and human health due to misuse, overutilization, and their long-lasting persistence in soil and groundwater which may affect negatively all food chains (Jatala 1986; Schneider et al. 2003; Ntalli and Menkissoglu-Spiroudi 2011). In this respect, in recent years, both scientists and other stakeholders are focusing on eco-friendly alternative tools that may help in RKNs management without harming non-targeted organisms (Degenkolb and Vilcinskas 2016). Thus, the present review describes the main agricultural practices and, their effect in terms of Meloidogyne management. In addition, it presents the main research and advances dealing with the use of plant essential oils and microorganisms for the control of root-knot nematodes with a specific focus on Meloidogyne incognita and Meloidogyne javanica.
Background on Meloidogyne spp.
The genus Meloidogyne is an obligate sedentary endoparasite of plants and a serious pathogen of crops worldwide in all temperate and tropical areas (Abad and Williamson 2010). RKNs are extremely polyphagous infecting a wide range of species belonging to all botanical families (Jones et al. 2013). The sexual maturity of Meloidogyne shows a distinct dimorphism (Castagnone-Sereno et al. 2013). Females are pear-shaped distended bodies and males are vermiform in shape and motile (Eisenback and Triantaphyllou 1991; Truong et al. 2015). Meloidogyne spp. are known to show different varieties of reproductive strategies, varying between amphimixis and facultative meiotic parthenogenesis or obligatory mitotic parthenogenesis. Most species are parthenogenetic and males are only formed under unfavorable conditions, e.g., when there is higher population density and limitation in nutrients. Meloidogyne incognita, Meloidogyne javanica, and Meloidogyne arenaria are instances of these nematodes, which are obligatory mitotic parthenogenetic species (Castagnone-Sereno et al. 1993). There are cross-fertilizing species which have balanced sex ratios such as Meloidogyne carolinensis and Meloidogyne spartinae. However, Meloidogyne hapla can reproduce by both cross-fertilization and meiotic parthenogenesis (Perry et al. 2010; Liu and Park 2018). For most Meloidogyne species, adult males are largely functionless and Meloidogyne spp. reproduction is almost always parthenogenetic (James et al. 2019).
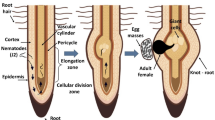
A life cycle of Meloidogyne spp. (Fig. 1) takes 3–4 weeks under optimal conditions, depending on nematode species, type of host and soil, and weather conditions (Stirling et al. 2002). Many plant nematodes complete their life cycle in about 4 weeks, during summer months when the soil temperature is high (Singh and Phulera 2015). Meloidogyne spp. are characterized by four juvenile stages that undergo four molts before achieving the adult stage (male or female) (Abad and Williamson 2010). The first-stage juvenile (J1) develops within the egg and molts once to become the second-stage juvenile (J2), which emerges from the egg. The second-stage juvenile, the only infectious form, is attracted by the root plant: chemotaxis (Zuckerman 1984). This phenomenon is the key means for locating a host plant by RKNs and it could happen as different forms of stimuli and signal gradients of chemical, volatile and non-volatile exudates (Bais et al. 2006; Hamada et al. 2020; Oota et al. 2020). All those factors play an important role in sensing the host (Hassan and Mathesius 2012; Hida et al. 2015; Kaloshian and Teixeira 2019; Tsai et al. 2019). Nevertheless, the chemotactic movement differs in rate for species due to variation in genetic mobility (Kaloshian and Teixeira 2019). After the attraction of the nematode to the host root, J2 initiates the parasitic relationship and penetrates the elongation zone of the host root, using its stylet, the needle like-structure. Meloidogyne spp. produce principally a mixture of hydrolytic enzymes and inject them into plant cells. They are cell wall-degrading enzymes such as endoglucanase, endoxylanase, pectatelyase, amylase, cellulase, and pectinase (Hussey 1989; Béra-Maillet et al. 2000; Jones and Goto 2011; Perry and Moens 2011; Malinovsky et al. 2014). These cellulolytic and pectinolytic enzymes are secreted from specialized esophageal gland cells and can degrade the structural plant cell walls for migration within their tissues (Gheysen and Fenoll 2002; Huang et al. 2005; Davis et al. 2008). In addition, these secretions are implied in the formation and maintenance of the nematode feeding sites (NFS), which are multinucleate and hypertrophied feeding cells called ‘‘giant cells” (Huettel 1986; Abad and Williamson 2010; Jones et al. 2013; Truong et al. 2015). These cells constitute the sole source of nutrients to the nematode (Rodiuc et al. 2014; Favery et al. 2016; Mejias et al. 2019). The J2 remains at the feeding site within the gall and develops into the adult stage (male or female) after three more molts (Abad and Williamson 2010). Both J3 and J4 do not feed as they are devoid of the stylet and their lifespan is short (no more than 4–6 days) (Moens et al. 2009; Jones et al. 2013). Males leave the root and stand free in the rhizosphere or near the female body (James et al. 2019). Female adults are generally embedded within host roots. They lay eggs in a gelatinous sac composed of a glycoprotein matrix, which is attached to the females’ posterior, in order to protect them against extreme conditions and predators. Egg masses may be found on the surface of galled roots, or embedded within the gall tissue (Moens et al. 2009; Perry et al. 2010; Jones et al. 2013).
Life cycle of Meloidogyne spp.(Stirling and Reay 2002)
Management of root-knot nematodes
Cropping practices
Since a long time ago, cropping practices had been among the most strategies used for the management of plant-parasitic nematodes. However, they have been rekindled a great interest in the recent few years due to the overuse of chemical pesticides and the need to find safer alternatives.
For an effective integrated management, it is vitally important to gather information about the plant infecting nematode, before proceeding to a management practice. Information include nematode species, favorable development conditions, population level, and the host plant (Hill 1988).
Crop rotation
Crop rotation is the most practical activity for managing Meloidogyne spp. and among the oldest one (Trivedi and Barker 1986; Chen and Tsay 2006). It is a practice of growing different types of crops, susceptible host plants with non-host, in succession on the same field (Thenail et al. 2009; Ansari et al. 2020). Rotation is considered good only when resistant or tolerant plants are used in the rotation cycle (Ansari et al. 2020). This practice serves to disturb the organism life cycle and allows a sufficient period after each hosting crop to maintain the nematode below a damaging level before the next culture (Trivedi and Barker 1986; Baldwin 2006). Also, it improves the effectiveness of control based on resistance to parasitic nematodes in plants (Gullino and Albajes 2001). Indeed, many considerations must be taken into account when planning a rotation cycle. It must be carefully selected so that it keeps maintaining beneficial soil microorganisms, soil fertility, and its structure (Thenail et al. 2009). Moreover, the economic benefit of the rotation cycle is estimated to be important (Hill 1988). For example, one of the most common integrated rotation plans for the management of Meloidogyne hapla is corn, wheat, barley, and other small grains (Hill 1988). Pankaj et al. (2010) affirmed that the infestation with Meloidogyne sp. can be reduced by crop rotation with non-host crops like mustard, jute, and chickpea. Unfortunately, plant resistance or tolerance to nematodes is species dependent, and consequently, this management approach seems to be limited when different nematode species are present. Hence, the selection of a practical rotation cycle is challenging (Barker 1991; Viaene et al. 2013). Keeping this in mind, getting information about species and the host spectrum of targeted nematode before elaborating the rotation schema is a must (Ansari et al. 2020). In general, for economically important plant-parasitic nematodes worldwide, at least a 4-year rotation cycle of cultivating non-host plants is necessary for a significant attenuation of nematode density (Trivedi and Barker 1986).
Fallowing
Fallowing is one of the main cultural practices which are used in the management of PPNs by starvation (Trivedi and Barker 1986). It is a farming practice in which no crop (including weeds) is planted for a period of time to ensure that the PPNs do not find hosts (Viaene et al. 2013; Nielsen and Calderón 2015; Ansari et al. 2020). Fallowing is a very effective method especially when RKNs are in the most critical stage of their life cycle because they cannot complete it during host absence. Thus, the fallowing aims to stress the nematode and reducing its population density (Hill 1988). Nevertheless, fallowing has adverse effects on soil degradation, erosion, quality, and formation of its organic matter and also affects the growth of microorganisms that need vegetation. Besides, fallowing is less efficient or even inefficient under certain conditions such as dry weather. An additional limitation is the negative impact due to loss of production (Hill 1988; Ansari et al. 2020).
Flooding
Flooding soil might limit and destroy RKNs multiplication due to the high moisture level, soil anaerobic conditions, low pH, and the development of toxic substances (Trivedi and Barker 1986). To ensure that flooding will lead to nematodes’ death, the soil must be flooded for a long time. Besides, this measure can be used only in fields that are not sloping and where water is not a limiting factor. For this reason, this method is not a practical strategy in many countries due to water deficiency. Another downside is that the inundated field will be exempted from agricultural activities for a long period, and will become not productive (Viaene et al. 2013). According to Trivedi and Barker (1986), only 23% of root-knot nematode juveniles can survive over 2 to 5 days in saturated soil. A study of Sikora et al. (2005) revealed that RKNs damage was undetectable on the paddy rice fields where the soil was flooded for 3 months.
Trap cropping
Trap cropping is mostly used for targeting Meloidogyne spp. (Koch et al. 1998; Scholte 2000; Westerdahl 2011). It is a practice based on planting a susceptible host plant characterized by quick and extensive root system growth in an infested soil for a short period of time (Sikora et al. 2005; Westerdahl 2011). Larvae of the nematode invade the roots and complete its cycle of development. Thereafter, nematodes are trapped inside the root tissues, destructing plant by tillage, before nematodes within the root reach maturity and start reproduction (Westerdahl 2011). The trap crop can be any nematode susceptible plant easily available to a cultivator, but it should be grown very densely in a nematode-infested soil to be sure that a maximum of individuals reach host roots and enter within it (Trivedi and Barker 1986). Carrots, tomatoes, and beans are believed to be a good choice for this purpose (Westerdahl 2011). Moreover, potato is considered as the best trapping crop for the management of Meloidogyne hapla (Scholte 2000).
