Abstract
Purpose of Review
Neonatal sepsis is a diagnosis made in infants less than 28 days of life and consists of a clinical syndrome that may include systemic signs of infection, circulatory shock, and multisystem organ failure.
Recent Findings
Commonly involved bacteria include Staphylococcus aureus and Escherichia coli. Risk factors include central venous catheter use and prolonged hospitalization. Neonates are at significant risk of delayed recognition of sepsis until more ominous clinical findings and vital sign abnormalities develop. Blood culture remains the gold standard for diagnosis.
Summary
Neonatal sepsis remains an important diagnosis requiring a high index of suspicion. Immediate treatment with antibiotics is imperative.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neonatal sepsis is a diagnosis made in infants less than 28 days of life and consists of a clinical syndrome that may include systemic signs of infection, circulatory shock, and multisystem organ failure. Neonatal sepsis may be divided into two types: early-onset neonatal sepsis (EONS) and late-onset neonatal sepsis (LONS). EONS is typically described as infection and sepsis occurring within the first 24 hours to first week of life [1,2,3]. LONS has been labeled as after 24 hours or after the first week of life, up to 28 days or 1 month [4,5,6]. The literature varies in the definition of EONS and LONS, but most categorize EONS as within the first 72 hours of life and LONS as after this time period up to 28 days [7,8,9,10]. Some have proposed the need to create a unified definition worldwide to further develop accuracy in the diagnosis and treatment of EONS and LONS [11, 12•].
Classifications of neonates can be separated even further depending on age and weight. A newborn is an infant within the first 24 hours of life, while a neonate is up to 28 days old. Preterm infants are those born at a gestational age less than 37 weeks, and term infants are those born at or after 37 weeks of gestation. Low birth weight (LBW) is considered less than 2,500 grams and very low birth weight (VLBW) is less than 1,500 g. Extremely low birth weight (ELBW) is used to describe neonates less than 1,000 g. These designations become significant when discussing the etiology of and risk factors for neonatal sepsis. Neonatal sepsis can also be defined as clinically diagnosed or confirmed by positive culture in a typically sterile bodily fluid. The gold standard for the diagnosis of neonatal sepsis is a positive culture in the blood, urine, cerebrospinal fluid, peritoneal fluid, or any other sterile tissues [13, 14].
Methods
We performed a search of the MEDLINE database for the keywords and titles, “neonatal sepsis,” “neonatal fever,” “newborn sepsis,” and “newborn fever,” covering the period January 1, 2018 to December 31, 2018, which resulted in a total of 1,107 citations. Limiting the search to articles published in the English language and including only human studies yielded a total of 1,055 citations. These 1,055 articles were manually reviewed for relevance and there were a total of 64 citations. The remaining 1,003 were not cited as they did not contain relevant information, contained outdated information, or were superseded by more recent articles.
Epidemiology
Worldwide, neonatal sepsis occurs in about 1 to 50 out of 1,000 live births and accounts for 3 to 30% of infant and child deaths annually [15, 16]. In a prospective study performed between 1997 and 1999 at several neonatal centers in South Korea, the incidence rate of neonatal sepsis was 6 per 1,000 live births in those with positive cultures and 30 per 1,000 live births in clinically diagnosed neonatal sepsis [7]. Fatality rates in this study were 2.2% in culture-confirmed neonatal sepsis and 4.7% in clinically diagnosed neonatal sepsis [7]. An analysis performed in Taiwan from 2001 to 2006 found an incidence rate of 4 out of 1,000 live births in all those diagnosed with neonatal sepsis either clinically or by positive culture [8].
A retrospective study from the Netherlands showed a decrease in the incidence of EONS from 4% between 1978 to 1982 to 1.2% from 2003 to 2006 [17]. The incidence of LONS in this study increased from 7.1% between 1978 to 1982 to 13.9% from 2003 to 2006 [17]. A review from the United States (US) in 2012 reported that EONS occurs in 1.5 to 2% of VLBW infants and LONS in 21% of VLBW infants [18]. In an epidemiological study of culture positive diagnoses of neonatal sepsis in Switzerland from 2011 to 2015, the national incidence was 1.43 out of 1,000 live births with a mortality rate of up to 18% [19]. A systematic review that investigated the global burden of neonatal sepsis from 1979 to 2016 showed an annual incidence of three million cases of neonatal sepsis worldwide with a mortality rate of 19% [20].
