Abstract
The progress of neurosurgery as a specialty is intrinsically related to the history of treatment for acoustic neuroma (vestibular schwannoma). Dealing with vestibular schwannomas (VS) has developed from almost a death sentence at the beginning of the century to the current concept of “functional microsurgery.” In this interim, several developments regarding imaging modalities and microsurgical techniques, as well as intraoperative neuromonitoring, were responsible for significant reductions in the morbidity of patients suffering from cerebellopontine angle diseases. Herein, we provide an overview of the VS’s state of the art emphasizing aspects such as surgical results, intraoperative monitoring techniques and prediction criteria, the vasoactive treatment, and the current role of radiosurgery from the surgeon’s perspective.
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
If any neurologic surgeon were asked to name the most difficult tumor to extirpate, his answer would doubtless be the acoustic tumor. Dandy, 1941 [1]
Approximately seven decades separate this comment from current days. In this interim, several developments regarding imaging modalities and microsurgical techniques, as well as intraoperative neuromonitoring, were responsible for significant reductions in the morbidity of patients suffering from cerebellopontine angle diseases [2–6].
The progress of neurosurgery as a specialty is intrinsically related to the history of treatment for acoustic neuroma (vestibular schwannoma) [5]. Dealing with vestibular schwannomas (VS) has developed from almost a death sentence at the beginning of the century [2, 4, 7] to the current concept of “functional microsurgery” [8]:
A few surgeons have thought that the attempt to preserve hearing is useful in every case, because each functionally preserved cochlear nerve might be useful now or later in a patient’s life, in view of further technological development, and because of the tremendous educational benefit of collecting experiences and gaining microsurgical skills. If all microsurgeons could agree on this, the last decade of the century would be the era not only of microsurgery but also of functional microsurgery. Samii and Matthies [8]
Herein, we provide an overview of VSs focusing on current aspects of intraoperative monitoring, treatment modalities, and decision-making.
2 Histopathological Remarks
VSs are benign neoplasms that are caused by an overproliferation of Schwann cells on the vestibular branches of the vestibulocochlear nerve [5, 9–11]. The vestibular nerves are enclosed by a proximal neuroglial and a distal neurilemmal or Schwann cell sheaths [12]. From the distal sheath arise the VSs at or close to the neuroglial-neurilemmal junction [13]. The transition zone is encountered 1 cm away from the pons commonly occurring at or just the internal auditory canal (IAC) [5]. However, the position of the central-peripheral interface, as well as the site of origin of these tumors, may vary [11] contributing to different clinical and audiological presentation of medially and laterally arising tumors [14, 15].
Macroscopically, VSs appear as lobular in form, well encapsulated, and solid tumors [5]. Cystic formation is well recognized, although being uncommon [5]. The nerves are stretched over the tumor surface by tumor growth [5]. In addition, surrounding blood vessels such as anterior inferior and posterior inferior cerebellar arteries may also be adherent to the tumor capsule [5]. Tumor consistency differs from very soft to firm and adherent [5].
Microscopically, VS consists of two types of tissues, namely, those with Antoni A or Antoni B fibers [5]. Antoni B fibers are loose, semipalisading arrangements of Schwann cells, wherein the Antoni A fibers are observed in a denser arrangement showing more nuclei and firmer cytoplasm [5].
Histologically, the differential diagnosis is limited to meningiomas due to occurrence in both tumors of xanthomatous cells and lipofuscin, as well as palisading of cells [5]. However, the presence of whorls and psammoma bodies may be useful for the diagnosis of meningioma [5]. VSs containing islets of meningioma are usually described related to neurofibromatosis type 2 (NF2), although it may also be reported in sporadic VS [16]. NF2 is a severe autosomal dominant disease predisposing to multiple tumors of the peripheral and central nervous systems [17, 18]. The hallmark of the disease is the occurrence of bilateral VS [17].
3 Clinical Remarks
The clinical presentation of VSs may vary broadly [3, 5, 11, 15, 19–21]. In part, the wide variety of symptoms is due to different stages of tumor growth. The evolution comprises four different stages [11]. Initially, the tumor produces otological symptoms such as high-frequency sensorineural hearing loss, tinnitus, vertigo, and disequilibrium [11, 22]. This stage is also known as otological [11] or intracanalicular stage [22]. As the tumor grows out from the porus acusticus, worsening of the auditory symptoms is observed together with the onset of headache and facial hypoesthesia due to trigeminal compression, when the tumor reaches 2 cm in size [11, 22]. The third stage is characterized by filling of the cerebellopontine cistern compressing the brainstem, ultimately involving the facial nerve, the cerebellum, as well as the lower cranial nerves [11, 22]. Finally, the tumor causes a shift of fourth ventricle and obstructive hydrocephalus [11]. Fortunately, nowadays even the largest tumors seldom achieve this stage [5].
Besides, as aforementioned, the site of origin of these tumors may vary [11] contributing to different clinical and audiological presentation [14, 15]. VSs may arise laterally, intermediately (nearby), or medially to IAC [11, 15]. In patients affected of medially arising tumors, hearing is significantly better preserved in comparison to intermediate and laterally arising tumors [11, 15]. On the other hand, these patients present with larger tumors which develop without significant auditory symptoms [11, 15]. Smaller tumors along with early hearing complaints are frequently observed in patients affected of laterally arising tumors [11, 15]. The relationship of tumor clinical presentation and tumor morphology would be able to explain some differences regarding signs and symptoms, tumor size, and time of disease. Nevertheless, this association is not corroborated by other authors [21].
Hearing loss is the most frequent clinical symptom of VS [3, 5, 11, 15, 20, 21, 23, 24]. Cochlear nerve disturbances affect 91 % of patients suffering from VS [3]. Normal audiological presentations are rarely observed, comprising 2.7–12 % of patients with detected VS [21]. Preoperative deafness occurs chronically in 23 % [3] or acutely in 3–13 % of patients [3, 14, 20, 21, 23]. The vestibular system is subjectively affected in 61 % of patients, followed by the trigeminal nerve in 9 %, the facial nerve in 6 %, the caudal nerves in 2.7 %, and the abducens nerve in 1.8 % of patients [3]. The cranial nerves are objectively more involved than noted by the patients [3]. Trigeminal neuralgia and facial hemispasm are rarely reported as presenting symptom of VS [3, 25]. Concerning trigeminal neuralgia, it is considered to be a sign of large tumors; however it may also be associated with small tumors as a part of typical vascular compression pathology [25].
VS-associated symptomatic hydrocephalus is a well-recognized phenomenon occurring in circa 13 % of patients [26]. It is worth noting that the pathogenesis of hydrocephalus in VS patients can result of tumor-induced obstruction of the CSF flow or as a consequence of CSF malabsorption in 21 and 79 % of patients, respectively [26]. Pronounced signs of intracranial hypertension are an otherwise rare event affecting 9.4 % of patients [26].
4 Diagnosis and Classification
High degree of clinical suspicion is the best key for prompt diagnosis [3, 5, 20]. The clinician should be aware of VS diagnosis in patients complaining of unilateral hearing loss, tinnitus, and vertigo in any combination [5].
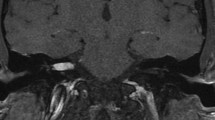
Magnetic resonance imaging (MRI) has evolved to become the “gold standard” for VS diagnosis [11, 20, 21, 23]. Tumors small as 2–3 mm may be diagnosed using MRI [23]. Tumors appear isointense on T1-weighted images enhancing uniformly after contrast injection [5]. Eventually, tumors exhibit heterogeneous contrast enhancement or cystic degeneration. Hemorrhage is rarely observed [5]. T2-weighted gradient-echo image (3DFT-CISS – three-dimensional Fourier transformation-constructive interference in steady state) is particularly useful because of its high contrast and spatial resolution showing with precision tumor extension to the fundus of the IAC and the signal intensity of the intralabyrinthine fluids [27] (Fig. 23.1). Hearing is less preserved in tumors that obliterate the fundus in comparison to tumors that do not reach the fundus [27]. In addition, a low intralabyrinthine signal is followed by preserved hearing in solely 20 % of cases [27]. Somers et al. [27] believe that the hypointense intralabyrinthine signal is related to vascular compromise of the labyrinth structures caused by mechanical compression.