A good management by trap cropping depends on effective cultivation methods, timing, and destroying completely the susceptible crop (Viaene et al. 2013). However, when destroying the trap crops is done tardy, the nematode life cycle could be completed and its increases (Westerdahl 2011).
Resistant plant
An efficient measure against root-knot nematodes is the use of resistant varieties. These do not allow the development of nematode density while plants could grow normally (Roberts 2003; Noling 2016). Indeed, with resistant plants, the targeted nematode may be managed similarly as or better than with chemical products (Fassuliotis 1985). Another benefit is that after harvesting the resistant variety, the nematode density is reduced, and it might be possible growing a susceptible crop without using other control measures (Westerdahl 2011).
The major disadvantage of using nematode-resistant plants is that they are not always available for several cropping systems. Also, the production of resistant varieties is time-consuming. Resistant varieties to a wide scope of Meloidogyne species have been developed for tomato, pepper, snap bean, sweet potato, sweet corn, cowpea, potato, carrot, soybean, and tobacco (Hill 1988; Westerdahl 2011).
Biofumigation
Biofumigation is a promising cultural practice that has attracted the attention of the research community. It has been viewed as an effective alternative method to the use of chemical pesticides (Dutta et al. 2019). The concept of this method is based on the suppressive effects of Brassica species on plant-nematodes by biocidal compounds, mainly isothiocyanates. These plants produce glucosinolates that are hydrolyzed by myrosinase isoenzymes in soil and converted into biocidal isothiocyanates, the active ingredient in the most commonly used nematicides (Sikora et al. 2005; Matthiessen and Kirkegaard 2006; Motisi et al. 2010). Moreover, this technique efficacy could be improved by the addition of a trap (Stapleton and Duncan 1998). Additionally, soil amended with fresh or dried residues of the cruciferous plant at 38 °C daytime and 27 °C night temperatures decreased the Meloidogyne incognita galling by 95–100% after 7 days of incubation in controlled environmental tests (Stapleton and Duncan 1998). It could delay the biofumigant persistence in the soil (Westerdahl 2011).
Organic amendment
Many studies were done to evaluate the effect of organic amendments in controlling RKN. This strategy is considered as a promising alternative practice to enhance soil quality, crop production, and diseases suppression of soil-borne pathogens such as root-knot nematode (Bonilla et al. 2012). In addition to their high content in organic matter, organic amendments contain substances resulting from animal and plant residues which have biocide activity (Collange et al. 2011). The mechanism of action by which organic amendments act on nematodes is done following three principal processes: (1) enhancing tolerance to nematode damage and increasing soil nutrient retention and water holding capacity, (2) improving specific compounds having bioactivity against nematodes, and (3) activating the microbial activity of soil microorganisms. Thus, there is indirect stimulation of antagonist organisms (Collange et al. 2011). However, there is a high variability in the effectiveness of suppression depending on the nature of the amendment, the crop, the pathogen, and the environmental conditions (Bonilla et al. 2012).
Nematicidal effect of plant essential oils and their compounds
Essential oils (EOs), also called volatile or ethereal oils, are complex mixtures of odorous, natural volatile compounds found in more than 3000 plants and they are responsible for their distinctive flavor or scent. EOs are secondary metabolites that have a powerful role in plant defense mechanisms against plant pathogens. They could be obtained from different parts of the plant like flowers, stems, buds, seeds, leaves, wood, fruits, and roots (Van de Braak and Leijten 1999; Bakkali et al. 2008; Figueiredo et al. 2008; Christaki et al. 2012; Negi 2012).
For a long time, many studies showed that EOs and their main constituents have biological activity against bacteria, fungi, and viruses, as well as cytotoxic activity against PPN (Avato et al. 2000; Isman 2000; Bakkali et al. 2008; Echeverrigaray et al. 2010; Andrés et al. 2012; Sharar et al. 2017). Moreover, the effect of EOs from different plant species on Meloidogyne spp. was extensively investigated. These experiments were conducted both in vitro and in vivo (Isman 2000; Bakkali et al. 2008). Instances of studied plant families include Lamiaceae, Apiaceae, Myrtaceae, and Rutaceae; with species of the genera Thymus, Rosmarinus, Artemisia, and Mentha (Table 1). For example, an in vitro test of Eupatorium viscidum at 1000 ppm caused mortality of 100% Meloidogyne javanica second-stage juveniles (Sosa et al. 2012). Moreover, Artemisia herba-alba and Rosmarinus officinalis inhibited more than 94%, and 98% of Meloidogyne incognita respectively in an in vitro study (Avato et al. 2017).
EOs include many nematicide compounds like terpenes, terpenoids, sesquiterpenes, and phenols (Table 2). Constituents with hydroxyl and carbonyl groups are the most active such as geraniol, carvacrol, and thymol (Echeverrigaray et al. 2010; Ntalli et al. 2010; Andrés et al. 2012; Santana et al. 2014; Avato et al. 2017). Thymol is the most effective nematicide compound of both Meloidogyne incognita and Meloidogyne javanica (Ntalli et al. 2010; Santana et al. 2014; Avato et al. 2017). For instance, thymol caused 100% mortality of Meloidogyne javanica J2 at 500 ppm concentration (Santana et al. 2014). Other studies showed that EOs and their compounds act on egg-hatching inhibition of Meloidogyne spp. by penetrating the gelatinous matrix (Oka 2001; Santana et al. 2014; Laquale et al. 2018). Bakkali et al. (2008) suggested that the nematoxic activity of essential oils is mainly attributed to their chemical compositional profile, mostly the main compounds (Oka et al. 2000; Sacchetti et al. 2005; Ntalli et al. 2010; Ntalli and Menkissoglu-Spiroudi 2011; Laquale et al. 2015). According to Avato et al. (2017), the bioactivity of EOs mainly relies on the type and amount of active constituents. Other authors affirmed that both major and minor compounds are involved in EOs’ bioactivity and act synergistically or antagonistically. The contribution of further minor compounds is necessary to achieve the full toxic effect of the EOs (Ntalli et al. 2010; Santana et al. 2014; Laquale et al. 2018).
On another hand, it must be noted that EO’s nematicidal effect may also rely on the extraction method (Laquale et al. 2018) and the application technique (Avato et al. 2017). For example, when applying directly to the soil, the EOs of Artemisia herba-alba and Rosmarinus officinalis displayed a significant suppressive effect on Meloidogyne incognita in comparison with the in vitro test, and the fumigation was revealed to be the most effective method because of the high volatility of their active constituents (Laquale et al. 2015). Indeed, the mechanism of action of EOs and their constituents against RKNs is still unclear. However, many hypotheses were suggested in this regard. It was demonstrated that EOs cause inhibition of AChE activity which is a necessary enzyme for the degradation of acetylcholine (Ach), the main neurotransmitter in the nervous system of nematodes (Oka 2001). Thus, acetylcholine concentration increases and leads to the death of the nematode. Besides, EOs may disrupt the cell membrane and change irreversibly its permeability by changing protein structures of nematode surface like formaldehyde and other aldehydes (Oka et al. 2000; Oka 2001). Another mechanism of action of EOs is the production of intracellular reactive oxygen species (ROS) in nematode cells, which leads to severe oxidative damages and the activation of the apoptotic pathway (Kalaiselvi et al. 2019).
Microbial control of RKN
Nematodes in soil and rhizosphere zone are subjected to microorganism infections including bacteria and fungi through antagonistic behavior. Hence, soil bacteria and fungi might be used as potential biocontrol agents (BCAs) for plant-parasitic nematodes (Tian et al. 2007; Almaghrabi et al. 2013; Askary and Martineli 2015). Soil microorganisms are considered as the most efficient natural eco-friendly way for controlling PPN (Borah et al. 2018).
Nematophagous bacteria as biocontrol agents of RKNs
Nematophagous bacteria are natural antagonists suppressing root-knot nematodes by diverse mechanisms of action. Their antagonistic effect involves parasitizing and producing nematicidal toxins. In addition, they can control nematode by interfering with nematode-plant-host recognition, competing for nutrients, and promoting plant health. The antagonistic bacteria can also increase host defense mechanisms of the infested plant by inducing systemic resistance of host plants (Siddiqui and Mahmood 1999; Tian et al. 2007; Dihingia et al. 2017; Abd-Elgawad and Askary 2018; Uysal and Özdemir 2020). This section discusses different mechanisms implied by nematophagous bacteria towards the control Meloidogyne spp. Many in vitro and in vivo studies on RKN control by bacteria demonstrated promoting results (Table 3).
Bacteria parasitizing root-knot nematodes
One of the most important bacteria parasitizing root-knot nematodes is Pasteuria spp. They are obligate, mycelial, endospore-forming bacterial parasites of most plant-parasitic-nematodes (Sayre and Wergin 1977; Sayre and Starr 1985; Starr and Sayre 1988). They are parasitic to over 200 nematode species belonging to more than 90 genera (Mateille et al. 2002). Pasteuria spp. are widely present in the world and they were reported in at least 51 countries (Chen and Dickson 1998; Siddiqui and Mahmood 1999; Bird et al. 2003) and they have been reported from 323 nematodes including plant-parasitic nematodes (Stirling 2011).
For a long time, many species in this genus were regarded as promising biocontrol agents against Meloidogyne spp. on various annual and perennial crops (Mateille et al. 2002). Furthermore, Meloidogyne spp. is mostly attacked by Pasteuria penetrans which presents great specificity for this genus whether for one specie of Meloidogyne spp. or intra-specifically (Stirling 1985; Bird et al. 1990; Oostendorp et al. 1990; Iftikhar et al. 2020).