Etiology
The organisms and pathogens that are most associated with neonatal sepsis differ depending upon country involved. Pathogens range from gram positive and negative bacteria to viruses and fungi, with bacteria being the most frequently identified. The most commonly implicated bacteria include Staphylococcus aureus, coagulase negative staphylococci (CONS), Streptococcus pneumoniae, Streptococcus pyogenes, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Salmonella typhi, and Group B streptococcus (GBS) [21]. Viruses include echovirus, enterovirus, parechovirus, coxsackie virus, adenovirus, parainfluenza virus, rhinovirus, herpes simplex virus, respiratory syncytial virus, and coronavirus [21]. Candida albicans and other Candida species are the most common fungi associated with neonatal sepsis [22].
In the 1990s, the American Academy of Pediatrics (AAP) began to recommend the use of intrapartum antibiotic prophylaxis (IAP) to prevent perinatal GBS, and in 2002, the AAP and the American College of Obstetricians and Gynecologists instituted guidelines on the universal screening by culture of all pregnant women from 35- to 37-week gestation. Due to the widespread use of prophylactic antibiotics for neonates, particularly intrapartum antibiotic use in mothers with positive cultures for GBS, the incidence of GBS-associated neonatal sepsis has declined significantly, a decrease of 70% in the US [8, 23]. During the same period, other countries such as Canada and Taiwan have recommended the universal use of IAP and have seen a decline in the incidence of neonatal sepsis secondary to GBS infection as well [1, 24]. In such countries where IAP is utilized, the most common causative agents of neonatal sepsis are Escherichia coli and gram-positive organisms [1, 24].
Risk Factors
In EONS, which is typically associated with vertical transmission of pathogens from mother to child, the most common pathogens are GBS, Escherichia coli, CONS, Haemophilus influenzae, and Listeria monocytogenes [3, 5, 25, 26]. In LONS, which is most commonly associated with iatrogenic or nosocomial infections, the most common pathogens are CONS, followed by Staphylococcus aureus and Escherichia coli [3, 17, 19, 24]. Risk factors include central venous catheter use and other invasive medical devices as well as prolonged hospitalization [27]. Other risk factors include preterm rupture of membranes, amnionitis, meconium aspiration, LBW, VLBW, ELBW, preterm birth, greater than three vaginal examinations during labor, fever in the mother during labor, or any other infection in the mother during labor [14, 16, 28]. In full-term infants, males have a greater incidence of sepsis compared to female infants, an association not found in preterm infants [21]. A study performed in the US found significant disparity and increased incidence of mortality secondary to neonatal sepsis among children from low household income backgrounds versus those from affluent households [OR 1.19, 95% confidence interval (1.05, 1.35)] [29••].
Clinical Findings
Considering the relatively subtle findings seen during the clinical assessment, neonates are at significant risk of delayed recognition of sepsis until more ominous clinical findings and vital sign abnormalities develop. In the early onset type, they may have a history of fetal distress including fetal tachycardia in the peripartum period. Soon after delivery, there may be other clinical clues such as meconium-stained amniotic fluid and low Apgar scores on initial neonatal assessment. The caretaker may give a history of feeding intolerance, irritability, excessive sleepiness, or “just not looking right.”
Vital sign derangements include both hypothermia and fever. Fever is more common in term babies whereas preterm babies more often demonstrate hypothermia. There may be tachycardia or bradycardia, signs of poor perfusion including cool and pale extremities, and a rapid thready pulse. Respiratory symptoms and signs are common in neonatal sepsis, including grunting, nasal flaring, use of accessory muscles of respiration, cyanosis, and episodes of apnea. Neurological symptoms and signs include lethargy, seizures, irregular respiration, high pitched cry, hypotonia, hypoactive deep tendon reflexes, and abnormal primitive reflexes. Gastrointestinal signs include decreased feeding, vomiting, diarrhea, jaundice, abdominal distension, and hepatosplenomegaly. Skin findings include petechiae, impetigo, cellulitis, and abscess. Underlying metabolic acidosis secondary to poor perfusion can manifest as tachypnea and respiratory distress in the absence of respiratory tract infection.