MRI in the axial view showing a small left vestibular schwannoma extending to the cerebellopontine angle cistern. On T1- (a) and T2-weighted (b) images, the tumor appears isointense. Note the cerebrospinal fluid (CSF) collection between the tumor and the fundus of the internal auditory canal (IAC) (b). After gadolinium injection (c), homogeneous enhancement is typically observed
MR imaging with CISS sequences has also been applied for direct identification of cranial nerves in healthy individuals [28] and VS patients as well [29]. Recently, diffusion tensor imaging-based (DTI) fiber tracking was validated as a reliable tool for preoperative estimation of the position of the facial nerve in relation to large VSs [29]. In 91 % of patients, the position of the facial nerve was correctly predicted preoperatively. On the other hand, DTI was not useful for estimation of facial nerve morphological shape (compact or flat) [29]. This is a valuable information for surgical planning and facial nerve preservation which should be investigated in larger studies.
Computed tomography (CT) is still helpful for identifying bone destruction or expansion of the IAC [5, 30]. Besides, preoperative CT is important for surgical planning wherein the position of the labyrinth structures, their relationship to fundus, as well as the position of the sigmoid sinus and the emissary vein should be evaluated [5, 30]. Differential diagnosis comprises meningiomas, hemangiomas, and glomus tumors [5].
Evaluating NF2 patients, VSs are encountered in 96 % of them being mostly bilateral, although unilateral VS is also reported [17]. Tumors that appear multilobulated may designate a lesion of multiple nerve tumors arising from different nerves (from the vestibular, cochlear, and facial nerves) [31] (Fig. 23.2). Spinal tumors are more common than previously reported comprising 90 % of NF2 patients [17]. Meningiomas in 58 % of NF2 patients and trigeminal schwannomas in 29 % are also part of the clinical spectrum of the disease [17].
The patients are classified regarding the tumor extension as follows: class T1, purely intrameatal; T2, intra- and extrameatal; T3a, filling the cerebellopontine cistern; T3b, reaching the brainstem; T4a, compressing the brainstem; and T4b, severely dislocating the brainstem and compressing the fourth ventricle [3, 4, 8, 30, 32] (Figs. 23.3, 23.4, 23.5, 23.6 and 23.7) (Table 23.1). Patients affected by tumors larger than 4 cm in the maximum extrameatal diameter are considered to harbor giant lesions, which brings about a challenging situation for cranial nerve preservation due to severe nerve stretching [33].
A complete audiological evaluation is performed based on patient’s pure tone audiogram (PTA), speech discrimination scores (SDS), and brainstem auditory evoked potentials (BAEP). VS may cause hearing loss of any frequency; however hearing loss affecting high frequencies is the most common audiometric finding [15, 23, 34]. Flat loss, trough-shaped loss, and low tone loss have been also reported in evaluating PTA of patients suffering from VS [15, 23, 34]. High-frequency hearing loss together with a marked reduction in SDS is a landmark sign of an eighth nerve lesion, such as VS [34]. The auditory function is analyzed by using the new Hannover classification, as follows: H1 (normal hearing), 0–20 dB and 95–100 % SDS; H2 (useful hearing), 21–40 dB and 70–94 % SDS; H3 (moderate hearing), 41–60 dB and 40–69 % SDS; H4 (poor hearing), 61–80 dB and 10–39 % SDS; and H5 (no functional hearing), >80 dB and 0–9 % SDS [8] (Table 23.2).
BAEP is considered to be the most sensitive audiological test for diagnosis of VS [15, 32]. Nevertheless, a lower sensitivity is expected in patients with normal audiological findings [21] showing additionally a significant variation with tumor size [20]. In tumors smaller than 1 cm, the BAEP’s wave morphology was abnormal solely in 76.5 % of patients, whereas in tumors larger than 2 cm, it was abnormal in 100 % [20]. Given the relevance of early diagnosis of VS along with the aforementioned lower sensitivity, MRI is recommended whenever there is clinical suspicion of VS [20].
The patients are further classified according to Nordstadt’s BAEP typing system, consisting of types B1 to B5 [32] (Table 23.3). Preoperative prediction of hearing preservation may be more accurately evaluated when combining clinical data from the patients’ audiometry, the radiological data of tumor extension, and neurophysiological parameters [32]. These data are currently used for patient counseling regarding timing of surgery [32]. Patients suffering from small tumors, together with good to fair hearing and BAEP types B1 and B2, attain the best hearing preservation rates [32]. The House-Brackmann scale is used to assess facial function [35].
5 Treatment Options
The treatment comprises clinical and radiological observation, surgery, and radiation therapy. The patients are advised of all treatment modalities and the decision is taken together with the medical team.
The conservative management is a viable treatment alternative for VS being advocated by some authors in selected cases such as patients with advanced age, poor general health, small tumors with minimal or no symptoms (especially intracanalicular), tumors in the only or better-hearing ear, NF2, patient preference, and any combination of these [36–42]. Nevertheless, the conservative management carries a significant risk of hearing loss [36–42] even in nongrowing tumors [43] but particularly in those tumors with a growth rate greater than 2.5 mm/year at any point during follow-up [44, 45]. Therefore, when hearing preservation is considered, earlier intervention should be taken into account [36, 37]. Besides, the chances of hearing preservation are better in cases of short symptom duration [8, 31]. In addition to hearing deterioration, loss of patient’s compliance should be considered in choosing the conservative management [36, 39, 42].
In a systematic review, Sughrue et al. [44] analyzed 982 untreated patients and described a mean growth rate of 2.9 ± 1.2 mm/year in a 26–52-month period. A recent prospective study of 178 untreated small VS compared three different growth models, namely, a mm/year-based model, a cm3/year-based model, and a volume doubling time (VDT)-based model [46]. Varughese et al. [46] observed that the mean growth rate was of 0.66 mm/year, 0.19 cm3/year, and a VDT of 4.4 years, which was found as the most realistic VS growth model indicating a threshold of 5.22 years as the cutoff point to distinguish growing from nongrowing tumors [46].
5.1 Surgery
The surgical indications comprise, as follows:
-
Large tumors (brainstem compression)
-
Cystic tumors
-
Young patients (<60 years)
-
Vestibular-related main symptoms
-
Bilateral tumors in NF2 patients
-
Patient’s individual decision
Concerning NF2 patients, the first surgery is recommended as soon as possible. If hearing preservation is not a goal, the surgeon may adopt a waiting attitude for the second tumor while the patients are scheduled for lip-reading learning. On the other hand, if hearing preservation is a goal, the second surgery is planned for the next 2 or 3 months at which total or deliberate subtotal resection may be performed with the assumption of function preservation, particularly in the last hearing ear.
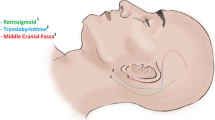
VSs may be operated on by the retrosigmoid approach, the middle fossa approach, and the translabyrinthine approach.
The risk implied for patients is minimized if the surgeon and the team use the approach with which they are most familiar. Samii and Matthies [8]
We perform solely the retrosigmoid approach at our department for VS because it provides a wide exposure of the cerebellopontine angle (CPA) allowing superior and inferior extensions of the approach for treating multiple tumors within a single procedure (as occur in NF2 patients), as well as preservation or reconstruction of the cranial nerves [8].
The operative nuances of the retrosigmoid approach for VS resection and auditory brainstem implant (ABI), as well as the preoperative and postoperative precautions, were detailed in Chap. 9.