These microorganisms act against Meloidogyne spp. either by attaching to the juvenile cuticle or parasitizing female eggs (Sayre and Wergin 1977; Bishop et al. 2007; Kariuki and Dickson 2007; Davies 2009). They produce dome-shaped non-motile spores (4 um in diameter) that attach to the cuticle of infective second-stage juveniles (Mateille et al. 2010; Iftikhar et al. 2020). A germ spore tube penetrates the cuticle after the juvenile has entered the plant root and vegetative microcolonies form and begin feeding through the body of the developing female. Many hundreds of Pasteuria penetrans spores may parasite one single nematode. Bacteria inhibit egg production and,sporulate inside the female body. Hence, instead of producing eggs, female produces a lot of mature endospores (Bird 1986; Iftikhar et al. 2020). Then, Pasteuria penetrans can decrease nematode infection of roots by sterilizing females and producing high spore densities in soil once the nematode cuticle is ripped. Thus, the second stage juveniles become heavily encumbered with spores, and motility is restricted (Sayre and Wergin 1977; Davies et al. 1991). Consequently, the nematode becomes sterile and fails to reach the host root (Iftikhar et al. 2020). The endospores are released into the soil for beginning a new cycle upon decomposition of the root parasitized by females and they may persist in soil for many years because of their resistance to environmental stresses like heat and drought (Giannakou et al. 1997; Chen and Dickson 1998).
At low spore densities, Pasteuria penetrans suppress nematode infection mainly by inhibiting the egg female production. Whereas, at high spore densities, the nematode is inhibited by J2 encumbered with spores (Timper 2009). Attachment of Pasteuria to the nematode cuticle is due to the presence of fibers surrounding the Pasteuria spore surface (Sayre and Wergin 1977; Stirling et al. 1986; Persidis et al. 1991; Davies and Opperman 2006; Mouton et al. 2009; Srivastava et al. 2018; Mhatre et al. 2020).
Bacteria producing nematicidal toxin
Bacillus thuringiensis (Bt) is a spore-forming bacterium that produces a large variety of crystal (Cry) and cytolytic (Cyt) toxins (also named δ-endotoxins) during the sporulation phase of its growth cycle (Schnepf et al. 1998). These bacteria show high toxicity against insects belonging to Lepidoptera, Diptera, and Coleoptera orders (Bravo et al. 2011). More than 200 isolates of Bt were isolated, and their biocontrol effect against insects was determined. Many Bt strains were recognized as an important antagonists of egg and juvenile of Meloidogyne spp. (Schnepf et al. 1998; De Maagd et al. 2001; Wei et al. 2003; Palma et al. 2014).
The screening of Bt strains and cry gene sequencing has led to the identification of more than 700 cry gene sequences that correspond to 70 different gene groups according to their amino acid sequence. Six cry proteins Cry5, Cry6, Cry12, Cry13, Cry14, and Cry21 were reported to possess nematicidal activity (Wei et al. 2003; Tian et al. 2007; zi-Quan et al. 2008; Bravo et al. 2012). However, Cry5B and Cry6A are two distinct representatives (Wei et al. 2003).
Indeed, many in vitro and in vivo experiments were conducted to evaluate the efficacy of Bacillus thuringiensis towards the hatching, motility, penetration, development, and reproduction of RKNs. It was reported that nematicidal toxins of Bt share similar modes of action as insecticidal toxins. After ingestion by the nematode larvae, they are solubilized within the gut of the nematode leading to their proteolytic activation (Zhang et al. 2012). Then, these Cry proteins form lytic pores in the epithelial cell membrane which led to cell perturbation, and then the death of the nematode (Schnepf et al. 1998; Crickmore 2005; Bravo et al. 2007). The cry protein binding to epithelial cell receptors is a significant action in this toxicity mechanism (Tian et al. 2007). However, the latter may differ according to the type of toxin and nematode species (Ravari and Moghaddam 2015).
Bacteria promoting plant growth and health
The rhizosphere zone is colonized by free-living bacteria, called plant growth-promoting rhizobacteria (PGPR) or plant health-promoting rhizobacteria (PHPR) that enhance plant growth, yield, and nutrient uptake, (Sikora 1988; Kloepper et al. 1991, 1992). They only represent a portion of 2% to 5% of global rhizobacteria (Siddiqui 2006; Lugtenberg and Kamilova 2009). Generally, PGPR can be subdivided into extracellular (ePGPRs) or intracellular plant growth-promoting rhizobacteria (iPGPRs). iPGPR may exist mainly inside root cells in the specialized nodular structures while ePGPR are located in the rhizosphere, on the root surface, or in the spaces between the cells of the root cortex colonizing the intercellular tissue (Gray and Smith 2005; Gupta et al. 2015).
PGPR confer defense against a variety of plant pathogens and it has been largely shown that they may act as natural enemies towards plant-parasitic nematodes, including root-knot nematodes (Krebs et al. 1998; Siddiqui and Mahmood 1999; Li et al. 2005; Siddiqui 2006; Lugtenberg and Kamilova 2009; Xiang et al. 2018).
Mechanisms of action of PGPR are whether direct or indirect (Glick 1995; Kundan et al. 2015). The direct mechanisms involve the production of phytohormones (auxins, cytokinins, ethylene), fixation of nitrogen, and solubilization of phosphate (Kundan et al. 2015; Vejan et al. 2016). The indirect mechanisms of PGPR include the production of secondary metabolites that inhibit soil pathogen proliferation, production of cell wall-degrading enzymes such as chitinase and 1,3-glucanase that can lyse pathogen cell walls, competing for niche and nutrients as well as the induction of plant resistance (Whipps 2001). Pseudomonas sp. and Bacillus sp. are the most PGPR having considerable action against Meloidogyne spp. (Siddiqui and Mahmood 1999; Tian et al. 2007; Mhatre et al. 2019; Iftikhar et al. 2020; Habazar et al. 2021).
Bacillus subtilis and Pseudomonas fluorescens were recorded to have a significant effect on Meloidogyne incognita on Vigna mungo. Also, Borrajo et al. (2021) reported that cell-free supernatants of Pseudomonas spp. and bacillus spp. reduced significantly egg hatching and juvenile mortality. Moreover, the biocontrol activity of pseudomonas spp. is manifested by the production of antinematode compounds, antibiotics, and hydrolytic enzymes like lipases, protease, and chitinases (Siddiqui et al. 2005a; Tian et al. 2007). Yet, Pseudomonas fluorescens and Bacillus spp. exert a nematicidal activity against Meloidogyne spp. by producing volatile organic compounds (VOCs) such as dimethyldisulfide, hydrogen cyanide, 2-nonanon, hydrogen sulfide (Huang et al. 2010; Cheng et al. 2017; Zhai et al. 2018),and hydrolytic enzymes like lipases, chitinases, and proteases (Siddiqui and Shaukat 2003; Siddiqui et al. 2005b; Tian et al. 2007; Borrajo et al. 2021).
Nematophagous fungi against Root-Knot-Nematodes
Nematophagous fungi (NFs) are carnivorous fungi that can feed on nematodes under unfavorable nutritional conditions at all stages of their life cycles (juveniles, adults, and eggs) (Dong and Zhang 2006; Braga and De Araújo 2014; Degenkolb and Vilcinskas 2016; Karthik Raja et al. 2020). They are the most diverse group of microorganisms (Dong and Zhang 2006; Nordbring-hertz 2006; Liu et al. 2009). NFs are accounting for more than 700 species, belonging to several phylogenetic groups, such as the Ascomycota, Basidiomycota, Chytridiomycota, and Zygomycota (Li et al. 2015).
NFs are natural antagonists of RKNs that are evolving sophisticated strategies of infection (Siddiqui and Mahmood 1996; Nordbring-hertz 2006; Lopez-llorca et al. 2008; Askary 2015; Soares et al. 2018). Based on their mechanisms of infection, nematophagous fungi are classified into nematode-trapping, egg- and female-parasitic, and endoparasitic organisms (Dong and Zhang 2006; Braga and De Araújo 2014; Stirling 2014). Recently, two new other groups, toxin-producing fungi and producers of special attack devices, were reported (Liu et al. 2009; Soares et al. 2018). However, a great interest is given in the recent studies on trapping nematodes and egg- and female-parasitic groups. These two groups are the subject of this current section, in which their mechanisms of action will be discussed. Many studies were carried out to investigate NFs efficacy against RKN (Table 4).
Nematode-trapping fungi
Nematode-trapping fungi, also called predacious or predatory fungi are the most studied group among the nematode antagonists (Jamshidnejad et al. 2013). They modify their hyphae into trapping structures of different shapes and sizes to capture the moving stages of the plant-parasitic-nematodes. Their structures vary widely even within a genus, from simple adhesive hyphae to much more complex structures with two-dimensional or three-dimensional networks (Moosavi and Zare 2012; Askary 2015; Soares et al. 2018). These trap formations were found to be extremely influenced by many factors such as nematode species, nematode population, pH, temperature, and nutritional level (Singh et al. 2012). Nematodes trapped are killed and digested by fungi making them as an alternative or supplementary source of nutrients (Nordbring-hertz 2006; Askary 2015; Soares et al. 2018). In addition, when trapping, predator fungi produce extracellular enzymes in order to facilitate the adhesion and digestion of the nematode larvae (Moosavi and Zare 2012; Jamshidnejad et al. 2013). Furthermore, nematode-trapping fungi have been shown to secrete nematicidal substances like linoleic acid (Anke et al. 1995).