Diagnostic Testing
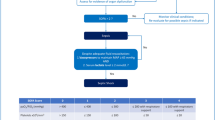
As the symptoms and signs of neonatal sepsis are often very subtle and vague, it is imperative to perform diagnostic testing in any neonate with significant risk factors and concerning signs and symptoms. There are various multivariate predictive scoring systems based on retrospective studies that may be used to predict the need for antibiotics and extensive laboratory evaluation of a neonate versus observation for concerning signs and symptoms. One such example is the EONS calculator based on a large retrospective population study performed in the US to support clinicians in the decision to start antibiotics in neonates suspected of having sepsis [30]. The newborn’s prior probability of EONS obtained from maternal risk factors such as chorioamnionitis and premature rupture of membranes is combined with findings based on the clinical examination, creating a scoring system that can determine the need for antibiotics and level of monitoring required (Table 1). This scoring system has been shown to reduce the proportion of newborns undergoing extensive laboratory evaluation and administration of antibiotics without any adverse effects [31••]. The number needed to treat (NNT) for the high-risk group requiring antibiotics determined by this scoring system was still 118, highlighting the challenges involved in coming up with better diagnostic tools in picking up EONS at an early stage [32].
A complete blood count (CBC) should be performed to assess for total and differential white blood cell count (WBC), absolute and immature neutrophil count, and the ratio of immature to total neutrophil count. Although an absolute leukocytosis has low sensitivity for neonatal sepsis, they may aid in clinical decision-making in cases where a low-to-moderate clinical suspicion for sepsis is present. Interestingly, a low WBC count, low absolute neutrophil count (ANC), and an immature to total neutrophil ratio (I/T) of 0.2 or greater have been shown to be highly predictive of infection [33]. Obtaining an I/T2 ratio by dividing I/T with the total neutrophil count has been shown to have better specificity and area under the curve than I/T and ANC alone in diagnosing EONS [33]. An I/T2 ratio would account for both the elevated immature neutrophils and any neutropenia which can be worrying in the background of sepsis. The sensitivity, specificity, likelihood ratios, and the area under the curve for ANC, I/T, and I/T2 were found to be highest after 4 hours of birth as compared to anytime earlier [33]. Limitations with I/T and I/T2 include the skill of the laboratory personnel performing the manual counts as well as their limited specificity. It is also important to note that there are multiple variables that can affect the various components of WBC, including a crying neonate, gestational age, and arterial versus venous sample [34].
Blood culture remains the gold standard for confirmation of sepsis but is limited by low sensitivity and duration of time before a culture is determined to be positive (often around 24 to 72 hours). Fastidious organisms, maternal antibiotics, and small sample collection limit the sensitivity of blood cultures. False positives may occur due to inadequate skin antisepsis prior to sample collection. At least 0.5 mL of blood should be collected to improve the diagnostic yield. Samples should be collected from two different sites to reduce false positive results. If a central venous catheter is present, blood culture should be taken from both the line and a separate peripheral source, to assess for the differential time to positivity. This helps in distinguishing catheter-associated infections from other sources of infection, which has implications in clinical management.
Swab cultures from surface sites such as the eyes, ears, umbilicus, groin, throat, pharynx, and rectum may provide information about colonizing organisms. They, however, do not contribute to the decision on starting antibiotics, especially if the neonate appears well on clinical examination. Placental cultures may indicate the possible pathogen the fetus was exposed to but does not indicate infection [21]. Placental culture results should not, therefore, be used as a reason for antibiotic therapy. Urinary tract infections are uncommon in the first 72 hours of life. Urine cultures are therefore only performed in the evaluation of LONS [35]. Lumbar puncture (LP) should be routinely performed in neonates showing signs of EONS or LONS. About 23% of neonates with culture-positive bacteremia will have concomitant meningitis [36]. If LP has not been performed in a neonate whose blood culture is positive, it should be performed promptly. Negative blood cultures do not rule out meningitis, as 38% of these individuals will have positive cerebrospinal fluid (CSF) gram stain or culture [37]. False negative CSF gram stain and culture may occur in neonates treated with antibiotics prior to LP.
Acute phase reactants such as C-reactive protein (CRP), procalcitonin, interleukin levels (IL-6 and IL-8), presepsin, haptoglobin, and neutrophil CD64 have been investigated as potential biomarkers for neonatal sepsis. CRP may not be elevated in early stages of infection, due to the time taken for its synthesis in the liver and eventual appearance in the blood. Serial measurements of CRP combined with other acute phase reactants such as procalcitonin, IL-6, and IL-8 may improve its diagnostic accuracy [38].
Procalcitonin (PCT) is more specific than CRP for bacterial infections and rises more rapidly in response to infection than CRP. In normal birth weight infants, a PCT level greater than 0.5 ng/mL is associated with a nosocomial infection, whereas a level of greater than 2.4 ng/mL in VLBW infants should prompt antibiotic therapy [39]. It has been shown that procalcitonin-guided decision making is superior to standard care in reducing antibiotic therapy in neonates with suspected EONS [40]. PCT levels, however, can be elevated with non-infectious conditions such as respiratory distress syndrome, pneumothorax, intracranial hemorrhage, and hemodynamic instability [41]. Serial PCT concentration may be of utility in the evaluation of neonatal sepsis although PCT physiologically increases in the absence of infection over the first 48 hours of life [42, 43].