5.1.1 Intraoperative Monitoring (IOM)
5.1.1.1 Cochlear Nerve Monitoring
The introduction of intraoperative neurophysiological monitoring technique for the auditory function emerged in the 1970s and at the beginning of the 1980s together with the first attempts of hearing preservation in CPA surgery [32, 47].
The goal of intraoperative auditory function monitoring is to provide information about the auditory pathway status during surgery in order to prevent damage to the auditory function [48] and consequently hearing loss. Early detection of the damage through a near-real-time recording technique is required for the electrophysiological method to alert the surgical team, resulting in immediate interruption or change of the microsurgical maneuvers with the assumption for reversal of the damaging process. This may enable wave recovery sparing hearing loss [48, 49].
The brainstem auditory evoked potential (BAEP) is the most widely used intraoperative electrophysiological method for auditory function monitoring, because it has a high sensitivity [48] and reliability to detect cochlear nerve damage [50]. However, BAEP requires 30 s up to 4 min to deliver reliable response of wave changes [34, 49], which is a disadvantage of the method. In this interim, a permanent damage to the cochlear nerve could happen, preventing the surgical team from detecting and avoiding it [48].
Direct auditory nerve manipulation [49, 51, 52], cerebellar retraction [49, 51, 52], and drilling of the internal auditory canal [49] are identified events that lead to intraoperative BAEP changes and, ultimately, could intervene with the postoperative hearing outcome.
During intraoperative BAEP monitoring, three changes can happen, as follows: latency increase, amplitude decrease, and wave loss [49]. Usually, latency increase and amplitude decrease are observed in the way to wave loss [49].
“When to warn the surgeon?” still is a matter of debate between authors. Empirical and divergent warning criteria have been used for different electrophysiological teams to warn the surgeon about deteriorating BAEP: (a) latency increase of 0.3 ms or amplitude decrease of 50 % [49], (b) latency increase of wave V of 0.5 ms [48], (c) latency increase of wave V of 0.07 ms/min or more than 1.5 ms (absolute value) [51], or (d) latency increase of wave V of 1.0 ms and amplitude decrease of 50 % [48, 53, 54].
Latency increase of wave V of 1.0 ms and amplitude decrease of 50 % are the most widely used criteria [48, 53, 54]. However, the observance of hearing impairment in CPA tumor surgeries following increased latency of less than 1 ms together or not with amplitude decrease smaller than 50 % of wave V showed that these criteria may be not sensitive enough as “warning sign” for hearing preservation in this group of patients [54]. Conversely, in patients affected of nonneoplastic CPA diseases, such as neurovascular compression (trigeminal neuralgia, facial hemispasm, etc.), these criteria seemed to be too sensitive in the form that latency increase of wave V of 1.0 ms and amplitude decrease of 50 % or even wave V loss did not predict hearing loss in this group of patients [54].
So, the reasonable answer for that question would be: “It depends on the disease that you are dealing with” [53, 54]. For CPA tumor a more strict criteria should be used and, therefore, we use latency increase of more than 0.3 ms or amplitude decrease greater than 50 % as our reference in such surgeries [49].
As previously reported by Matthies and Samii [49], each I, III, or V temporary or permanent wave loss carries a risk of deafness, as follows: for wave I loss is 65 %, for wave III loss is 65 %, and for wave V loss is 78 %. Whether these losses are concomitant, that is, permanent waves I, III, and V loss or type B5, it is associated with 96 % of deafness postoperatively [49]. The loss of wave III is the most precocious and sensitive sign for predicting postoperative deafness, whereas loss of wave V is the most definitive sign of postoperative deafness [49]. Other authors also emphasize the relevance of waves I and V loss for hearing preservation, alone or in combination [51, 52].
BAEP stability during CPA tumor surgeries indicates high hearing preservation rate [52, 55]. Patients with preoperative types B1 and B2 have a higher rate of hearing preservation (up to 80 %), whereas those with types B4 and B5 have reduced chances of hearing preservation (12–17 %, respectively) [32].
BAEP is performed throughout the procedure at our department in all patients that have types B1 to B4 BAEPs being the microsurgical maneuvers modified if BAEP changes happen that are known to have impact on postoperative hearing.
5.1.1.2 Facial Nerve Monitoring
Even though there is an increasing rate of reported facial nerve preservation following VS surgery [56], facial nerve paresis or paralysis remains a significant risk to the patients [57], being frequent postoperative sequelae of major concern [56]. Indeed, the increasing rates of facial nerve preservation are supported by the introduction of routine intraoperative facial nerve monitoring [56–59]. Conversely, some authors believe that monitoring serves only to speed up the dissection thereby reducing the operating time substantially [5].
Various monitoring techniques have become routine in dealing with complex cranial base lesions [57]. Direct electrical stimulation, continuous electromyography (EMG), and facial motor evoked potential (fMEP) are currently useful adjuncts to CPA surgery [57–60].
Direct electrical stimulation is performed by using a bipolar stimulation probe and the responses are recorded in the subdermally placed needle electrodes into the ipsilateral orbicularis oculi and orbicularis oris. The stimuli have the purpose to directly identify the facial nerve fibers over the tumor surface [58]. Hence, dissection can be performed without risking the flattened nerve bundle [58]. Besides, the stimuli may be intermittently applied assuring the surgeon that the nerve is preserved distal to stimulation [58]. Compound muscle action potentials comparing the response from stimulation at the brainstem to the response at the internal auditory canal may be predictive of functional integrity of the facial nerve [57]. Minimal stimulus intensity of 0.05 mA or less together with a response amplitude of 240 μV or greater is able to predict House-Brackmann grades I and II outcome in 85 % of patients at 1-year follow-up [59]. Disadvantages of the method include its intermittent use and difficulty to locate the facial nerve at the brainstem in large tumors [57, 58].
Continuous EMG is better than direct electrical stimulation alone, improving the rate of facial nerve preservation during cranial base surgery [57, 58]. The spontaneous facial activity registered by EMG shows five typical waveforms that could be classified into spikes, bursts, and A, B, and C trains [58]. A-trains are sustained periodic EMG activities presenting a sinusoidal pattern that produce a high-frequency sound from the loudspeaker [58]. They occur following tumor dissection in the vicinities of the facial nerve, as well as dissection nearby the brainstem and intrameatal decompression [58]. A-trains appearance has a sensitivity of 86 % and a specificity of 89 % in demonstrating postoperative facial function deterioration [58]. In that study, however, a correlation between the number of A-trains and postoperative facial function could not be demonstrated [58]. Thereafter, Prell et al. [61] showed that a quantitative EMG parameter, namely, the train time, is a reliable indicator of postoperative facial palsy. Interestingly, 2 time thresholds are described [61]. For patients with normal preoperative function, a train time of <0.5 s is strongly correlated with a good
postoperative outcome, whereas the 10-s time threshold is associated with facial deterioration in patients with normal preoperative function and those affected by preoperative facial palsy [61].
It is worth mentioning that both studies provided an offline analysis of the intraoperative findings so that waveforms are evaluated retrospectively after the end of the surgical procedure. Recently, the study was continued by developing software capable of recognizing and quantifying A-trains in real time [62]. The software shows train time activity continuously through a monitor inlet in the surgeon’s view depicted as a traffic light display (green, < 0.125 s; yellow, 0.125–2.5 s; red, 2.5–10 s; and black, > 10 s) [62]. Such threshold levels were compared with those obtained in the offline analysis, rendering lower amounts of time by a factor of 4, in which 0.5 s of offline analysis corresponded to 0.125 s of real-time analysis. For patients with normal preoperative facial function, train time activity lower than 0.125 s was strongly correlated with good postoperative outcome, whereas 2.5-s and 10-s thresholds mostly indicated good postoperative function and marked dysfunction, respectively [62]. Conversely, for patients with preoperative facial dysfunction, only the 2.5-s threshold was defined indicating favorable or unfavorable facial function. The surgical strategy was modulated according to intraoperative findings in a way that subtotal or near-total resection was considered when the red and black thresholds were achieved. This situation occurred in 4 of 30 studied patients, whereas in the other 4 patients, threshold levels were exceeded, but the surgical procedure was continued because of the younger age [62]. That study is unique due to first-time demonstration of real-time FN monitoring with interpretation independent from the electrophysiological team. It may contribute in the near future to increasing reliability of facial function prediction during surgical procedures. Nonetheless, a major limitation is that special software is necessary for either offline or real-time analysis.