Arthrobotrys spp. is one of the most studied nematode-trapping fungi (Hsueh et al. 2013; Jamshidnejad et al. 2013; Tazi et al. 2021). In vitro and in vivo studies done by Soliman et al. (2021) showed that Arthrobotrys oligospora Fresenius had a significant suppression effect by trapping and predating Meloidogyne incognita in addition to producing nematicidal metabolites. Arthrobotrys oligospora is considered as a nematode-trapping fungi model (Niu and Zhang 2011). This predacious fungus develops different trapping forms varying from simple traps by adhering to nematode cuticle, to a very sophisticated traps in form of networks entering inside the target nematode (Nordbring-hertz 2004). The predacious fungi are in a saprophytic lifestyle with plants and animal debris. When nematodes are present, the predacious fungi change their lifestyle into parasitic mode by parasitizing nematodes and destroying them (Soliman et al. 2021). According to Hyde et al. (2014), this change is probably due to metabolites produced by the nematodes and secreted outside their bodies.
Egg and female-parasitic fungi
Egg- and female-parasitic fungi are those infecting the eggs and females by using appressoria or zoospores (Nordbring-hertz 2006; Lopez-llorca et al. 2008). These fungi are parasitizing nonmotile stages of nematodes such as Pochonia chlamydospora, which use their hyphae to grow towards the eggs. At the hyphal tips, the fungi form an appressoria that penetrate the eggshell and digest the content of eggs regardless of their nature, whether mature (containing juveniles) or immature eggs (Nordbring-hertz 2006). In addition, in egg-parasitic fungi appressoria, there is a secretion of extracellular hydrolytic enzymes, such as chitinase, protease, and collagenase. These enzymes play an important role in the penetration and disintegration of eggshell layers, which consist principally of protein and chitin (Bedelu et al. 1998; Yang et al. 2007).
Conclusion
This review highlighted different biological approaches (cultural practices, plant EOs, bacteria, and fungi) adopted for the management of Meloidogyne spp. These strategies are getting great attention in plant protection research and they were proven to be promising alternatives to synthetic pesticides. Thanks to the advancement in technological and analytical tools, agricultural practices are becoming easier and mechanisms of action of biological control agents are more understood.
As reported, agricultural practices have an important role in perturbing RKNs’ reproduction. Additionally, essential oils present a natural source of nematicide compounds. Moreover, soil microorganisms, bacteria and fungi, contribute significantly to the management of such organisms via different processes. However, the adoption of such strategies for the management of Meloidogyne spp. in different cropping systems seems to be challenging. Additional studies are necessary to study the effect of the introduction of biocontrol agents on soil biodiversity. On another hand, the complex interactions between biological control agents (or their product) towards targeted pathogen, infected plant, environmental conditions, soil structure, and all other considerations in the field should be investigated. The biocontrol must be accomplished in a more precise and good timing for good management, taking into account the life cycle of nematodes in the optimal conditions of their development. Also, the biocontrol agents are affected by field conditions, soil’s chemical and physical properties, which may adversely impact their effectiveness. Hence, studies conducted on a laboratory scale must be confirmed under determined field conditions.
References
Abad P, Williamson VM (2010) Plant nematode interaction: a sophisticated dialogue. In: Advances in Botanical Research. Elsevier, pp 147–192
Abd-elgawad M, Askary TH (2015) Impact of Phytonematodes on Agriculture Economy. In: Askary TH, Martinelli PRP (eds) Biocontrol Agents of Phytonematodes. CAB International: Wallingford, UK, pp 3–49
Abd-Elgawad MMM, Askary TH (2018) Fungal and bacterial nematicides in integrated nematode management strategies. Egypt J Biol Pest Control 28:74. https://doi.org/10.1186/s41938-018-0080-x
Affokpon A, Coyne DL, Htay CC et al (2011) Biocontrol potential of native Trichoderma isolates against root-knot nematodes in West African vegetable production systems. Soil Biol Biochem 43:600–608. https://doi.org/10.1016/j.soilbio.2010.11.029
Ali N, Chapuis E, Tavoillot J, Mateille T (2014) Plant-parasitic nematodes associated with olive tree (Olea europaea L.) with a focus on the Mediterranean Basin: a review. Comptes Rendus - Biol 337:423–442. https://doi.org/10.1016/j.crvi.2014.05.006
Almaghrabi OA, Massoud SI, Abdelmoneim TS (2013) Influence of inoculation with plant growth promoting rhizobacteria (PGPR) on tomato plant growth and nematode reproduction under greenhouse conditions. Saudi J Biol Sci 20:57–61. https://doi.org/10.1016/j.sjbs.2012.10.004
Andrés MF, González-Coloma A, Sanz J et al (2012) Nematicidal activity of essential oils: a review. Phytochem Rev 11:371–390. https://doi.org/10.1007/s11101-012-9263-3
Anke H, Stadler M, Mayer A, Sterner O (1995) Secondary metabolites with nematicidal and antimicrobial activity from nematophagous fungi and Ascomycetes. Can J Bot 73:932–939. https://doi.org/10.1139/b95-341
Ansari RA, Rizvi R, Mahmood I (2020) Management of Phytonematodes : Recent Advances and Future Challenges. Springer: Singapore. https://doi.org/10.1007/978-981-15-4087-5
Askary TH (2015) Nematophagous fungi as biocontrol agents of phytonematodes. In: Askary TH, Martinelli PRP (eds) Biocontrol agents of phytonematodes. CAB International, Wallingford, Oxfordshire, pp 81–125
Askary TH, Martineli PRP (2015) Biocontrol agents of phytonematodes. CAB International, UK
Avato P, Tursi F, Vitali C et al (2000) Allylsulfide constituents of garlic volatile oil as antimicrobial agents. Phytomedicine 7:239–243. https://doi.org/10.1016/S0944-7113(00)80010-0
Avato P, Laquale S, Argentieri MP et al (2017) Nematicidal activity of essential oils from aromatic plants of Morocco. J Pest Sci (2004) 90:711–722. https://doi.org/10.1007/s10340-016-0805-0
Baghaee Ravari S., Mahdikhani Moghaddam E. (2016) Efficacy of Bacillus thuringiensis Cry14 toxin against root knot nematode, Meloidogyne javanica Plant Protection Science 51(No. 1):46–51. https://doi.org/10.17221/93/2013-PPS
Bais HP, Weir TL, Perry LG et al (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 57:233–266. https://doi.org/10.1146/annurev.arplant.57.032905.105159
Bakkali F, Averbeck S, Averbeck D, Idaomar M (2008) Biological effects of essential oils - a review. Food Chem Toxicol 46:446–475. https://doi.org/10.1016/j.fct.2007.09.106
Baldwin KR (2006) Crop rotations on Organic farms. Org Prod Publ Ser Environ Farming Syst 2–14
Barker KR (1991) Rotation and cropping systems for nematode control: the North Carolina experience-introduction. J Nematol 23:342–343
Bedelu T, Gessesse A, Abate D (1998) Relation of protease production to nematode-degrading ability of two Arthrobotrys spp. World J Microbiol Biotechnol 14:731–734. https://doi.org/10.1023/A:1008842706070
Béra-Maillet C, Arthaud L, Abad P, Rosso M-N (2000) Biochemical characterization of MI-ENG1, a family 5 endoglucanase secreted by the root-knot nematode Meloidogyne incognita. Eur J Biochem 267:3255–3263. https://doi.org/10.1046/j.1432-1327.2000.01356.x
Bird AF (1986) The influence of the actinomycete, Pasteuria penetrans, on the host-parasite relationship of the plant-parasitic nematode, Meloidogyne javanica. Parasitology 93:571–580. https://doi.org/10.1017/S0031182000081270
Bird DM, Opperman CH, Davies KG (2003) Interactions between bacteria and plant-parasitic nematodes: now and then. Int J Parasitol 33:1269–1276. https://doi.org/10.1016/S0020-7519(03)00160-7
Bird AF, Brisbane P., Mcclure S., Kimber RWL (1990) Studies on the properties of the spores of some populations Pasteuria penetrans. 55:169–178. https://doi.org/10.1016/0022-2011(90)90052-8
Bishop AH, Gowen SR, Pembroke B, Trotter JR (2007) Morphological and molecular characteristics of a new species of Pasteuria parasitic on Meloidogyne ardenensis. J Invertebr Pathol 96:28–33. https://doi.org/10.1016/j.jip.2007.02.008
Bonilla N, Gutiérrez-Barranquero JA, De Vicente A, Cazorla FM (2012) Enhancing soil quality and plant health through suppressive organic amendments. Diversity 4:475–491. https://doi.org/10.3390/d4040475
Bontempo AF, Fernandes RH, Lopes J, et al (2014) Pochonia chlamydosporia controls Meloidogyne incognita on carrot. Australas Plant Pathol 43:421–424. https://doi.org/10.1007/s13313-014-0283-x
