3.3: Transition Elements and Ionic Compounds
- Page ID
- 430554
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
- Name and write formula of the ions of the transition elements
- Name and write the formulas of simple ionic compounds from monoatomic ions
After learning a few more details about the names of individual ions, you will be a step away from knowing how to write the formula of ionic compounds and name them. This section begins the formal study of writing formula and nomenclature (the systematic naming of ionic compounds).
Naming Ions
The name of many monatomic cation is simply the name of the element followed by the word ion. Thus, Na+ is the sodium ion, Al3+ is the aluminum ion, Ca2+ is the calcium ion, and so forth.
However, most transitional elements, tin, and lead lose different numbers of electrons, producing ions of different charges. Iron, for example, can form two cations, each of which, when combined with the same anion, makes a different compound with unique physical and chemical properties. Thus, we need a different name for each iron ion to distinguish Fe2+ from Fe3+.
There are two ways to make this distinction. In the simpler, more modern approach, called the Stock system, an ion’s positive charge is indicated by a roman numeral in parentheses after the element name, followed by the word ion. Thus, Fe2+ is called the iron(II) ion, while Fe3+ is called the iron(III) ion. This system is used only for elements that form more than one common positive ion. We do not call the Na+ ion the sodium(I) ion because (I) is unnecessary. Sodium forms only a 1+ ion, so there is no ambiguity about the name sodium ion.
The second system, called the common system, is not conventional but is still prevalent and used in the health sciences. This system recognizes that many metals have two common cations. The common system uses two suffixes (-ic and -ous) that are appended to the stem of the element name. The -ic suffix represents the greater of the two cation charges, and the -ous suffix represents the lower one. In many cases, the stem of the element name comes from the Latin name of the element. This system does not work when there are more than two cations for a metal. We simply run out of names. The element manganese is an example. See table \(\PageIndex{1}\) for the list.
The element zinc in group 2B is an exception to the transitional elements. The metal loses two valence electrons to form the zinc ion (Zn2+). The most common ion for the element silver in group 1B is the silver ion (Ag+).
| Element | Stem/Root | Charge | Name | ||||||
|---|---|---|---|---|---|---|---|---|---|
| iron | ferr- | 2+ |
ferrous ion or iron(II) ion |
||||||
| 3+ | ferric ion or iron(III) ion | ||||||||
| copper | cupr- | 1+ | cuprous ion or copper(I) ion | ||||||
| 2+ | cupric ion or copper(II) ion | ||||||||
| tin | stann- | 2+ | stannous ion or tin(II) ion | ||||||
| 4+ | stannic ion or tin(IV) ion | ||||||||
| lead | plumb- | 2+ | plumbous ion or lead(II) ion | ||||||
| 4+ | plumbic ion or lead(IV) ion | ||||||||
| chromium | chrom- | 2+ | chromous ion or chromium(II) ion | ||||||
| 3+ | chromic ion or chromium(III) ion | ||||||||
| cobalt | colbalt- | 2+ | colbaltous ion or colbalt(II) ion | ||||||
| 3+ | Cobaltic ion or cobalt(III) ion | ||||||||
| manganese | - |
|
|
||||||
| nickel | - |
|
|
The name of a monatomic anion consists of the stem of the element name, the suffix -ide, and then the word ion. Thus, as we have already seen, Cl− is “chlor-” + “-ide ion,” or the chloride ion. Similarly, O2− is the oxide ion, Se2− is the selenide ion, and so forth. Table \(\PageIndex{2}\) lists the names of some common monatomic ions.
| Ion | Name |
|---|---|
| F− | fluoride ion |
| Cl− | chloride ion |
| Br− | bromide ion |
| I− | iodide ion |
| O2− | oxide ion |
| S2− | sulfide ion |
| P3− | phosphide ion |
| N3− | nitride ion |
The polyatomic ions have their own characteristic names. They will be discussed in the next section.
Name each ion.
- Ca2+
- S2−
- Br-
- K+
- Cu+
- Answer a
-
the calcium ion
- Answer b
-
the sulfide ion (from Table \(\PageIndex{2}\) )
- Answer c
-
the bromide ion
- Answer d
-
the potassium ion
- Answer e
-
the copper(I) ion or the cuprous ion (copper can form cations with either a 1+ or 2+ charge, so we have to specify which charge this ion has
Name each ion.