As aforementioned, both methods have limitations which have stimulated the development of alternative techniques for facial nerve monitoring. Facial motor evoked potential (FMEP), obtained by transcranial electrical stimulation (TES), has recently been introduced and may be considered the most promising method for facial nerve monitoring [57, 63–66] because it surpasses most of the disadvantages of standard techniques, namely, direct electrical stimulation and free-running EMG [65]. FMEP interpretation is independent from the surgeon’s ability to locate the proximal facial nerve at the brainstem and can facilitate recognition of waveform recordings obtained by free-running EMG [63, 64]. FMEP can be performed throughout the procedure at the demand of the surgeon, as well as following neurotonic discharges recorded by EMG or after extensive dissection on the facial nerve [57].
FMEP amplitude ratio reduction of 50 % at the end of the surgery has been identified as a good predictor for postoperative facial nerve outcome following CPA and skull-base surgeries [57, 63–65]. However, by calculating final-to-baseline amplitude ratios, only initial and final FMEP amplitude values are considered for function prediction. We hypothesized that intraoperative variation of FMEP amplitude could correlate with postoperative facial function, even if final-to-baseline amplitude ratios remain above the 50 % cutoff level. With this assumption, we have demonstrated the correlation of intraoperative FMEP deterioration with immediate and late postoperative outcome justifying changes in surgical strategy in order to avoid definitive facial nerve injury. Therefore, event-to-baseline FMEP monitoring is a better predictor of facial function than final-to-baseline isolated measurements [67].
In addition, amplitude has been the only quantitative criterion used to date for interpretation of FMEP [67]. Thus, we also investigated the intraoperative changes of the FMEP waveform morphology and their correlation to postoperative facial function [67]. We found that waveform complexity seems to represent an additional, independent quantitative parameter that can be used for FMEP monitoring, providing excellent results for prediction of postoperative facial function [67].
Just as for cochlear nerve monitoring, the surgical steps are modified or even interrupted if facial nerve changes happen that are known to be significant in postoperative deterioration of facial function. Table 23.4 summarizes prediction criteria for facial nerve monitoring which are related to a satisfactory functional outcome.
5.1.2 Surgical Outcomes
Evaluating a large series of 1,000 VSs, Samii and Matthies [4, 8, 56] achieved complete resection in 97.9 % of patients, whereas deliberate subtotal resection was performed in 2.1 %. The recurrence rate was 0.8 % in patients not affected of NF2 [8]. The mortality rate was 1.1 % [4]. Hemiparesis was observed in 10 patients (1 %), tetraparesis was found in 1 (0.1 %), and caudal cranial nerves palsies were documented in 5.5 % of the patients [4]. In addition, permanent ataxia was observed in 2 patients (0.2 % of the series) [4].
The cochlear nerve was anatomically preserved in 68 % of patients and functionally in 39.5 % [4, 8]. Considering solely small tumors (T1 and T2) and patients with good or normal hearing function, hearing was preserved in up to 88 % of patients [8]. The overall anatomical facial nerve preservation rate was 93 % [56]. Normal facial nerve function was documented in 51 % of the patients [4]. Forty-five percent of the patients showed some reduction of the facial nerve function (House-Brackmann grade 2 in 13 %, grade 3 in 15 %, grade 4 in 6 %, and grade 5 in 11 %); however they accomplished good recovery within 1 year postoperatively [4]. Facial paralysis was experienced in 4 % of the patients, although 1.7% did not show any recovery and were submitted to hypoglossal-facial anastomosis [4].
In 2002, this series was updated to 1,800 VSs considering only hearing function [68]. Again, the rates of hearing preservation showed significant differences regarding tumor extension and preoperative hearing. This work is worth mentioning because it showed that hearing preservation should be considered in patients affected of large VSs, such as classes T3 and T4. In patients with normal or good preoperative hearing, the chances of preserving hearing function comprise 58 % of T3 tumors and 29 % of T4 [68]. In summary, this work showed that:
The summit of hearing preservation has not been reached as improvements are still achieved even in most difficult conditions.
This sentence sounded somehow prophetic in the form that better results were then presented in evaluating the last 200 VSs [69]. Anatomical facial nerve preservation was reached in 98.5 % of patients together with an overall functional hearing preservation rate of 51 % [69]. Permanent surgery-related morbidity and mortality were null in this series [69]. Undoubtedly, the expertise gained over 3,200 patients operated on by Prof. Samii [70] may be one reason for these sounding results.
Giant VS comprises an otherwise special situation due to increased surgical difficulty and surgical-related morbidity and mortality as well [33]. Even though the best management option for such challenging tumors is highly controversial, we share Prof. Samii’s philosophy of complete surgical removal and cranial nerve preservation irrespective of their size [33]. The surgical results of giant VSs were separately analyzed according to this perception rendering also satisfactory results. Total tumor removal was achieved in all 50 patients evaluated with a 92 % anatomical preservation rate and a 75 % rate of excellent or good facial nerve functional outcome at 1-year follow-up [33]. In conclusion, the authors demonstrated that giant VSs can be removed safely via retrosigmoid approach with no mortality and low morbidity [33].
As discussed, anteriorly, the primary goal is total tumor resection. Subtotal removal can be preferred in selected patients, in whom tumor demonstrates brainstem adhesion, or in the case of facial nerve injury risk according to intraoperative inspection and electrophysiological observations. There is an increasing body of recent literature emphasizing a new tenet in VS surgery, namely, the facial nerve-sparing concept [71, 72]. Haque et al. [71] evaluated 151 VSs, in which the primary aim was gross total tumor resection, but it was achieved in only 36 % of patients. The remaining patients were followed and in the light of tumor growth, patients were scheduled for radiosurgery [71]. The authors claimed for the efficacy of such strategy due to excellent facial nerve preservation rates (House-Brackmann 1 and 2 in 96 % of patients) at 1-year follow-up [71]. Another study by van de Langenberg et al. [72], in which the primary aim was subtotal resection for cytoreduction and subsequent radiosurgery, also demonstrated satisfactory facial nerve long-term results and tumor control. It is noteworthy to discuss that in facial nerve-sparing surgery, patients have their status changed to a chronic disease, which deserves closer clinical and imaging follow-up, while after surgery patients are cured or experience a durable long-term freedom from any tumor growth in 89 % in the first 10 years and 81 % at 20-year follow-up [73]. We advise then that subtotal VS removal should be an option, but not a goal.
5.1.2.1 Neurofibromatosis Type 2 (NF2) and Cochlear Nerve-Sparing Concept
Concerning NF2, the surgical outcomes are beneath those reached in patients not affected by NF2. The completeness of resection was achieved in 87.5 % of the patients [31]. Facial nerve was anatomically preserved in 85 %, whereas the cochlear nerve was preserved in 59 % [31]. The overall hearing preservation rate was 36 % [31]. Just as for individuals not affected by NF2, the chances of hearing preservation are greater with early surgery and good preoperative hearing function [31].
Deliberate subtotal resection was performed for brainstem decompression and principally for hearing preservation in the last hearing ear being successful in 8 of 11 patients for this purpose [31]. Therefore, subtotal resection with functional preservation for the last hearing ear is recommended [31]. In addition, another strategy that we have adopted includes surgery for unroofing of the internal auditory canal in order to increase time to definite deafness in patients suffering from intracanalicular tumors.