Borah B, Ahmed R, Hussain M, et al (2018) Suppression of root-knot disease in Pogostemon cablin caused by Meloidogyne incognita in a rhizobacteria mediated activation of phenylpropanoid pathway. Biol Control 119:43–50. https://doi.org/10.1016/j.biocontrol.2018.01.003
Borrajo MP, Mondino EA, Maroniche GA et al (2021) Potential of rhizobacteria native to Argentina for the control of Meloidogyne javanica. Rev Argent Microbiol. https://doi.org/10.1016/j.ram.2021.02.010
Bouchagier P (2018) Survival of Root-Knot nematodes and their egg-parasitic fungus Pochonia chlamydosporia (Goddard) on weed roots. SDRP J Plant Sci 2:1–8. https://doi.org/10.25177/jps.2.2.4
Braga FR, De Araújo JV (2014) Nematophagous fungi for biological control of gastrointestinal nematodes in domestic animals. Appl Microbiol Biotechnol 98:71–82. https://doi.org/10.1007/s00253-013-5366-z
Bravo A, Gill SS, Soberón M (2007) Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 49:423–435. https://doi.org/10.1016/j.toxicon.2006.11.022
Bravo A, Likitvivatanavong S, Gill SS, Soberón M (2011) Bacillus thuringiensis: a story of a successful bioinsecticide. Insect Biochem Mol Biol 41:423–431. https://doi.org/10.1016/j.ibmb.2011.02.006
Bravo A, Gómez I, Porta H et al (2012) Evolution of Bacillus thuringiensis Cry toxins insecticidal activity. Microb Biotechnol 6:17–26. https://doi.org/10.1111/j.1751-7915.2012.00342.x
Caillaud MC, Dubreuil G, Quentin M et al (2008) Root-knot nematodes manipulate plant cell functions during a compatible interaction. J Plant Physiol 165:104–113. https://doi.org/10.1016/j.jplph.2007.05.007
Castagnone-Sereno P, Piotte C, Uijthof J et al (1993) Phylogenetic relationships between amphimictic and parthenogenetic nematodes of the genus Meloidogyne as inferred from repetitive DNA analysis. Heredity (edinb) 70:195–204. https://doi.org/10.1038/hdy.1993.29
Castagnone-Sereno P, Danchin EGJ, Perfus-Barbeoch L, Abad P (2013) Diversity and evolution of root-knot nematodes, genus Meloidogyne : new insights from the genomic era. Annu Rev Phytopathol 51:203–220. https://doi.org/10.1146/annurev-phyto-082712-102300
Chen ZX, Dickson DW (1998) Review of Pasteuria penetrans: biology, ecology, and biological control potential. J Nematol 30:313–340
Chen P, Tsay TT (2006) Effect of crop rotation on Meloidogyne spp. and Pratylenchus spp. populations in strawberry fields in Taiwan. J Nematol 38:339–344
Cheng W, Yang J, Nie Q et al (2017) Volatile organic compounds from Paenibacillus polymyxa KM2501-1 control Meloidogyne incognita by multiple strategies. Sci Rep 7:1–11. https://doi.org/10.1038/s41598-017-16631-8
Christaki E, Bonos E, Giannenas I, Florou-Paneri P (2012) Aromatic plants as a source of bioactive compounds. Agriculture 2:228–243. https://doi.org/10.3390/agriculture2030228
Chuan QB, Zhi LL, Qi ZL (2011) Nematicidal constituents from the essential oil of Chenopodium Ambrosioides aerial parts. E-Journal Chem 8:143–148. https://doi.org/10.1155/2011/470862
Collange B, Navarrete M, Peyre G et al (2011) Root-knot nematode (Meloidogyne) management in vegetable crop production: the challenge of an agronomic system analysis. Crop Prot 30:1251–1262. https://doi.org/10.1016/j.cropro.2011.04.016
Crickmore N (2005) Using worms to better understand how Bacillus thuringiensis kills insects. Trends Microbiol 13:347–350. https://doi.org/10.1016/j.tim.2005.06.002
Dalla Pasqua S, Dallemole-Giaretta R, dos Santos I, et al (2020) Combined application of Pochonia chlamydosporia and solid by-product of the wine industry for the control of Meloidogyne javanica. Appl Soil Ecol 147:103397. https://doi.org/10.1016/j.apsoil.2019.103397
Davies KG, Opperman CH (2006) A potential role for collagen in the attachment of Pasteuria penetrans to nematode cuticle. Multitrophic Interact Soil IOBC/wprs Bull 29:11–15
Davies K, Laird V, Kerry BR (1991) The motility, development and infection of Meloidogyne incognita encumbered with spores of the obligate hyperparasite Pasteuria penetrans. Rev Nématologie 14:611–618
Davies KG (2009) Understanding the interaction between an obligate hyperparasitic bacterium, Pasteuria penetrans and its obligate plant-parasitic nematode host, Meloidogyne spp. In: Advances in Parasitology, 1st edn. Elsevier Ltd, pp 211–245
Davis EL, Hussey RS, Mitchum MG, Baum TJ (2008) Parasitism proteins in nematode-plant interactions. Curr Opin Plant Biol 11:360–366. https://doi.org/10.1016/j.pbi.2008.04.003
de F Soares FE, Sufiate BL, de Queiroz JH (2018) Nematophagous fungi: far beyond the endoparasite, predator and ovicidal groups. Agric Nat Resour 52:1–8. https://doi.org/10.1016/j.anres.2018.05.010
De Maagd RA, Bravo A, Crickmore N (2001) How Bacillus thuringiensis has evolved specific toxins to colonize the insect world. Trends Genet 17:193–199. https://doi.org/10.1016/S0168-9525(01)02237-5
Degenkolb T, Vilcinskas A (2016) Metabolites from nematophagous fungi and nematicidal natural products from fungi as an alternative for biological control. Part I: metabolites from nematophagous ascomycetes. Appl Microbiol Biotechnol 100:3799–3812. https://doi.org/10.1007/s00253-015-7233-6
Dihingia S, Das D, Bora S (2017) Effect of microbial secrection on innhibotory effect of phytnematode: a review. Int J Inf Res Rev 04:4275–4280
Dong LQ, Zhang KQ (2006) Microbial control of plant-parasitic nematodes: a five-party interaction. Plant Soil 288:31–45. https://doi.org/10.1007/s11104-006-9009-3
Dong L, Huang C, Huang L et al (2012) Screening plants resistant against Meloidogyne incognita and integrated management of plant resources for nematode control. Crop Prot 33:34–39. https://doi.org/10.1016/j.cropro.2011.11.012
Du B, Xu Y, Dong H, et al (2020) Phanerochaete chrysosporium strain B-22, a nematophagous fungus parasitizing Meloidogyne incognita. PLoS One 15:1–14. https://doi.org/10.1371/journal.pone.0216688
Dutta TK, Khan MR, Phani V (2019) Plant-parasitic nematode management via biofumigation using brassica and non-brassica plants: current status and future prospects. Curr Plant Biol 17:17–32. https://doi.org/10.1016/j.cpb.2019.02.001
Echeverrigaray S, Zacaria J, Beltrão R (2010) Nematicidal activity of monoterpenoids against the root-knot nematode Meloidogyne incognita. Phytopathology 100:199–203. https://doi.org/10.1094/PHYTO-100-2-0199
Eisenback JD, Triantaphyllou HH (1991) Root-knot Nematodes: Meloidogyne species and races. In: Manual of Agricultural Nematology, WR Nickle. Marcel Dekker, New York, pp 191 – 274
Fassuliotis G (1985) The role of the nematologist in the development of resistant cultivars. In: J.N. Sasser and C.C. Carter (ed) An advanced treatise on Meloidogyne, Vol. I. Biology and control. N. C. State Univ., Graphics, Raleigh. pp 233–240
Favery B, Quentin M, Jaubert-Possamai S, Abad P (2016) Gall-forming root-knot nematodes hijack key plant cellular functions to induce multinucleate and hypertrophied feeding cells. J Insect Physiol 84:60–69. https://doi.org/10.1016/j.jinsphys.2015.07.013
Figueiredo AC, Barroso JG, Pedro LG, Scheffer JJC (2008) Chemical variability of the leaf oil of 113 hybrids from. 152–163. https://doi.org/10.1002/ffj
Gheysen G, Fenoll C (2002) Gene expression in nematode feeding sites. Annu Rev Phytopathol 40:191–219. https://doi.org/10.1146/annurev.phyto.40.121201.093719
Giannakou I, Pembroke B, Gowen S, Davies K (1997) Effects of long term storage and above normal temperatures on spore adhesion of Pasteuria penetrans and infection of the root-knot nematode Meloidogyne javanica. Nematologica 43:185–192
Glick BR (1995) The enhancement of plant growth by free-living bacteria. Can J Microbiol 41:109–117. https://doi.org/10.1139/m95-015
Gray EJ, Smith DL (2005) Intracellular and extracellular PGPR: commonalities and distinctions in the plant-bacterium signaling processes. Soil Biol Biochem 37:395–412
Gullino ML, Albajes R (2001) Integrated Pest and Disease Management in Greenhouse Crops. In: Gullino ML, Albajes R, Nicot PC (eds) Plant Pathology in 21st Century, 2nd edn. Springer, pp 759–760
Gupta G, Parihar SS, Ahirwar NK, et al (2015) Plant growth promoting Rhizobacteria (PGPR): current and future prospects for development of sustainable agriculture. J Microb Biochem Technol 07: https://doi.org/10.4172/1948-5948.1000188
Habazar T, Winarto Obel et al (2021) Biocontrol of Meloidogyne sp. on tomato plants by selected Bacillus spp. IOP Conf Ser Earth Environ Sci 757:012019. https://doi.org/10.1088/1755-1315/757/1/012019
Hamada N, Yimer HZ, Williamson VM, Siddique S (2020) Chemical hide and seek: nematode’s journey to its plant host. Mol Plant 13:541–543. https://doi.org/10.1016/j.molp.2020.03.005
Hassan S, Mathesius U (2012) The role of flavonoids in root-rhizosphere signalling: opportunities and challenges for improving plant-microbe interactions. J Exp Bot 63:3429–3444. https://doi.org/10.1093/jxb/err430