- Fe2+
- Fe3+
- N3−
- Ba2+
- F-
- Answer a
-
the iron (II) or ferrous ion
- Answer b
-
the iron (III) or ferric ion
- Answer c
-
the nitride ion
- Answer d
-
the barium ion
- Answer e
-
the fluoride ion
Write the formula for each ion.
- the bromide ion
- the phosphide ion
- the cupric ion
- the magnesium ion
- Answer a
-
Br−
- Answer b
-
P3−
- Answer c
-
Cu2+
- Answer d
-
Mg2+
Write the formula for each ion.
- the fluoride ion
- the oxide ion
- the ferrous ion
- the potassium ion
- Answer a
-
F−
- Answer b
-
O2-
- Answer c
-
Fe2+
- Answer d
-
K+
Formulae and Names for Ionic Compounds from Monoatomic Ions
The formula for an ionic compound follows several conventions. First, the cation is written before the anion. Because most metals form cations and most nonmetals form anions, formulas typically list the metal first and then the nonmetal. Second, charges are not written in a formula. Remember that in an ionic compound, the components are ions, not neutral atoms, even though the formula does not contain charges. Finally, the proper formula for an ionic compound always has a net zero charge, meaning the total positive charge must equal the total negative charge. To determine the proper formula of any combination of ions, determine how many of each ion is needed to balance the total positive and negative charges in the compound.
This rule is ultimately based on the fact that matter is, overall, electrically neutral.
If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1− charge. Only one ion of each is needed to balance these charges. The formula for lithium bromide is \(\ce{LiBr}\). By convention, assume that there is only one atom if a subscript is not present. We do not use 1 as a subscript.
When an ionic compound is formed from magnesium ion and oxide ion, the magnesium ion has a 2+ charge, and the oxide has a 2− charge. Although both of these ions have higher charges than the ions in lithium bromide, they still balance each other in a one-to-one ratio. Therefore, the proper formula for this magnesium oxide is \(\ce{MgO}\).
Now consider the ionic compound formed by magnesium ion and chloride ion. A magnesium ion has a 2+ charge, while a chloride ion has a 1− charge:
\[\ce{Mg^{2+}Cl^{−}} \nonumber \]
Combining one ion of each does not completely balance the positive and negative charges. The easiest way to balance these charges is to assume the presence of two chloride ions for each magnesium ion:
\[\ce{Mg^{2+} Cl^{−} Cl^{−}} \nonumber \]
Now the positive and negative charges are balanced, the convention is to use a numerical subscript when there is more than one ion of a given type: so \(\ce{MgCl2}\). This chemical formula says that there are one magnesium ion and two chloride ions in the formula for magnesium chloride. (Do not read the “Cl2” part of the formula as a molecule of the diatomic elemental chlorine. (Chlorine does not exist as a diatomic element in this compound. Rather, it exists as two individual chloride ions.) By convention, the lowest whole number ratio is used in the formulas of ionic compounds. The formula \(\ce{Mg2Cl4}\) has balanced charges with the ions in a 1:2 ratio, but it is not the lowest whole number ratio.
By convention, the lowest whole-number ratio of the ions is used in ionic formulas. There are exceptions for certain ions, such as \(\ce{Hg2^{2+}}\) known as the mercurous ion.
For compounds in which the ratio of ions is not as obvious, the subscripts in the formula can be obtained by crossing charges: use the absolute value of the charge on one ion as the subscript for the other ion. This method is shown schematically in Figure \(\PageIndex{1}\).
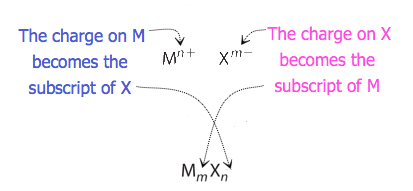
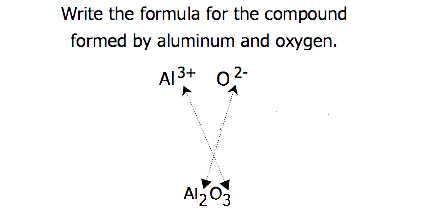
When crossing charges, it is sometimes necessary to reduce the subscripts to their simplest ratio to write the formula. Consider, for example, the compound formed by Pb4+ and O2−. Using the absolute values of the charges on the ions as subscripts gives the formula Pb2O4. This simplifies to its correct formula PbO2 for lead(IV) oxide. The formula has one Pb4+ ion and two O2− ions.
Write the chemical formula and name for an ionic compound composed of each pair of ions.