5.1.2.2 Trigeminocardiac Reflex (TCR) and Vasoactive Treatment
TCR is a phenomenon occurring during intraoperative manipulations or traction of the fifth cranial nerve during its intra- or extracranial course [74–77]. TCR is defined, for neurosurgical procedures, as a decrease of the main arterial blood pressure (MABP) of 20 % or more associated with bradycardia lower than 60 beats/min after direct or indirect traction of the trigeminal nerve [74–76]. TCR occurs in 11 % of the patients undergoing VS surgery [76]. The hypotension caused by this reflex has a negative prognostic impact for hearing preservation in patients harboring large tumors, such as classes T3 and T4 [76].
In patients affected by VS, there is an increased internal auditory canal pressure and thereby compromised vascular supply to the auditory apparatus [24, 76, 78]. The acute hypotension that follows TCR occurs in this ischemic environment and may lead to further decrease in the vascular supply and ultimately to cochlear nerve damage [76]. This is the most likely mechanism to explain the negative impact on the auditory function following TCR [76].
In order to elucidate this issue, we conducted a detailed investigation of intraoperative BAEP changes after TCR during CPA tumor surgery [79]. In a consecutive series of 102 patients, 5 had one or more episodes of intraoperative TCR. Although no direct cause-effect relationship was shown, we suggest TCR as an additional event that may cause BAEP alterations since temporary and permanent wave deterioration occurred minutes after this reflex [79].
Since 2001, there is an increasing interest in the study of the vasoactive treatment for hearing preservation following VS surgery [80]. The rationale for such study was that surgical manipulation elicits nerve edema and vasospasm and somehow triggers a chain mechanism of microcirculation disturbances, nerve ischemia, and, consequently, delayed hearing loss [80, 81]. This process begins during surgery and remains thereafter [82]. According to BAEP at the end of tumor resection, the patients were enrolled to a medical therapy with hemodilution, attempting to improve the microcirculation, and a calcium-channel blocker schema with the assumption to serve as a neuronal protective agent [80, 82]. By using hydroxyethyl starch and nimodipine, Strauss et al. [80] were able to achieve 66.6 % of hearing preservation in the treated group, whereas in the nontreated group, the hearing preservation rate was 30 %.
The significant increase in hearing preservation observed motivated retrospective evaluation of the facial function in those patients [83]. Patients were scheduled for topical and systemic treatment for 10 days. At the end of the surgery, a nimodipine-soaked gel foam patty was applied over the cranial nerves together with the infusion of intravenous nimodipine (15–30 μg/kg/h). At the first postoperative day, hydroxyethyl starch 6 % (500 ml, twice per day) was added for hemodilution. The results were encouraging, especially for patients suffering from complete facial palsy (House-Brackmann 6), in whom 6.7 % of the treated group maintained such clinical grades after 1-year follow-up, whereas in the untreated group, 62.5 % had complete facial dysfunction [83].
The assumption was further investigated with a prospective and randomized design [84]. Patients were stratified in either prophylactic or therapeutic groups according to intraoperative deterioration of facial nerve monitoring [84]. Prophylactic vasoactive treatment showed significantly better results in terms of cranial nerve preservation during VS resection [84].
Several authors studied the hemorheological and hemodynamic effects of the hypervolemic hemodilution [85] and isovolemic hemodilution [86]. During hypervolemic hemodilution, the systolic blood pressure increases up to 20 % in these patients. Induced hypertension, hemodilution, and calcium-channel blocker are also widely used for treating cerebral arterial vasospasm following subarachnoid hemorrhage in order to avoid neurological deficits [86]. Perhaps, the induced hypertension that joins the hemodilution might also play a role in the so-called vasoactive treatment for hearing and facial function preservation, not only postoperatively, but also intraoperatively. At the moment, we take part in a prospective randomized multicenter phase III study to investigate the effect of prophylactic “vasoactive treatment” on the functional preservation of facial and cochlear nerves during VS surgery (Study AKNipro – EudraCT number – 2009-012088-32).
5.1.3 Auditory Brainstem Implant (ABI) and Future Perspectives
Despite the reports of hearing preservation among NF2 patients [31, 87], they have typically became deaf as a result of tumor progression or surgical removal of bilateral VSs [31, 87–90]. These patients develop neural deafness because of the loss of continuity along the cochlear nerve and thereby are unable to profit from cochlear implants [88–90]. Solely a central auditory prosthesis that stimulates proximally to the damaged cochlear nerve is the treatment modality for hearing restoration among NF2 patients [90]. The current option for these patients is ABI, which directly stimulates the cochlear nucleus [87–90].
The electrode is placed at the end of the first- or second-side VS removal into the lateral recess of the fourth ventricle, particularly over the cochlear nucleus complex that bulges in the floor of the lateral recess [87, 88]. Then, the correct position of the electrode is evidenced by using electrically brainstem auditory evoked potentials thereby detecting the auditory pathways response and avoiding false stimulation of the cranial nerve nuclei or long sensory or motor tracts [87, 91]. Facial and glossopharyngeal nerve monitoring by EMG warrants that these nerves are not activated by ABI stimulation [87].
Six weeks postoperatively, the patient is submitted to initial stimulations [87, 88]. Channels that induce unpleasant sounds or nonauditory effects are excluded [87]. ABI provides useful auditory sensations improving the patients’ ability to recognize environmental sounds and lip-reading capacities [87–90]. However, only a few patients are able to detect auditory-only word recognition [87–90].
The irregular tonotopic arrangement of the cochlear nucleus complex is believed to be the reason of these results [87, 89, 90]. Nevertheless, a special device, namely, penetrating ABI that was designed for better alignment of the tonotopic gradient of the cochlear nucleus, did not improve hearing performance significantly [89, 90]. This observation together with the fact that some of ABI recipients not affected of NF2 achieved higher levels of speech perception gives rise to the possibility that these limitations observed in NF2 patients may be associated with cochlear nucleus damage caused by the tumor or tumor resection [90].
So, a new device that bypasses the damaged cochlear nucleus was developed in order to overcome these limitations, called the auditory midbrain implant (AMI) [89, 90]. The inferior colliculus, more specifically the inferior colliculus central nucleus, is a capable site for central auditory prosthesis because it collects almost every kind of preprocessed auditory information that comes from the brainstem, and it has a well-defined tonotopic organization [89, 90]. Animal studies suggest that behavioral responses produced by inferior colliculus central nucleus stimulation are due to detected auditory sensations [89]. Of course, further studies are necessary to give evidence of the usefulness of this auditory device.
Besides NF2 patients, additional indications of ABI comprise VS in the last hearing ear, total deafness in patients with head trauma or cochlear nerve aplasia [87]. At the moment, solely NF2 patients are enrolled for ABI at our department.
5.2 Stereotactic Radiotherapy
Alternatively to conservative and microsurgical treatments, other treatment options for VS include fractionated stereotactic radiotherapy [92] and stereotactic radiosurgery by using both linear accelerator (linac) [93] and gamma-knife-based approaches [94–97] or even with CyberKnife fractionated radiosurgery [98, 99]. Initially, the indications for VS radiosurgery included elderly patients, patients with high surgical risk, and patients with residual or recurrent tumors following surgical resection [95, 100]. Thereafter, some authors extended these concepts recommending radiosurgery as the first-line treatment for VS [94].
Undoubtedly, there have been many advances regarding radiosurgery in the form that better results are currently achieved [94, 96–99]. The reduction of treatment doses to 12–13 Gy showed that tumor growth was similarly controlled while inducing less side effects in terms of hearing loss and function of the trigeminal and facial nerves [101, 102]. Staging the dose as performed in the CyberKnife radiosurgery regimen may reduce injury to adjacent normal cranial nerves thereby improving hearing preservation in VS patients undergoing radiosurgery [98, 99].