Hida H, Nishiyama H, Sawa S et al (2015) Chemotaxis assay of plant-parasitic nematodes on a gel-filled microchannel device. Sensors Actuators, B Chem 221:1483–1491. https://doi.org/10.1016/j.snb.2015.07.081
Hill NS (1988) Cultural practices for the management of plant parasitic nematodes. Ornamentals Northwest Arch 12:7–9
Hsueh YP, Mahanti P, Schroeder FC, Sternberg PW (2013) Nematode-trapping fungi eavesdrop on nematode pheromones. Curr Biol 23:83–86. https://doi.org/10.1016/j.cub.2012.11.035
Huang G, Dong R, Allen R et al (2005) Two chorismate mutase genes from the root-knot nematode Meloidogyne incognita. Mol Plant Pathol 6:23–30. https://doi.org/10.1111/J.1364-3703.2004.00257.X
Huang Y, Xu CK, Ma L et al (2010) Characterisation of volatiles produced from Bacillus megaterium YFM3.25 and their nematicidal activity against Meloidogyne incognita. Eur J Plant Pathol 126:417–422. https://doi.org/10.1007/s10658-009-9550-z
Huettel RN (1986) Chemical communicators in nematodes. J Nematol 18:3–8
Hussey RS (1989) Disease-inducing secretions of plant-parasitic nematodes. Annu Rev Phytopathol 27:123–141. https://doi.org/10.1146/annurev.py.27.090189.001011
Hyde K, Swe A, Zhang K (2014) Nematode-trapping fungi. In: KQ Z, K H (eds) Nematode-trapping fungi, Fungal Div. Springer
Iftikhar Y, Sajid A, Shakeel Q et al (2020) Biological antagonism: a safe and sustainable way to manage plant diseases. In: Haq IU, Ljaz S (eds) Plant Disease Management Strategies for Sustainable Agriculture through Traditional and Modern Approaches. Springer, Cham, pp 137–172
Isman MB (2000) Plant essential oils for pest and disease management. Crop Prot 19:603–608. https://doi.org/10.1016/S0261-2194(00)00079-X
James K, Back M, Prior T (2019) A literature review of the root-knot nematodes (Meloidogyne species) that pose a threat to potato production in GB
Jamshidnejad V, Sahebani N, Etebarian H (2013) Potential biocontrol activity of Arthrobotrys oligospora and Trichoderma harzianum BI against Meloidogyne javanica on tomato in the greenhouse and laboratory studies. Arch Phytopathol Plant Prot 46:1632–1640. https://doi.org/10.1080/03235408.2013.778476
Jatala P (1986) Biological control of plant-parasitic nematodes. Ann Rev Pathopathol 24:453–489
Jones JT, Haegeman A, Danchin EGJ et al (2013) Top 10 plant-parasitic nematodes in molecular plant pathology. Mol Plant Pathol 14:946–961. https://doi.org/10.1111/mpp.12057
Jones MGK, Goto DB (2011) Root-knot nematodes and giant cells. In: J. J, G. G, C. F (eds) genomics and molecular genetics of plant-nematode interactions. Springer, Dordrecht, pp 83–100
Kalaiselvi D, Mohankumar A, Shanmugam G et al (2019) Altitude-related changes in the phytochemical profile of essential oils extracted from Artemisia nilagirica and their nematicidal activity against Meloidogyne incognita. Ind Crops Prod 139:111472. https://doi.org/10.1016/j.indcrop.2019.111472
Kaloshian I, Teixeira M (2019) Advances in plant_nematode interactions with emphasis on the notorious nematode genus Meloidogyne. Phytopathology 109:1988–1996. https://doi.org/10.1094/PHYTO-05-19-0163-IA
Kamran M, Javed N, Khan SA, et al (2014) Efficacy of Pasteuria penetrans on Meloidogyne incognita reproduction and growth of tomato. Pak J Zool 46:1651–1655
Kariuki GM, Dickson DW (2007) Transfer and development of Pasteuria penetrans. J Nematol 39:55–61
Karthik Raja R, Arun A, Touray M et al (2020) Antagonists and defense mechanisms of entomopathogenic nematodes and their mutualistic bacteria. Biol Control 152:104452. https://doi.org/10.1016/j.biocontrol.2020.104452
Kimbaris AC (2017) Biocidal compounds from Mentha sp. essential oils and their structure–activity relationships. Chem Biodivers 14(3):e1600270. https://doi.org/10.1002/cbdv.201600270
Khyami-horani H, Al-Banna L (2006) Efficacy of Bacillus thuringiensis jordanica against Meloidogyne javanica infecting tomato. Phytopathol Mediterr 45:153–157. https://doi.org/10.14601/Phytopathol_Mediterr-1826
Kloepper JW, Zablotowicz RM, Tipping EM, Lifshitz R (1991) Plant growth promotion mediated by bacterial rhizosphere colonizers. Rhizosph Plant Growth 1978:315–326. https://doi.org/10.1007/978-94-011-3336-4_70
Kloepper JW, Rodríguez-Kábana R, McInroy JA, Young RW (1992) Rhizosphere bacteria antagonistic to soybean cyst (Heterodera glycines) and root-knot (Meloidogyne incognita) nematodes: identification by fatty acid analysis and frequency of biological control activity. Plant Soil 139:75–84. https://doi.org/10.1007/BF00012844
Koch DW, Gray FA, Krall JM (1998) Nematode-resistant oil radish for Heterodera schachtii control. J Sugarbeet Res 35:63–75. https://doi.org/10.5274/jsbr.35.1.63
Krebs B, Höding B, Kübart S et al (1998) Use of Bacillus subtilis as biocontrol agent. I. Activities and characterization of Bacillus subtilis strains. Z Pflanzenkrankh Pflanzenschutz 105:181–197
Kundan R, Pant G, Jadon N, Agrawal PK (2015) Plant growth promoting Rhizobacteria: mechanism and current prospective. J Biofertilizers Biopestic 06: https://doi.org/10.4172/jbfbp.1000155
Kyndt T, Vieira P, Gheysen G, de Almeida-Engler J (2013) Nematode feeding sites: unique organs in plant roots. Planta 238:807–818. https://doi.org/10.1007/s00425-013-1923-z
Laquale S, Candido V, Avato P et al (2015) Essential oils as soil biofumigants for the control of the root-knot nematode Meloidogyne incognita on tomato. Ann Appl Biol 167:217–224. https://doi.org/10.1111/aab.12221
Laquale S, Avato P, Argentieri MP et al (2018) Nematotoxic activity of essential oils from Monarda species. J Pest Sci 91:1115–1125. https://doi.org/10.1007/s10340-018-0957-1
Li B, Xie GL, Soad A, Coosemans J (2005) Suppression of Meloidogyne javanica by antagonistic and plant growth-promoting rhizobacteria. J Zhejiang Univ Sci 6(B):496–501. https://doi.org/10.1631/jzus.2005.B0496
Li J, Zou C, Xu J et al (2015) Molecular mechanisms of nematode-nematophagous microbe interactions: basis for biological control of plant-parasitic nematodes. Annu Rev Phytopathol 53:67–95. https://doi.org/10.1146/annurev-phyto-080614-120336
Liu W, Park SW (2018) Underground mystery: interactions between plant roots and parasitic nematodes. Curr Plant Biol 15:25–29. https://doi.org/10.1016/j.cpb.2018.11.004
Liu X, Xiang M, Che Y (2009) The living strategy of nematophagous fungi. Mycoscience 50:20–25. https://doi.org/10.1007/s10267-008-0451-3
Lopez-llorca LV, Macia-vicente J, Jansson H-B (2008) Mode of action and interactions of nematophagous fungi bt - integrated management of plant pests and diseases. Integrated Management and Biocontrol of Vegetable and Grain Crops Nematodes. Springer, Dordrecht, pp 51–76
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting Rhizobacteria. Annu Rev Microbiol 63:541–556. https://doi.org/10.1146/annurev.micro.62.081307.162918
Malinovsky FG, Fangel JU, Willats WGT (2014) The role of the cell wall in plant immunity. Front Plant Sci 5:1–12. https://doi.org/10.3389/fpls.2014.00178
Mateille T, Trudgill DL, Trivino C et al (2002) Multisite survey of soil interactions with infestation of root-knot nematodes (Meloidogyne spp.) by Pasteuria penetrans. Soil Biol Biochem 34:1417–1424. https://doi.org/10.1016/S0038-0717(02)00085-8
Mateille T, Dabiré KR, Fould S, Diop MT (2010) Host-parasite soil communities and environmental constraints: modelling of soil functions involved in interactions between plant-parasitic nematodes and Pasteuria penetrans. Soil Biol Biochem 42:1193–1199. https://doi.org/10.1016/j.soilbio.2010.04.010
Matthiessen J, Kirkegaard J (2006) Biofumigation and enhanced biodegradation: opportunity and challenge in soilborne pest and disease management. CRC Crit Rev Plant Sci 25:235–265. https://doi.org/10.1080/07352680600611543
Mejias J, Truong NM, Abad P, et al (2019) Plant proteins and processes targeted by parasitic nematode effectors. Front Plant Sci 10: https://doi.org/10.3389/fpls.2019.00970
Messa VR, Torres da Costa AC, Kuhn OJ, Stroze CT (2020) Nematophagous and endomycorrhizal fungi in the control of Meloidogyne incognita in soybean. Rhizosphere 15:100222. https://doi.org/10.1016/j.rhisph.2020.100222
Mhatre PH, Karthik C, Kadirvelu K et al (2019) Plant growth promoting rhizobacteria (PGPR): a potential alternative tool for nematodes bio-control. Biocatal Agric Biotechnol 17:119–128. https://doi.org/10.1016/j.bcab.2018.11.009
Mhatre PH, Eapen SJ, Chawla G, et al (2020) Isolation and characterization of Pasteuria parasitizing root-knot nematode, Meloidogyne incognita, from black pepper fields in India. Egypt J Biol Pest Control 30: https://doi.org/10.1186/s41938-020-00296-z