Presepsin has been found to have a high level of diagnostic accuracy and has been recommended as a valuable marker in neonatal sepsis, albeit not as a single diagnostic test [44•]. A meta-analysis performed to investigate the potential of IL-6 concluded that it could be used as a valid marker for early diagnosis of sepsis in neonatal care units [45].
Newer Diagnostic Techniques
Automated blood culture systems monitor continuously for positive signals, which improves time to detection of pathogens. Matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectroscopy can identify organisms in blood cultures much earlier, allowing antibiotic therapy specific to the organism(s) involved [46]. Multiplex polymerase chain reaction (PCR) can detect the identity of the bacteria or fungi, as well as the presence of antimicrobial resistance genes within hours of identification of the pathogen [47].
PCR can be performed on blood and other body fluids directly without the need to first culture causative organisms. Quantitative real-time amplification systems, known as qPCR, can be used to rapidly rule out the presence of organisms in body fluids, considering its high negative predictive value and a short time to results. The technique is based on 16S ribosomal deoxyribonucleic acid (DNA) amplification. qPCR utilizes a small sample volume and can be used for other bodily fluids such as pleural or peritoneal fluid. Disadvantages include the inability to perform antibiotic susceptibility testing, difficulty in differentiating a recent infection from an active infection, and the presence of contaminants that can give false positive results. Hence, clinical correlation should be made in the interpretation of these results.
Treatment and Management
Management varies depending on a number of factors including age, site of infection, suspected causative organism, microbial resistance patterns, and available resources. Consensus among authors exists that antibiotic therapy should be initiated as soon as neonatal sepsis is suspected, but there is no consensus regarding duration of treatment.
EONS Empiric Antibiotic Therapy
Recommendations from the Canadian Pediatric Society (CPS) and the AAP recommend initiating antibiotic therapy if clinical symptoms are present, with the AAP also recommending antibiotics in the presence of abnormal laboratory values or more than one risk factor (Table 2) [48]. The presence of maternal chorioamnionitis with no neonatal clinical signs warrants antibiotic initiation as per the AAP and only if present with laboratory abnormalities per the CPS. The US Center for Disease Control and Prevention (CDC) recommends empiric antibiotic therapy for all newborns with a maternal diagnosis of chorioamnionitis, regardless of the infant’s clinical condition [48, 49]. Reevaluation at 48 hours and discontinuation of antibiotics if infection was unlikely was universally recommended [48].
Antibiotic therapy should include intravenous ampicillin for GBS, and coverage for Escherichia coli and other gram-negative bacteria implicated in neonatal sepsis, such as gentamicin, with local antibiotic resistance patterns considered [49, 50]. The routine empirical use of broad-spectrum antibiotic agents should only be considered among term newborn infants who are critically ill until culture results are available. Elective genetic testing prior to aminoglycoside use is increasingly being considered to decrease the incidence of permanent hearing loss [51]. Further studies are required as this has not been evaluated in the neonatal setting. In low-resource settings, or when hospitalization is not possible, the use of intramuscular gentamicin and oral amoxicillin in lieu of intravenous medications has been recommended [52].
Treatment of LONS
Early diagnosis, appropriate antibiotic administration, and timely supportive management are the keys to successful treatment [53]. Most cases are attributable to Staphylococcus species and GBS, but about one-third are caused by gram-negative organisms. Most empiric antibiotic regimens include ampicillin, a third-generation cephalosporin, or meropenem, plus an aminoglycoside or vancomycin. In preterm infants, the most common isolates are CONS [54]. Vancomycin and teicoplanin are the antibiotics of choice for a proven and significant CONS infection, but their excessive use has been associated with the development of vancomycin-resistant enterococcus (VRE) infections and gram-negative infections. Their use as first-line antibiotics for nosocomial infection should be avoided. A combination of flucloxacillin and gentamicin can be used to treat the majority of cases caused by other organisms [51]. Clindamycin or metronidazole are sometimes added to cover anaerobic organisms in cases of necrotizing enterocolitis. Cefotaxime is commonly reserved for the treatment of infants with meningitis [53]. Infants with risk factors for candidal sepsis should receive fungal empiric therapy [10].