The rates of 5-year tumor control following radiosurgery are approximately 95 % [93, 94, 97]. Hearing preservation rates range from 51 to 93 % [93, 94, 96–99]. Worsening of facial nerve function varies from 0 to 21 % [93, 94, 96–99]. Trigeminal dysfunction is also broadly described varying from 0 to 27 % [93, 94, 96–99]. Lobato-Polo et al. [102] evaluated separately patients having 40 years of age or younger and described that the 10-year rate of freedom from additional management was 96 %, the hearing preservation rate was 93 %, the facial nerve preservation rate was 100 %, and there was no incidence of secondary malignancy [102]. However, outcomes at 20-year follow-up are yet to be defined, especially the exact long-term durability and safety (i.e., secondary malignancy), which comprise the primary argument against radiosurgery in young patients [73].
It is worth mentioning that up to 5 % of patients may fail radiosurgery and tumors keep growing beyond expected swelling [100, 103, 104]. For these patients, the surgery is more difficult to be performed due to destroyed arachnoid plane [8, 104, 105]. Conversely, other authors believe that there is no relationship between ease and difficulty in surgery following radiosurgery [100]. Apart from that controversy, Gerganov et al. [105] divided patients into two groups, namely, radiosurgery prior to microsurgery and partial tumor removal followed by radiosurgery prior to current microsurgery. Interestingly, total tumor resection was accomplished in all cases, but the second group carries a higher risk of new cranial nerve deficits and CSF leakage, as well as postoperative hematomas in the tumor bed and cerebellum [105]. Even though surgery was considered more demanding than in nontreated patients, the rate of facial nerve anatomical and functional preservation was roughly the same for the first group and significantly decreased in the second group [105].
Proponents of radiosurgery continue to compare results of the earlier surgical series with more recent radiosurgery clinical studies. Interestingly, they did not realize that microsurgical treatment is also under continuous development toward the concept of functional microsurgery. More strict neurophysiological criteria have been developed improving the rates of hearing and facial nerve preservation. As aforementioned, a recent surgical study showed complete tumor resection in 98 % of patients together with 100 % anatomical facial nerve preservation considering only tumors with Hannover classes T1 to T3. At the last follow-up examination, excellent or good facial nerve function was obtained in 81 % of the patients with no total facial palsy [69]. Moreover, in patients affected of small tumors along with normal to moderate preoperative hearing, the hearing preservation rates reached up to 88 % [8]. So, the indications of radiosurgery remain elderly patients, patients with poor general clinical conditions, patients with residual or recurrent tumors following surgery, and patients’ decision.
References
Dandy WE (1941) Results of removal of acoustic tumours by the unilateral approach. Arch Surg 42:1026–1033
Penzholz H (1984) Development and present state of cerebellopontine angle surgery from the neuro- and otosurgical point of view. Arch Otorhinolaryngol 240:167–174
Matthies C, Samii M (1997) Management of 1000 vestibular schwannomas (acoustic neuromas): clinical presentation. Neurosurgery 40:1–10
Samii M, Matthies C (1997) Management of 1000 vestibular schwannomas (acoustic neuromas): surgical management and results with an emphasis on complications and how to avoid them. Neurosurgery 40:11–23
Sampath P, Long DM (2004) Acoustic neuroma. In: Winn HR (ed) Youmans neurological surgery. Saunders, Philadelphia, pp 1147–1168
Koerbel A, Gharabaghi A, Safavi-Abbasi S, Tatagiba M, Samii M (2005) Evolution of vestibular schwannoma surgery: the long journey to current success. Neurosurg Focus 18:e10
Krause F (1911) Surgery of the brain and spinal cord, vol II. Rebmann, New York, pp 3–32
Samii M, Matthies C (1997) Management of 1000 vestibular schwannomas (acoustic neuromas): hearing function in 1000 tumor resections. Neurosurgery 40:248–262
Luse SA (1960) Electron microscopic studies of brain tumors. Neurology 10:881–905
Rand RW, Kurze T (1968) Preservation of vestibular, cochlear, and facial nerves during microsurgical removal of acoustic tumors. Report of two cases. J Neurosurg 28:158–161
Moffat DA, Golledge J, Baguley DM, Hardy DG (1993) Clinical correlates of acoustic neuroma morphology. J Laryngol Otol 107:290–294
Nager GT, Baltimore MD (1969) Acoustic neuromas. Arch Otolaryngol 89:68–95
Skinner HA (1929) The origin of acoustic nerve tumours. Br J Surg 16:440–463
Moffat DA, Hardy DG, Baguley DM (1989) Strategy and benefits of acoustic neuroma searching. J Laryngol Otol 103:51–59
Berrettini S, Ravecca F, Sellari-Franceschini S, Bruschini P, Casani A, Padolecchia R (1996) Acoustic neuroma: correlations between morphology and otoneurological manifestations. J Neurol Sci 144:24–33
Ludemann W, Stan AC, Tatagiba M, Samii M (2000) Sporadic unilateral vestibular schwannoma with islets of meningioma: case report. Neurosurgery 47:451–454
Mautner VF, Lindenau M, Baser ME et al (1996) The neuroimaging and clinical spectrum of neurofibromatosis 2. Neurosurgery 38:880–886
Kluwe L, Mautner V, Heinrich B et al (2003) Molecular study of frequency of mosaicism in neurofibromatosis 2 patients with bilateral vestibular schwannomas. J Med Genet 40:109–114
Olivecrona H (1967) Acoustic tumors. J Neurosurg 26:6–13
Chandrasekhar SS, Brackmann DE, Devgan KK (1995) Utility of auditory brainstem response audiometry in diagnosis of acoustic neuromas. Am J Otol 16:63–67
Magdziarz DD, Wiet RJ, Dinces EA, Adamiec LC (2000) Normal audiologic presentations in patients with acoustic neuroma: an evaluation using strict audiologic parameters. Otolaryngol Head Neck Surg 122:157–162
Selesnick SH, Jackler RK (1992) Clinical manifestations and audiologic diagnosis of acoustic neuromas. Otolaryngol Clin North Am 25:521–551
Neary WJ, Newton VE, Laoide-Kemp SN, Ramsden RT, Hillier VF, Kan SW (1996) A clinical, genetic and audiological study of patients and families with unilateral vestibular schwannomas. II. Audiological findings in 93 patients with unilateral vestibular schwannomas. J Laryngol Otol 110:1120–1128
Badie B, Pyle GM, Nguyen PH, Hadar EJ (2001) Elevation of internal auditory canal pressure by vestibular schwannomas. Otol Neurotol 22:696–700
Samii M, Matthies C (1995) Acoustic neurinomas associated with vascular compression syndromes. Acta Neurochir (Wien) 134:148–154
Gerganov VM, Pirayesh A, Nouri M et al (2011) Hydrocephalus associated with vestibular schwannomas: management options and factors predicting the outcome. J Neurosurg 114:1209–1215
Somers T, Casselman J, de Ceulaer G, Govaerts P, Offeciers E (2001) Prognostic value of magnetic resonance imaging findings in hearing preservation surgery for vestibular schwannoma. Otol Neurotol 22:87–94
Kakizawa Y, Seguchi T, Kodama K et al (2008) Anatomical study of the trigeminal and facial cranial nerves with the aid of 3.0-tesla magnetic resonance imaging. J Neurosurg 108:483–490