- the sodium ion and the sulfide ion
- the aluminum ion and the fluoride ion
- the iron(III) ion and the oxide ion
Solution
- To obtain an octet, sodium forms an ion with a 1+ charge, while the sulfide ion has a 2− charge. Two sodium 1+ ions are needed to balance the 2− charge on the sulfide ion. Rather than writing the formula as \(\ce{NaNaS}\), we shorten it by convention to \(\ce{Na2S}\). The name of the compound is sodium sulfide.
- The aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1− charge. Three fluoride ions are needed to balance the 3+ charge on the aluminum ion. This combination is written as \(\ce{AlF3}\). The name of the compound is aluminum fluoride.
- Iron can form two possible ions, but the ion with a 3+ charge is specified here. The oxide has a 2− charge as an ion. To balance the positive and negative charges, we look to the least common multiple (6): two iron 3+ ions will give 6+, while three oxide ions will give 6−, thereby balancing the overall positive and negative charges. Thus, the formula for this ionic compound is \(\ce{Fe2O3}\). Alternatively, use the crossing charges method shown in Figure 3.3.1.
Write the chemical formula for an ionic compound composed of each pair of ions.
- the calcium ion and the oxide ion
- the copper(II) ion and the sulfide ion
- the copper(I) ion and the sulfide ion
- Answer a:
-
CaO
- Answer b:
-
CuS
- Answer c:
-
Cu2S
Name each ionic compound, using both Stock and common systems if necessary.
- Ca3P2
- Fe3N2
- KCl
- CuCl
- SnF2
- Answer a
-
calcium phosphide
- Answer b
-
iron(II) nitride or ferrous nitride
- Answer c
-
potassium chloride
- Answer d
-
copper(I) chloride or cuprous chloride
- Answer e
-
tin(II) fluoride or stannous fluoride
Name each ionic compound, using both Stock and common systems if necessary.
- ZnBr2
- FeN
- Al2O3
- CuF2
- AgF
- Answer a
-
zinc bromide
- Answer b
-
iron (III) nitride or ferric nitride
- Answer c
-
aluminum oxide
- Answer d
-
copper (II) fluoride or cupric fluoride
- Answer e
-
silver fluoride
KEY TAKEAWAY
- Each ionic compound has its own unique name and formula that comes from the names and formulae of the ions.
EXERCISES
- Briefly describe the process for naming an ionic compound.
2. In what order do the names of ions appear in the names of ionic compounds?
3. Which ionic compounds can be named using two different systems? Give an example.
4. Name each ion.
- Ba2+
- P3−
- S2-
- Sn4+
5. Name each ion.
- Cs+
- Al3+
- I-
- Sn2+
6. Name the ionic compound formed by each pair of ions.
- Na+ and Br−
- Mg2+ and Br−
- Mg2+ and S2−
7. Name the ionic compound formed by each pair of ions.
- K+ and Cl−
- Mg2+ and Cl−
- Mg2+ and S2−
8. Name the ionic compound formed by each pair of ions.
- Na+ and N3−
- Mg2+ and N3−
- Al3+ and S2−
9. Name the ionic compound formed by each pair of ions.
- Li+ and N3−
- Mg2+ and P3−
- Li+ and P3−
10. Name the ionic compound formed by each pair of ions. Use both the Stock and common systems, where appropriate.
- Fe3+ and Br−
- Fe2+ and Br−
- Au3+ and S2−
- Au+ and S2−
11. Name the ionic compound formed by each pair of ions. Use both the Stock and common systems, where appropriate.
- Cr3+ and O2−
- Cr2+ and O2−
- Pb2+ and Cl−
- Pb4+ and Cl−
Answers
- Name the cation and then the anion but don’t use numerical prefixes.
- the cation name followed by the anion name
- Ionic compounds in which the cation can have more than one possible charge have two naming systems. FeCl3 is either iron(III) chloride or ferric chloride (answers will vary).
4.
- the barium ion
- the phosphide ion
- the sulfide ion
- the tin(IV) ion or the stannic ion
5.
- the cesium ion
- the aluminum ion
- the iodide ion
- the tin(II) ion or the stannous ion
6.
- sodium bromide
- magnesium bromide
- magnesium sulfide
7.
- potassium chloride
- magnesium chloride
- magnesium sulfide
8.
- sodium nitride
- magnesium nitride
- aluminum sulfide
9.
- lithium nitride
- magnesium phosphide
- lithium phosphide
10.
- iron(III) bromide or ferric bromide
- iron(II) bromide or ferrous bromide
- gold(III) sulfide or auric sulfide
- gold(I) sulfide or aurous sulfide


