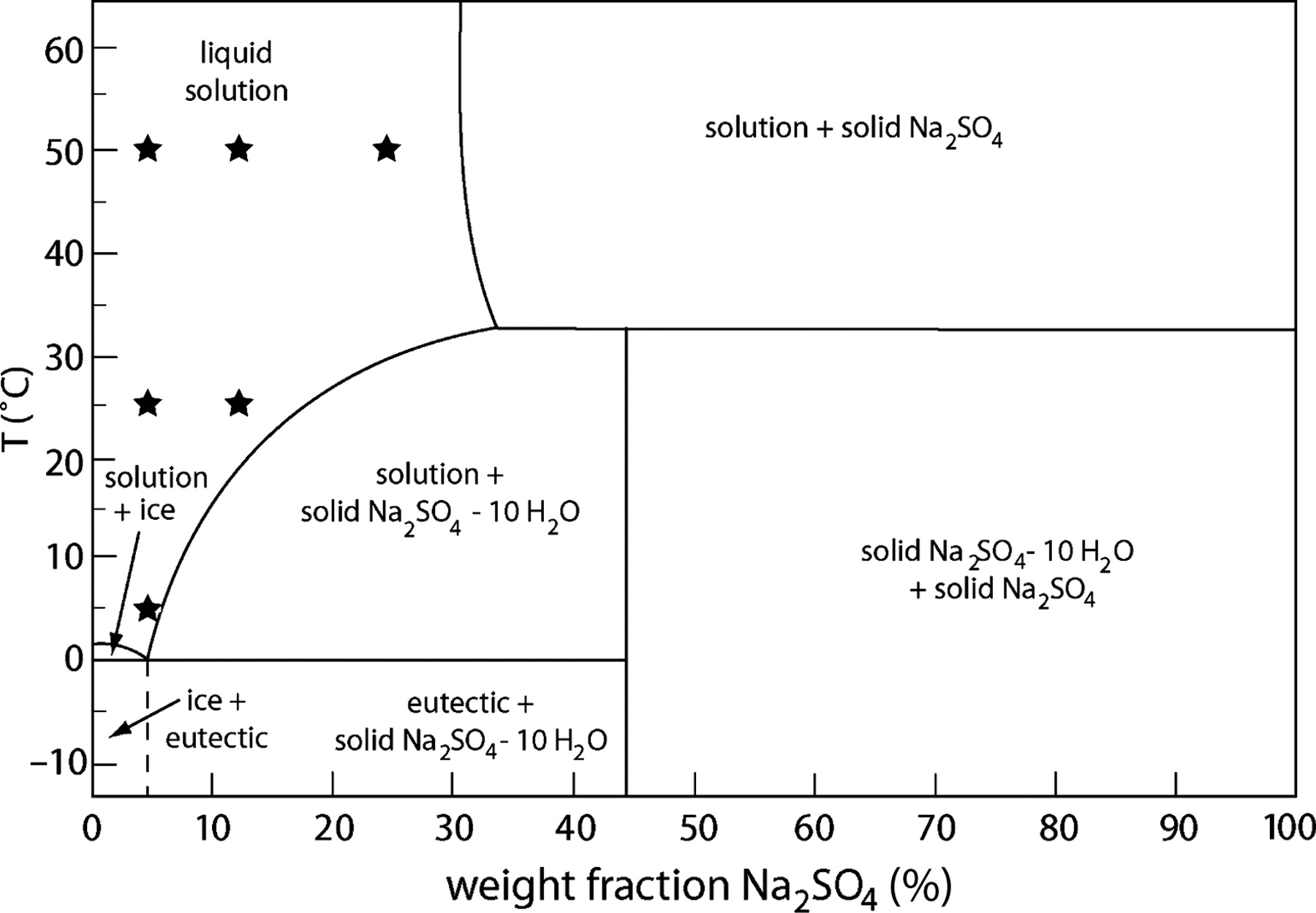
For many salts there are solubility curves as a function of temperature that are smooth (don't have any kinks). Sodium sulfate, however, has a kink in the solubility-T curve at 30 degrees as shown below:

What happens when the temperature is about 30 degree?