Treatment with a beta-lactam or beta-lactamase inhibitor combined with an aminoglycoside for Enterobacter, Serratia, or Pseudomonas sepsis is recommended by many experts [53]. Meropenem is recommended for preterm infants with systemic extended-spectrum beta-lactamase infections. In one study, prolonged intravenous infusion of meropenem (over 4 hours every 8 hours) in neonates with gram-negative LONS was associated with better clinical outcome compared to the conventional strategy (over 30 min every 8 h) [55].
Proven Bacterial Sepsis Without Meningitis
In blood culture-proven sepsis, it is reasonable to treat for 10–14 days. A shorter duration (7–10 days) of treatment may be considered in select situations, provided appropriate follow-up can be ensured [56]. Serial daily blood cultures should be performed until blood cultures are negative. Serial CRP measurements may also be used in deciding to discontinue antibiotics [56, 57]. An infant with symptoms can have a false-negative blood culture if antibiotics are given prenatally to the mother or if the blood sample is collected improperly. Hence, antibiotics should be continued for symptomatic infants and those with positive blood culture [56]. Continuing empirical antibiotic therapy in response to laboratory test abnormalities alone is rarely justified, particularly among well-appearing term infants [50]. Prolonged duration of initial empirical antibiotic treatment has been associated with death and necrotizing enterocolitis among premature infants [58].
In resource-poor countries, empiric antibiotic therapy should be individualized for each hospital or region [56]. Consultation with a pediatric infectious disease specialist is warranted for failure of sterilization of the bloodstream (i.e., resistant or atypical organisms) and site-specific infections [50]. The use of pentoxifylline in neonatal sepsis was demonstrated to significantly decrease all-cause mortality during hospital stays in underdeveloped or developing countries, which warrants further investigation in large randomized clinical trials in capable countries [53].
Hydrocortisone has cytokine-suppressing effects, and may improve patient’s cardiovascular status, but has not been evaluated in prospective randomized clinical trials for the treatment of neonatal septic shock [53]. Immunotherapeutic interventions such as intravenous immunoglobulin (IVIG) infusion, IgM-enriched intravenous immunoglobulin, and granulocyte-macrophage colony-stimulating factor are not recommended [53].
Bacterial Meningitis
Intensive care with maintenance of cerebral perfusion, oxygenation, and prevention of hypoglycemia are crucial aspects of management [53]. Combinations of ampicillin, cefotaxime, and aminoglycosides have been suggested by different authors [51, 53, 56]. Cefotaxime plus an aminoglycoside is a good choice for the initial treatment of gram-negative meningitis, due to adequate central nervous system (CNS) penetration. Ceftriaxone may increase the risk of kernicterus in the first week of life and is to be avoided in that age range [59]. For uncomplicated meningitis, the duration of treatment is 14 days for GBS, Listeria monocytogenes, and Streptococcus pneumoniae, and 21 days for Pseudomonas aeruginosa and gram-negative enteric bacteria such as Escherichia coli. Longer duration of therapy is recommended in complicated cases or for delayed clinical improvement [60]. Consultation with a pediatric infectious disease specialist is warranted for cases that are complicated by meningitis. Neuroimaging options include cranial sonography and magnetic resonance imaging and may provide prognostic information [53].
Herpes Simplex Virus (HSV) Infection
Empiric treatment with intravenous acyclovir (20 mg/kg/dose every 8 h) is recommended in cases of aseptic meningitis or suspected meningoencephalitis. Dosage adjustments are warranted in patients less than 34 weeks gestational age or in patients with significant hepatic or renal failure. Treatment is continued for 14 days in localized infections, or 21 days for disseminated disease or CNS infections. In all cases of neonatal HSV, suppressive therapy with acyclovir (300 mg/m2 per dose, orally, 3 times per day for 6 months) immediately following parenteral treatment may improve outcomes in CNS disease and reduce recurrence [53].
Congenital Pneumonia
Prompt diagnosis with recognition of risk factors, early administration of antibiotics, and supportive treatment are important for successfully treating congenital pneumonia. Commonly used antibiotics include ampicillin and gentamicin. Cephalosporins may be considered with failure of therapy with the aforementioned drugs or if Streptococcus pneumoniae is suspected. Supportive therapy includes surfactant replacement and nitric oxide inhalation for persistent pulmonary hypertension of the newborn [53].