Moens M, Perry RN, Starr JL (2009) Meloidogyne species - a diverse group of novel and important plant parasites. Root-knot Nematodes 1–17. https://doi.org/10.1079/9781845934927.0001
Mohammed SH, El Saedy MA, Enan MR, et al (2008) Biocontrol efficiency of Bacillus thuringiensis toxins against root-knot nematode, meloidogyne incognita. J Cell Mol Biol 7:57–66
Moosavi MR, Zare R (2012) Fungi as biological control agents of plant-parasitic nematodes. In: Plant Defence: Biological Control. pp 67–107
Motisi N, Doré T, Lucas P, Montfort F (2010) Dealing with the variability in biofumigation efficacy through an epidemiological framework. Soil Biol Biochem 42:2044–2057. https://doi.org/10.1016/j.soilbio.2010.08.016
Mostafanezhad H, Sahebani N, Nourinejhad Zarghani S (2014) Control of root-knot nematode (Meloidogyne javanica) with combination of Arthrobotrys oligospora and salicylic acid and study of some plant defense responses. Biocontrol Sci Technol 24:203–215. https://doi.org/10.1080/09583157.2013.855166
Mouton L, Traunecker E, McElroy K et al (2009) Identification of a polymorphic collagen-like protein in the crustacean bacteria Pasteuria ramosa. Res Microbiol 160:792–799. https://doi.org/10.1016/j.resmic.2009.08.016
Mukhtar T, Arshad Hussain M, Zameer Kayani M (2013) Biocontrol potential of Pasteuria penetrans, Pochonia chlamydosporia, Paecilomyces lilacinus and Trichoderma harzianum against Meloidogyne incognita in okra. Phytopathol Mediterr 52:66–76. https://doi.org/10.14601/Phytopathol_Mediterr-11305
Nasu É das GC, Amora DX, Monteiro TSA, et al (2018) Pochonia chlamydosporia applied via seed treatment for nematode control in two soil types. Crop Prot 114:106–112. https://doi.org/10.1016/j.cropro.2018.08.010
Negi PS (2012) Plant extracts for the control of bacterial growth: efficacy, stability and safety issues for food application. Int J Food Microbiol 156:7–17. https://doi.org/10.1016/j.ijfoodmicro.2012.03.006
Nielsen DC, Calderón FJ (2015) Fallow effects on soil. Soil Manag Build a Stable Base Agric 287–300. https://doi.org/10.2136/2011.soilmanagement.c19
Niu XM, Zhang KQ (2011) Arthrobotrys oligospora: a model organism for understanding the interaction between fungi and nematodes. Mycology 2:59–78. https://doi.org/10.1080/21501203.2011.562559
Noling JW (2016) Nematode management in tomatoes, peppers and eggplant. Univ Florida, IFAS Ext ENY-032:1–15
Nordbring-hertz B (2004) Morphogenesis in the nematode-trapping fungus. 18:125–133. https://doi.org/10.1017/S0269915XO4003052
Nordbring-hertz B (2006) Nematophagous fungi. 1–11. https://doi.org/10.1038/npg.els.0004293
Ntalli NG, Ferrari F, Giannakou I, Menkissoglu-Spiroudi U (2010) Phytochemistry and nematicidal activity of the essential oils from 8 greek lamiaceae aromatic plants and 13 terpene components. J Agric Food Chem 58:7856–7863. https://doi.org/10.1021/jf100797m
Ntalli NG, Menkissoglu-Spiroudi U (2011) Pesticides of botanical origin: a promising tool in plant protection. In: Prof. Margarita Stoytcheva (ed) Pesticides - Formulations, Effects, Fate. InTech
Ntalli NG, Nasiou E, Menkissoglu-Spiroudi U (2013) Evaluation of Essential Oils from Rosemary, Orange, Lavandula and False Yellowhead on Hatching and Motility of Root-Knot Nematode. J Agric Sci Technol A 3:603–616
Oerke EC (2006) Crop losses to pests. J Agric Sci 144:31–43. https://doi.org/10.1017/S0021859605005708
Oka Y (2001) Nematicidal activity of essential oil components against the root-knot nematode Meloidogyne javanica. Nematology 3:159–164. https://doi.org/10.1163/156854101750236286
Oka Y, Nacar S, Putievsky E, et al (2000) Nematicidal activity of essential oils and their components against the root- nematicidal activity of essential oils and their components against the root-knot nematode. https://doi.org/10.1094/PHYTO.2000.90.7.710
Onkendi EM, Kariuki GM, Marais M, Moleleki LN (2014) The threat of root-knot nematodes (Meloidogyne spp.) in Africa: A review. Plant Pathol 63:727–737. https://doi.org/10.1111/ppa.12202
Oostendorp M, Dickson DW, Mitchell DJ (1990) Host range and ecology of isolates of Pasteuria spp. from the southeastern United States. J Nematol 22:525–52531
Oota M, Tsai AYL, Aoki D et al (2020) Identification of naturally occurring polyamines as root-knot nematode attractants. Mol Plant 13:658–665. https://doi.org/10.1016/j.molp.2019.12.010
Palma L, Muñoz D, Berry C et al (2014) Bacillus thuringiensis toxins: an overview of their biocidal activity. Toxins (basel) 6:3296–3325. https://doi.org/10.3390/toxins6123296
Palomares-Rius JE, Escobar C, Cabrera J et al (2017) Anatomical alterations in plant tissues induced by plant-parasitic nematodes. Front Plant Sci 8:1–16. https://doi.org/10.3389/fpls.2017.01987
Pankaj S, Sharma HK, Prasad J (2010) The rice root-knot nematode, Meloidogyne graminicola: an emerging problem in rice-wheat cropping system. Indian J Nematol 40:1–11
Perry RN, Moens M, Starr JL (2010) Root-knot nematodes. In: Nematology. pp 483–484
Perry RN, Moens M (2011) Introduction to plant-parasitic nematodes; modes of parasitism. Genomics Mol Genet Plant-Nematode Interact. https://doi.org/10.1007/978-94-007-0434-3
Persidis A, Lay JG, Manousis T et al (1991) Characterisation of potential adhesins of the bacterium Pasteuria penetrans, and of putative receptors on the cuticle of Meloidogyne incognita, a nematode host. J Cell Sci 100:613–622
Quentin M, Abad P, Favery B (2013) Plant parasitic nematode effectors target host defense and nuclear functions to establish feeding cells. Front Plant Sci 4:1–8. https://doi.org/10.3389/fpls.2013.00053
Ravari SB, Moghaddam EM (2015) Efficacy of bacillus thuringiensis Cry14 toxin against root knot nematode, meloidogyne javanica. Plant Prot Sci 51:46–51. https://doi.org/10.17221/93/2013-PPS
Roberts P. (2003) Concepts and consequences of resistance. In: J.L. Starr, R. Cook and J B (ed) Plant resistance to parasitic nematodes. pp 6–8
Rodiuc N, Vieira P, Banora MY, de Almeida EJ (2014) On the track of transfer cell formation by specialized plant-parasitic nematodes. Front Plant Sci 5:1–14. https://doi.org/10.3389/fpls.2014.00160
Sacchetti G, Maietti S, Muzzoli M et al (2005) Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem 91:621–632. https://doi.org/10.1016/j.foodchem.2004.06.031
Santana O, Andrés MF, Sanz J et al (2014) Valorization of essential oils from Moroccan aromatic plants. Nat Prod Commun 9:1109–1114. https://doi.org/10.1177/1934578x1400900812
Savary S, Ficke A, Aubertot J-N, Hollier C (2012) Crop losses due to diseases and their implications for global food production losses and food security. Food Secur 4:519–537. https://doi.org/10.1007/s12571-012-0200-5
Sayre RM, Starr MP (1985) Pasteuria penetrans (ex Thorne, 1940) nom. rev., comb. n., sp. n., a mycelial and endospore-forming bacterium parasitic in plant-parasitic nematodes. Proc Helminthol Soc Wash 52:149–165
Sayre RM, Wergin WP (1977) Bacterial parasite of a plant nematode: morphology and ultrastructure. J Bacteriol 129:1091–1101. https://doi.org/10.1128/jb.129.2.1091-1101.1977
Schneider SM, Rosskopf EN, Leesch JG et al (2003) United States Department of Agriculture - Agricultural Research Service research on alternatives to methyl bromide: pre-plant and post-harvest. Pest Manag Sci 59:814–826. https://doi.org/10.1002/ps.728
Schnepf E, Crickmore N, Van Rie J et al (1998) Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Biol Rev 62:775–806. https://doi.org/10.1128/mmbr.62.3.775-806.1998
Scholte K (2000) Effect of potato used as a trap crop on potato cyst nematodes and other soil pathogens and on the growth of a subsequent main potato crop. Ann Appl Biol 136:229–238. https://doi.org/10.1111/j.1744-7348.2000.tb00029.x
Sharar M, Bozeya A, Al-Banna L, Al-Bawab A (2017) Fungicidal and nematicidal activities for essential oils formulated in Janus emulsion. Green Chem Lett Rev 10:121–128. https://doi.org/10.1080/17518253.2017.1306613
Siddiqui ZA, Mahmood I (1996) Biological control fo plant parasitic nematodes by fungi: a review. Bioresour Technol 58:229–239
Siddiqui ZA, Mahmood I (1999) Role of bacteria in the management of plant parasitic nematodes: a review. Bioresour Technol 69:167–179. https://doi.org/10.1016/S0960-8524(98)00122-9
Siddiqui IA, Haas D, Heeb S (2005b) Extracellular protease of Pseudomonas fluorescens CHA0, a biocontrol factor with activity against the root-knot nematode Meloidogyne incognita. Appl Environ Microbiol 71:5646–5649. https://doi.org/10.1128/AEM.71.9.5646-5649.2005
Siddiqui IA, Shaukat SS (2003) Suppression of root-knot disease by Pseudomonas fluorescens CHA0 in tomato : importance of bacterial secondary metabolite, 35:1615–1623. https://doi.org/10.1016/j.soilbio.2003.08.006