Gerganov VM, Giordano M, Samii M, Samii A (2011) Diffusion tensor imaging-based fiber tracking for prediction of the position of the facial nerve in relation to large vestibular schwannomas. J Neurosurg 115:1087–1093
Matthies C, Samii M, Krebs S (1997) Management of vestibular schwannomas (acoustic neuromas): radiological features in 202 cases – their value for diagnosis and their predictive importance. Neurosurgery 40:469–482
Samii M, Matthies C, Tatagiba M (1997) Management of vestibular schwannomas (acoustic neuromas): auditory and facial nerve function after resection of 120 vestibular schwannomas in patients with neurofibromatosis 2. Neurosurgery 40:696–706
Matthies C, Samii M (1997) Management of vestibular schwannomas (acoustic neuromas): the value of neurophysiology for evaluation and prediction of auditory function in 420 cases. Neurosurgery 40:919–930
Samii M, Gerganov VM, Samii A (2010) Functional outcome after complete surgical removal of giant vestibular schwannomas. J Neurosurg 112:860–867
Moller MB (1994) Audiological evaluation. J Clin Neurophysiol 11:309–318
House JW, Brackmann DE (1985) Facial nerve grading system. Otolaryngol Head Neck Surg 93:146–147
Bozorg Grayeli A, Kalamarides M, Ferrary E et al (2005) Conservative management versus surgery for small vestibular schwannomas. Acta Otolaryngol 125:1063–1068
Rosenberg SI (2000) Natural history of acoustic neuromas. Laryngoscope 110:497–508
Walsh RM, Bath AP, Bance ML, Keller A, Rutka JA (2000) Consequences to hearing during the conservative management of vestibular schwannomas. Laryngoscope 110:250–255
Smouha EE, Yoo M, Mohr K, Davis RP (2005) Conservative management of acoustic neuroma: a meta-analysis and proposed treatment algorithm. Laryngoscope 115:450–454
Shin YJ, Lapeyre-Mestre M, Gafsi I et al (2003) Neurotological complications after radiosurgery versus conservative management in acoustic neuromas: a systematic review-based study. Acta Otolaryngol 123:59–64
Yamakami I, Uchino Y, Kobayashi E, Yamaura A (2003) Conservative management, gamma-knife radiosurgery, and microsurgery for acoustic neurinomas: a systematic review of outcome and risk of three therapeutic options. Neurol Res 25:682–690
Hoistad DL, Melnik G, Mamikoglu B, Battista R, O’Connor CA, Wiet RJ (2001) Update on conservative management of acoustic neuroma. Otol Neurotol 22:682–685
Graamans K, Van Dijk JE, Janssen LW (2003) Hearing deterioration in patients with a non-growing vestibular schwannoma. Acta Otolaryngol 123:51–54
Sughrue ME, Yang I, Aranda D et al (2010) The natural history of untreated sporadic vestibular schwannomas: a comprehensive review of hearing outcomes. J Neurosurg 112:163–167
Sughrue ME, Kane AJ, Kaur R et al (2011) A prospective study of hearing preservation in untreated vestibular schwannomas. J Neurosurg 114:381–385
Varughese JK, Breivik CN, Wentzel-Larsen T, Lund-Johansen M (2012) Growth of untreated vestibular schwannoma: a prospective study. J Neurosurg 116:706–712
Moller AR, Jannetta PJ (1983) Monitoring auditory functions during cranial nerve microvascular decompression operations by direct recording from the eighth nerve. J Neurosurg 59:493–499
Colletti V, Fiorino FG, Mocella S, Policante Z (1998) ECochG, CNAP and ABR monitoring during vestibular Schwannoma surgery. Audiology 37:27–37
Matthies C, Samii M (1997) Management of vestibular schwannomas (acoustic neuromas): the value of neurophysiology for intraoperative monitoring of auditory function in 200 cases. Neurosurgery 40:459–466
Rosahl SK, Tatagiba M, Gharabaghi A, Matthies C, Samii M (2000) Acoustic evoked response following transection of the eighth nerve in the rat. Acta Neurochir (Wien) 142:1037–1045
Grundy BL, Jannetta PJ, Procopio PT, Lina A, Boston JR, Doyle E (1982) Intraoperative monitoring of brain-stem auditory evoked potentials. J Neurosurg 57:674–681
Watanabe E, Schramm J, Strauss C, Fahlbusch R (1989) Neurophysiologic monitoring in posterior fossa surgery. II. BAEP-waves I and V and preservation of hearing. Acta Neurochir (Wien) 98:118–128
Loiselle DL, Nuwer MR (2005) When should we warn the surgeon? Diagnosis-based warning criteria for BAEP monitoring. Neurology 65:1522–1523
James ML, Husain AM (2005) Brainstem auditory evoked potential monitoring: when is change in wave V significant? Neurology 65:1551–1555
Nakamura M, Roser F, Domiani M, Samii M, Matthies C (2005) Intraoperative auditory brainstem responses in patients with cerebellopontine angle meningiomas involving the inner auditory canal: analysis of the predictive value of the responses. J Neurosurg 102:637–642
Samii M, Matthies C (1997) Management of 1000 vestibular schwannomas (acoustic neuromas): the facial nerve-preservation and restitution of function. Neurosurgery 40:684–695
Akagami R, Dong CC, Westerberg BD (2005) Localized transcranial electrical motor evoked potentials for monitoring cranial nerves in cranial base surgery. Neurosurgery 57:ONS78–ONS85
Romstock J, Strauss C, Fahlbusch R (2000) Continuous electromyography monitoring of motor cranial nerves during cerebellopontine angle surgery. J Neurosurg 93:586–593
Neff BA, Ting J, Dickinson SL, Welling DB (2005) Facial nerve monitoring parameters as a predictor of postoperative facial nerve outcomes after vestibular schwannoma resection. Otol Neurotol 26:728–732
Acioly MA, Liebsch M, de Aguiar PH, Tatagiba M (2013) Facial nerve monitoring during cerebellopontine angle and skull base tumor surgery: a systematic review from description to current success on function prediction. World Neurosurg 80(6):e271–e300
Prell J, Rampp S, Romstöck J, Fahlbusch R, Strauss C (2007) Train time as a quantitative electromyographic parameter for facial nerve function in patients undergoing surgery for vestibular schwannoma. J Neurosurg 106:826–832
Prell J, Rachinger J, Scheller C, Alfieri A, Strauss C, Rampp S (2010) A real-time monitoring system for the facial nerve. Neurosurgery 66:1064–1073
Acioly MA, Liebsch M, Carvalho CH, Gharabaghi A, Tatagiba M (2010) Transcranial electrocortical stimulation to monitor the facial nerve motor function during cerebellopontine angle surgery. Neurosurgery 66:354–361
Dong CC, MacDonald DB, Akagami R et al (2005) Intraoperative facial motor evoked potential monitoring with transcranial electrical stimulation during skull base surgery. Clin Neurophysiol 116:588–596
Fukuda M, Oishi M, Takao T, Saito A, Fujii Y (2008) Facial nerve motor evoked potential monitoring during skull base surgery predicts facial nerve outcome. J Neurol Neurosurg Psychiatry 79:1066–1070
Sala F, Manganotti P, Tramontano V, Bricolo A, Gerosa M (2007) Monitoring of motor pathways during brain stem surgery: what we have achieved and what we still miss? Neurophysiol Clin 37:399–406
Acioly MA, Gharabaghi A, Liebsch M, Carvalho CH, Aguiar PH, Tatagiba M (2011) Quantitative parameters of facial motor evoked potential during vestibular schwannoma surgery predict postoperative facial nerve function. Acta Neurochir (Wien) 153:1169–1179
Matthies C, Samii M (2002) Vestibular schwannomas and auditory function: options in large T3 and T4 tumors? Neurochirurgie 48:461–470