Prevention Strategies
The only proven preventive strategy for EONS is the appropriate administration of maternal IAP [50]. Measures that have been postulated to decrease neonatal infection in intensive care units include the consumption of 50 mL/kg/day of fresh (non-donor) human milk, and probiotics, as well as the restriction of H2-blockers, fluconazole, and lactoferrin [61]. Neither GBS IAP nor the aforementioned preventive measures will prevent bacterial LONS [50]. Guidelines for prevention of perinatal transmission of HSV recommend cesarean delivery for women with active genital lesions or prodromal symptoms. It is also recommended that pregnant women with a history of genital herpes infection begin taking oral suppressive therapy at 36 weeks of gestation [62].
Conclusion
Though rates of neonatal sepsis have declined in some parts of the world, globally, it continues to be a significant problem. Testing modalities for the identification and diagnosis of neonatal sepsis continue to be developed, with new laboratory techniques still being tested. Monitoring and management of risk factors as well as IAP remain highly important in the prevention and control of infection in this vulnerable population. Treatment includes prompt antibiotic administration and supportive care in the appropriate hospital setting. Continued vigilance will be key in the diagnosis and management of neonatal sepsis.
Abbreviations
- RF:
-
Risk factor
- AAP:
-
American Academy of Pediatrics
- PROM:
-
Prolonged rupture of membranes
- GBS:
-
Group B streptococcus
- abx:
-
Antibiotics
- h:
-
Hour
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Sgro M, Yudin MH, Lee S, Sankaran K, Tran D, Campbell D. Early-onset neonatal sepsis: it is not only group B streptococcus. Paediatr Child Health. 2011;16(5):269.
Shim GH, Kim SD, Kim HS, Kim ES, Lee HJ, Lee JA, et al. Trends in epidemiology of neonatal sepsis in a tertiary center in Korea: a 26-year longitudinal analysis, 1980-2005. J Korean Med Sci. 2011;26:284–9.
Shah BA, Padbury JF. Neonatal sepsis: an old problem with new insights. Virulence. 2014;5(1):170–8.
Klingenberg C, Komelisse RF, Buonocore G, et al. Culture-negative early-onset neonatal sepsis – at the crossroad between efficient sepsis care and antimicrobial stewardship. Front Pediatr. 2018;6(285):1–9.
Shermadou ES, Mavrogeorgos G Neonatal sepsis. StatPearls. 2018; https://www.ncbi.nlm.nih.gov.
Ng S, Strunk T, Jiang P, et al. Precision medicine for neonatal sepsis. Front Mol Biosci. 2018, 5(70):1–12.
Shin Y, Ki M, Foxman B. Epidemiology of neonatal sepsis in South Korea. Pediatr Int. 2009;51:225–32.
Wu J, Chen C, Tsao P, et al. Neonatal sepsis: a 6-year analysis in a neonatal care unit in Taiwan. Pediatr Neonatol. 2009;50(3):88–95.
Sharma CM, Agrawal RP, Sharan H, Kumar B, Sharma D, Bhatia SS. “Neonatal sepsis”: bacteria and their susceptibility pattern towards antibiotics in neonatal intensive care unit. J Clin Diagn Res. 2013;7(11):2511–3.
Simonsen KA, Anderson-Berry AL, Delair SF, Davies HD. Early-onset neonatal sepsis. Clin Microbiol Rev. 2014;27(1):21–47. https://doi.org/10.1128/cmr.00031-13.
Wynn JL. Defining neonatal sepsis. Curr Opin Pediatr. 2016;28(2):135–40.
• Wynn JL, Polin RA. Progress in the management of neonatal sepsis: the importance of a consensus definition. Pediatr Res. 2017;83(1):13–5 Describes limitations of current diagnostic techniques and potential future directions to improve diagnostic accuracy.
Tam PI, Bendel CM. Diagnostics for neonatal sepsis: current approaches and future directions. Pediatr Res. 2017;82(4):574–83.
Cortese F, Scicchitano P, Gesualdo M, Filaninno A, de Giorgi E, Schettini F, et al. Early and late infections in newborns: where do we stand? A review. Pediatr Neonatol. 2016;57:265–73.
Sherman MP. Long-term epidemiology of neonatal sepsis: benefits and concerns. Neonatology. 2010;97:29–30.
Zhou B, Liu X, Wu J, et al. Clinical and microbiological profile of babies born with risk of neonatal sepsis. Exp Ther Med. 2016;12:3621–5.
Van de Hoogen A, Gerards LJ, Verboon-Maciolek MA, et al. Long-term trends in epidemiology of neonatal sepsis and antibiotic susceptibility of causative agents. Neonatology. 2010;97:22–8.