Siddiqui IA, Haas D, Heeb S (2005a) Extracellular protease of Pseudomonas fluorescens CHA0 , a biocontrol factor with activity against the root-knot nematode Meloidogyne incognita. 71:5646–5649. https://doi.org/10.1128/AEM.71.9.5646
Siddiqui ZA (2006) PGPR: Biocontrol and Biofertilization. Springer, Dordrecht, The Netherlands
Sikora RA, Bridge J, Starr JL (2005) Management Practices: an Overview of Integrated Nematode Management Technologies. In: M. L, R.A. S, J. B (eds) Plant parasitic nematodes in subtropical and tropical agriculture. pp 793–825
Sikora RA, Molendijk LPG, Desaeger J (2021) Integrated nematode management and crop health: future challenges and opportunities. Integr nematode Manag state-of-the-art visions Futur 3–10. https://doi.org/10.1079/9781789247541.0001
Sikora R (1988) Interrelationship between plant health promoting rhizobacteria, plant parasitic nematodes and soil microorganisms. Commun Agric Appl Biol Sci 53:867–878
Singh UB, Sahu A, Singh RK et al (2012) Evaluation of biocontrol potential of Arthrobotrys oligospora against Meloidogyne graminicola and Rhizoctonia solani in rice (Oryza sativa L.). Biol Control 60:262–270. https://doi.org/10.1016/j.biocontrol.2011.10.006
Singh R, Phulera S (2015) Plant parasitic nematodes: the hidden enemies of farmers. In: Environmental Issues for Socio-ecological Development. pp 68–81
Soliman MS, El-Deriny MM, Ibrahim DSS et al (2021) Suppression of root-knot nematode Meloidogyne incognita on tomato plants using the nematode trapping fungus Arthrobotrys oligospora Fresenius. J Appl Microbiol. https://doi.org/10.1111/jam.15101
Sosa ME, Lancelle HG, Tonn CE et al (2012) Insecticidal and nematicidal essential oils from Argentinean Eupatorium and Baccharis spp. Biochem Syst Ecol 43:132–138. https://doi.org/10.1016/j.bse.2012.03.007
Srivastava A, Mohan S, Mauchline TH, Davies KG (2018) Evidence for diversifying selection of genetic regions of encoding putative collagen-like host-adhesive fibers in Pasteuria penetrans. FEMS Microbiol Ecol 95:1–8. https://doi.org/10.1093/femsec/fiy217
Stapleton JJ, Duncan RA (1998) Soil disinfestation with cruciferous amendments and sublethal heating: effects on Meloidogyne incognita, Sclerotium rolfsii and Pythium ultimum. Plant Pathol 47:737–742
Starr MP, Sayre RM (1988) Pasteuria thornei sp. nov. and Pasteuria penetrans sensu stricto emend., mycelial and endospore-forming bacteria parasitic, respectively, on plant-parasitic nematodes of the genera Pratylenchus and Meloidogyne. Птицы 139:11–31
Stirling G, Nicol J, Reay F (2002) Advisory Services for Nematode Pests - Operational Guidelines. Biol Crop Prot Pty Ltd 111
Stirling G, Bird A, Cakurs A (1986) Attachment of Pasteuria panetrans spores to the cuticle of root-knot nematodes. Rev Nematol.9: 251–260
Stirling GR (1985) Host specificity of Pasteuria Penetrans within the genus Meloidogyne. 31:203–209
Stirling GR (2011) Biological Control of Plant-Parasitic Nematodes: An Ecological Perspective, a Review of Progress and Opportunities for Further Research. In: Davies, K., Spiegel Y (eds) Biological Control of Plant-Parasitic Nematodes: Progress in Biological Control. Springer, Dordrecht, p 311
Stirling GR (2014) Biological control of plantparasitic nematodes: soil ecosystem management in sustainable agriculture, 2nd edition
Subedi S, Thapa B, Shrestha J (2020) Root-knot nematode (Meloidogyne incognita) and its management: a review. J Agric Nat Resour 3:21–31. https://doi.org/10.3126/janr.v3i2.32298
Tazi H, Hamza MA, Hallouti A, et al (2021) Biocontrol potential of nematophagous fungi against Meloidogyne spp. infecting tomato. Org Agric 11:63–71. https://doi.org/10.1007/s13165-020-00325-z
Tazi H, Hamza MA, Hallouti A et al (2021) Biocontrol potential of nematophagous fungi against Meloidogyne spp. infecting tomato. Org Agric 11:63–71. https://doi.org/10.1007/s13165-020-00325-z
Thenail C, Joannon A, Capitaine M et al (2009) The contribution of crop-rotation organization in farms to crop-mosaic patterning at local landscape scales. Agric Ecosyst Environ 131:207–219. https://doi.org/10.1016/j.agee.2009.01.015
Tian B, Yang J, Zhang K-Q (2007) Bacteria used in the biological control of plant-parasitic nematodes: populations, mechanisms of action, and future prospects. FEMS Microbiol Ecol 61:197–213. https://doi.org/10.1111/j.1574-6941.2007.00349.x
Timper P (2009) Population dynamics of meloidogyne arenaria and pasteuria penetrans in a long-term crop rotation study. J Nematol 41:291–299
Trivedi P, Barker K (1986) Management of nematodes by cultural practices. Nematropica 16:213–236
Truong NM, Nguyen C-N, Abad P, et al (2015) Function of root-knot nematode effectors and their targets in plant parasitism. In: Carolina Escobar CF (ed) Plant Nematode Interactions A View on Compatible Interrelationships. Elsevier Ltd, pp 293–324
Tsai AYL, Higaki T, Nguyen CN et al (2019) Regulation of root-knot nematode behavior by seed-coat mucilage-derived attractants. Mol Plant 12:99–112. https://doi.org/10.1016/j.molp.2018.11.008
Uysal G, Özdemir FGG (2020) Bacteria in the management of plant parasitic. Turkish J Sci Rev 13:53–72
Van de Braak SAA., Leijten GCJ. (1999) Essential oils and oleoresins : a survey in the Netherlands and other major markets in the European Union. CBI, Cent Promot imports from Dev Ctries 116
Vejan P, Abdullah R, Khadiran T et al (2016) Role of plant growth promoting rhizobacteria in agricultural sustainability-a review. Molecules 21:1–17. https://doi.org/10.3390/molecules21050573
Verdejo-Lucas S, Talavera M (2019) Root-knot nematodes on zucchini (Cucurbita pepo subsp. pepo): pathogenicity and management. Crop Prot 126:104943. https://doi.org/10.1016/j.cropro.2019.104943
Viaene N, Coyne DL, Davies KG (2013) Biological and cultural management. Plant nematology. CABI, Wallingford, pp 383–410
Vikram, Walia RK (2015) Efficacy of Bacterial Parasite, Pasteuria penetrans Application as Seed Treatment against Root-Knot Nematode, Meloidogyne javanica. Indian J Nematol 45:1–6
Wei B-Q, Xue Q-Y, Wei L-H, Niu D-D, Liu H-X, Chen L-F, Guo J-H (2009) A novel screening strategy to identify biocontrol fungi using protease production or chitinase activity against Meloidogyne root-knot nematodes. Biocontrol Science and Technology 19(8):859–870. https://doi.org/10.1080/09583150903165636
Wei JZ, Hale K, Carta L et al (2003) Bacillus thuringiensis crystal proteins that target nematodes. Proc Natl Acad Sci U S A 100:2760–2765. https://doi.org/10.1073/pnas.0538072100
Westerdahl BB (2011) Cultural methods for managing nematodes on vegetables and ornamentals. Acta Hortic 911:185–198. https://doi.org/10.17660/ActaHortic.2011.911.18
Whipps JM (2001) Microbial interactions and biocontrol in the rhizosphere. J Exp Bot 52:487–511
Xiang N, Lawrence KS, Donald PA (2018) Biological control potential of plant growth-promoting rhizobacteria suppression of Meloidogyne incognita on cotton and Heterodera glycines on soybean: a review. J Phytopathol 166:449–458. https://doi.org/10.1111/jph.12712
Yang J, Tian B, Liang L, Zhang KQ (2007) Extracellular enzymes and the pathogenesis of nematophagous fungi. Appl Microbiol Biotechnol 75:21–31. https://doi.org/10.1007/s00253-007-0881-4
Yang JI, Loffredo A, Borneman J, Becker JO (2012) Biocontrol efficacy among strains of pochonia chlamydosporia obtained from a root-knot nematode suppressive soil. J Nematol 44:67–71
Zhai Y, Shao Z, Cai M, et al (2018) Multiple modes of nematode control by volatiles of Pseudomonas putida 1A00316 from Antarctic soil against Meloidogyne incognita. Front Microbiol 9: https://doi.org/10.3389/fmicb.2018.00253
Zhang F, Peng D, Ye X, et al (2012) In vitro uptake of 140 kDa Bacillus thuringiensis nematicidal crystal proteins by the second stage juvenile of Meloidogyne hapla. PLoS One 7: https://doi.org/10.1371/journal.pone.0038534
zi-Quan Y, Qian-Lan W, Bin L et al (2008) Bacillus thuringiensis crystal protein toxicity against plant-parasitic nematodes. Chinese J Agric Biotechnol 5:13–17. https://doi.org/10.1017/S1479236208002003
Zuckerman BM (1984) Nematode chemotaxis of host/prey recognition. Annu Rev Phytopathol 95:95–113
Author information
Authors and Affiliations
Contributions
L.A. wrote the manuscript text. M.E.M.E.B read the article, reviewed it, and provided feedback before the manuscript’s submission. E.H.M and M.B reviewed the plan of the review and provided guidance throughout the process of creating this review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Azlay, L., El Boukhari, M., Mayad, E. et al. Biological management of root-knot nematodes (Meloidogyne spp.): a review. Org. Agr. 13, 99–117 (2023). https://doi.org/10.1007/s13165-022-00417-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13165-022-00417-y