Samii M, Gerganov V, Samii A (2006) Improved preservation of hearing and facial nerve function in vestibular schwannoma surgery via the retrosigmoid approach in a series of 200 patients. J Neurosurg 105:527–535
Gharabaghi A, Samii A, Koerbel A, Rosahl SK, Tatagiba M, Samii M (2007) Preservation of function in vestibular schwannoma surgery. Neurosurgery 60:ONS124–ONS128
Haque R, Wojtasiewicz TJ, Gigante PR et al (2011) Efficacy of facial nerve-sparing approach in patients with vestibular schwannomas. J Neurosurg 115:917–923
van de Langenberg R, Hanssens PE, van Overbeeke JJ et al (2011) Management of large vestibular schwannoma. Part I. Planned subtotal resection followed by Gamma Knife surgery: radiological and clinical aspects. J Neurosurg 115:875–884
Sughrue ME, Kaur R, Rutkowski MJ et al (2010) A critical evaluation of vestibular schwannoma surgery for patients younger than 40 years of age. Neurosurgery 67:1646–1654
Schaller B, Probst R, Strebel S, Gratzl O (1999) Trigeminocardiac reflex during surgery in the cerebellopontine angle. J Neurosurg 90:215–220
Koerbel A, Gharabaghi A, Samii A et al (2005) Trigeminocardiac reflex during skull base surgery: mechanism and management. Acta Neurochir (Wien) 147:727–733
Gharabaghi A, Koerbel A, Samii A et al (2006) The impact of hypotension due to the trigeminocardiac reflex on auditory function in vestibular schwannoma surgery. J Neurosurg 104:369–375
Gharabaghi A, Acioly de Sousa MA, Tatagiba M (2006) Detection and prevention of the trigeminocardiac reflex during cerebellopontine angle surgery. Acta Neurochir (Wien) 148:1223
Lapsiwala SB, Pyle GM, Kaemmerle AW, Sasse FJ, Badie B (2002) Correlation between auditory function and internal auditory canal pressure in patients with vestibular schwannomas. J Neurosurg 96:872–876
Acioly MA, Carvalho CH, Koerbel A, Löwenheim H, Tatagiba M, Gharabaghi A (2010) Intraoperative brainstem auditory evoked potential observations after trigeminocardiac reflex during cerebellopontine angle surgery. J Neurosurg Anesthesiol 22:347–353
Strauss C, Bischoff B, Neu M, Berg M, Fahlbusch R, Romstock J (2001) Vasoactive treatment for hearing preservation in acoustic neuroma surgery. J Neurosurg 95:771–777
Strauss C, Fahlbusch R, Romstock J et al (1991) Delayed hearing loss after surgery for acoustic neurinomas: clinical and electrophysiological observations. Neurosurgery 28:559–565
Neu M, Strauss C, Romstock J, Bischoff B, Fahlbusch R (1999) The prognostic value of intraoperative BAEP patterns in acoustic neurinoma surgery. Clin Neurophysiol 110:1935–1941
Strauss C, Romstock J, Fahlbusch R, Rampp S, Scheller C (2006) Preservation of facial nerve function after postoperative vasoactive treatment in vestibular schwannoma surgery. Neurosurgery 59:577–584
Scheller C, Richter HP, Engelhardt M, Koenig R, Antoniadis G (2007) The influence of prophylactic vasoactive treatment on cochlear and facial nerve functions after vestibular schwannoma surgery: a prospective and open-label randomized pilot study. Neurosurgery 61:92–97
Mori K, Arai H, Nakajima K, Tajima A, Maeda M (1995) Hemorheological and hemodynamic analysis of hypervolemic hemodilutiontherapy for cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke 26:1620–1626
Ekelund A, Reinstrup P, Ryding E et al (2002) Effects of iso- and hypervolemic hemodilution on regional cerebral blood flow and oxygen delivery for patients with vasospasm after aneurysmal subarachnoid hemorrhage. Acta Neurochir (Wien) 144:703–713
Colletti V, Fiorino FG, Carner M, Giarbini N, Sacchetto L, Cumer G (2000) The retrosigmoid approach for auditory brainstem implantation. Am J Otol 21:826–836
Otto SR, Brackmann DE, Hitselberger WE, Shannon RV, Kuchta J (2002) Multichannel auditory brainstem implant: update on performance in 61 patients. J Neurosurg 96:1063–1071
Lenarz T, Lim HH, Reuter G, Patrick JF, Lenarz M (2006) The auditory midbrain implant: a new auditory prosthesis for neural deafness-concept and device description. Otol Neurotol 27:838–843
Samii A, Lenarz M, Majdani O, Lim HH, Samii M, Lenarz T (2007) Auditory midbrain implant: a combined approach for vestibular schwannoma surgery and device implantation. Otol Neurotol 28:31–38
Matthies C, Thomas S, Moshrefi M et al (2000) Auditory brainstem implants: current neurosurgical experiences and perspective. J Laryngol Otol Suppl 27:32–36
Koh ES, Millar BA, Menard C et al (2007) Fractionated stereotactic radiotherapy for acoustic neuroma: single-institution experience at The Princess Margaret Hospital. Cancer 109:1203–1210
Rutten I, Baumert BG, Seidel L et al (2007) Long-term follow-up reveals low toxicity of radiosurgery for vestibular schwannoma. Radiother Oncol 82:83–89
Kondziolka D, Lunsford LD, McLaughlin MR, Flickinger JC (1998) Long-term outcomes after radiosurgery for acoustic neuromas. N Engl J Med 339:1426–1433
Pollock BE, Lunsford LD, Flickinger JC, Clyde BL, Kondziolka D (1998) Vestibular schwannoma management. Part I. Failed microsurgery and the role of delayed stereotactic radiosurgery. J Neurosurg 89:944–948
Chung WY, Liu KD, Shiau CY et al (2005) Gamma knife surgery for vestibular schwannoma: 10-year experience of 195 cases. J Neurosurg 102(Suppl):87–96
Hasegawa T, Fujitani S, Katsumata S, Kida Y, Yoshimoto M, Koike J (2005) Stereotactic radiosurgery for vestibular schwannomas: analysis of 317 patients followed more than 5 years. Neurosurgery 57:257–265
Ishihara H, Saito K, Nishizaki T et al (2004) CyberKnife radiosurgery for vestibular schwannoma. Minim Invasive Neurosurg 47:290–293
Chang SD, Gibbs IC, Sakamoto GT, Lee E, Oyelese A, Adler JR Jr (2005) Staged stereotactic irradiation for acoustic neuroma. Neurosurgery 56:1254–1263
Pollock BE, Lunsford LD, Kondziolka D et al (1998) Vestibular schwannoma management. Part II. Failed radiosurgery and the role of delayed microsurgery. J Neurosurg 89:949–955
Flickinger JC, Kondziolka D, Niranjan A, Lunsford LD (2001) Results of acoustic neuroma radiosurgery: an analysis of 5 year’s experience using current methods. J Neurosurg 94:1–6
Lobato-Polo J, Kondziolka D, Zorro O, Kano H, Flickinger JC, Lunsford LD (2009) Gamma knife radiosurgery in younger patients with vestibular schwannomas. Neurosurgery 65:294–301
Iwai Y, Yamanaka K, Yamagata K, Yasui T (2007) Surgery after radiosurgery for acoustic neuromas: surgical strategy and histological findings. Neurosurgery 60:ONS75–ONS82
Sekhar LN (2007) Surgery after radiosurgery for acoustic neuromas: surgical strategy and histological findings. Neurosurgery 60:ONS75–ONS82
Gerganov VM, Giordano M, Samii A, Samii M (2012) Surgical treatment of patients with vestibular schwannomas after failed previous radiosurgery. J Neurosurg 116:713–720
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Tatagiba, M., Acioly, M.A. (2014). Vestibular Schwannoma: Current State of the Art. In: Ramina, R., de Aguiar, P., Tatagiba, M. (eds) Samii's Essentials in Neurosurgery. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-54115-5_23
Download citation
DOI: https://doi.org/10.1007/978-3-642-54115-5_23
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-54114-8
Online ISBN: 978-3-642-54115-5
eBook Packages: MedicineMedicine (R0)