Srinivasan L, Harris MC. New technologies for the rapid diagnosis of neonatal sepsis. Curr Opin Pediatr. 2012;24:165–71.
Giannoni E, Agyeman PK, Stocker M, Posfay-Barbe KM, Heininger U, Spycher BD, et al. Neonatal sepsis of early onset, and hospital-acquired and community-acquired late onset: a prospective population-based cohort study. J Pediatr. 2018;201:106–14.
Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, Schlapbach LJ, Reinhart K, Kissoon N. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet. 2018;6:223–30.
Shane AL, Sanches PJ, Stoll BJ. Neonatal sepsis. Lancet. 2017;390(10104):1770–80.
Turhan EE, Gusoy T, Ovali F. Factors which affect mortality in neonatal sepsis. Turk Pediatr Ars. 2015;50:170–5.
Fenton-Jones M, Cannon A, Paul SP. Recognition and nursing management of sepsis in early infancy. Emerg Nurse. 2017;25(6):23–8.
Lim WH, Lien R, Huang Y, et al. Prevalence and pathogen distribution of neonatal sepsis among very-low-birth-weight infants. Pediatr Neonatol. 2012;53:228–34.
El-Din S, Rabie EM, El-Sokkary A, et al. Epidemiology of neonatal sepsis and implicated pathogens: a study from Egypt. Biomed Res Int. 2015;2015:1–11.
Schrag SJ, Farley MM, Petit S, et al. Epidemiology of invasive early-onset neonatal sepsis, 2005 to 2014. Pediatrics. 2016;138(6):1–9.
Heo JS, Shin SH, Jung YH, Kim EK, Choi EH, Kim HS, et al. Neonatal sepsis in a rapidly growing, tertiary neonatal intensive care unit: trends over 18 years. Pediatr Int. 2015;57:909–16.
Ocviyanti D, Wahono WT. Risk factors for neonatal sepsis in pregnant women with premature rupture of the membrane. J Pregnancy. 2018;2018:1–5.
•• Bohanon FJ, Lopez ON, Adhikari D, et al. Race, income and insurance status affect neonatal sepsis mortality and healthcare resource utilization. Pediatr Infect Dis J. 2018;37(7):e178–84 This large population-based study described how environmental determinants of health affect healthcare utilization patterns and neonatal sepsis mortality.
Kasier Permanente Division of Research. Neonatal Early-Onset Sepsis Calculator: Infection Probability Calculator. Accessed 1 April 2019. https://neonatalsepsiscalculator.kaiserpermanente.org/InfectionProbabilityCalculator.aspx
•• Kuzniewicz MW, et al. A quantitative, risk-based approach to the management of neonatal early-onset sepsis. JAMA Pediatr. 2017;171(4):365–71 This large cohort study used multivariate regression to identify risk factors for neonatal sepsis resulting in the creation of a sepsis probability calculator that can reduce the need for invasive diagnostic testing and empiric antibiotic administration.
Escobar GJ, Puopolo KM, Wi S, Turk BJ, Kuzniewicz MW, Walsh EM, et al. Stratification of risk of early-onset sepsis in newborns >/= 34 weeks’ gestation. Pediatrics. 2014;133(1):30–6.
Newman TB, Draper D, Puopolo KM, Wi S, Escobar GJ. Combining immature and total neutrophil counts to predict early onset sepsis in term and late preterm newborns: use of the I/T2. Pediatr Infect Dis J. 2014;33(8):798–802.
Chiesa C, Panero A, Osborn JF, Simonetti AF, Pacifico L. Diagnosis of neonatal sepsis: a clinical and laboratory challenge. Clin Chem. 2004;50(2):279–87.
Ruangkit C, Satpute A, Vogt BA, Hoyen C, Viswanathan S. Incidence and risk factors of urinary tract infection in very low birth weight infants. J Neonatal-Perinatal Med. 2016;9:83–90.
Isaacs D, et al. Systemic bacterial and fungal infections in infants in Australian neonatal units. Australian Study Group for Neonatal Infections. Med J Aust. 1995;162(4):198–201.
Garges HP, Moody MA, Cotten CM, Smith PB, Tiffany KF, Lenfestey R, et al. Neonatal meningitis: what is the correlation among cerebrospinal fluid cultures, blood cultures, and cerebrospinal fluid parameters? Pediatrics. 2006;117(4):1094–100.
Hofer N, Zacharias E, Müller W, Resch B. An update on the use of C-reactive protein in early-onset neonatal sepsis: current insights and new tasks. Neonatology. 2012;102(1):25–36.
Auriti C, Fiscarelli E, Ronchetti MP, Argentieri M, Marrocco G, Quondamcarlo A, et al. Procalcitonin in detecting neonatal nosocomial sepsis. Arch Dis Child Fetal Neonatal Ed. 2012;97(5):F368–70.
Stocker M, van Herk W, el Helou S, Dutta S, Fontana MS, Schuerman FABA, et al. Procalcitonin-guided decision making for duration of antibiotic therapy in neonates with suspected early-onset sepsis: a multicentre, randomised controlled trial (NeoPIns). Lancet. 2017;390(10097):871–81.
Chiesa C, Pellegrini G, Panero A, Osborn JF, Signore F, Assumma M, et al. C-reactive protein, interleukin-6, and procalcitonin in the immediate postnatal period: influence of illness severity, risk status, antenatal and perinatal complications, and infection. Clin Chem. 2003;49(1):60–8.
Gkentzi D, Dimitriou G. Procalcitonin use for shorter courses of antibiotic therapy in suspected early-onset neonatal sepsis: are we getting there? J Thorac Dis. 2017;9(12):4899–902. https://doi.org/10.21037/jtd.2017.11.80.
Monneret G, Labaune JM, Isaac C, Bienvenu F, Putet G, Bienvenu J. Increased serum procalcitonin levels are not specific to sepsis in neonates. Clin Infect Dis. 1998;27(6):1559–61.
• Parri N, Trippella G, Lisi C, de Martino M, Galli L, Chiappini E. Accuracy of presepsin in neonatal sepsis: systematic review and meta-analysis. Expert Rev Anti-Infect Ther. 2019;17(4):223–32 A meta-analysis evaluating the value of presepsin as a novel biomarker for neonatal sepsis that reported that use of presepsin can improve diagnostic accuracy in neonatal sepsis.
Shahkar L, Keshtkar A, Mirfazeli A, Ahani A, Roshandel G. The role of IL-6 for predicting neonatal sepsis: a systematic review and meta-analysis. Iran J Pediatr. 2011;21(4):411–7.
Singhal N, et al. MALDI-TOF mass spectrometry: an emerging technology for microbial identification and diagnosis. Front Microbiol. 2015;6:791.
Liesenfeld O, Lehman L, Hunfeld KP, Kost G. Molecular diagnosis of sepsis: new aspects and recent developments. Eur J Microbiol Immunol (Bp). 2014;4(1):1–25. https://doi.org/10.1556/EuJMI.4.2014.1.1.
. Herk WV, Helou SE, Janota J, et al. Variation in Current Management of Term and Late- preterm Neonates at Risk for Early-onset SepsisAn excellent review article describing and comparingn international neonatal sepsis management guidelines. Pediatr Infect Dis J. 2016;35(5):494–500. https://doi.org/10.1097/inf.0000000000001063.
Morbidity and Mortality Weekly Report (MMWR). Centers for Disease Control and Prevention. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5910a1.htm?s_cid = rr5910a1_w. Published November 19, 2010. Accessed 10 May 2019.
Puopolo KM, Benitz WE, Zaoutis TE, COMMITTEE ON FETUS AND NEWBORN, COMMITTEE ON INFECTIOUS DISEASES. Management of neonates born at ≥35 0/7 weeks’ gestation with suspected or proven early-onset bacterial sepsis. Pediatrics. 2018;142:e20182894. https://doi.org/10.1542/peds.2018-2894.
Russell AB, Sharland M. Heath PT Improving antibiotic prescribing in neonatal units: time to act. Arch Dis Child Fetal Neonatal Ed. 2012;97:F141–6.
World Health Organization (2019) Managing possible serious bacterial infection in young infants when referral is not feasible. [online] Available at: https://www.who.int/maternal_child_adolescent/documents/bacterial-infection-infants/en/ Accessed 10 May 2019.
Huang F, Chen H, Yang P, Lin H. Birds eye view of a neonatologist: clinical approach to emergency neonatal infection. Pediatr Neonatol. 2016;57(3):167–73. https://doi.org/10.1016/j.pedneo.2015.06.004.
Pont-Thibodeau GD, Joyal J, Lacroix J. Management of neonatal sepsis in term newborns. F1000Prime Reports. 2014;6. https://doi.org/10.12703/p6-67.
Shabaan AE, Nour I, Eldegla HE, Nasef N, Shouman B, Abdel-Hady H. Conventional versus prolonged infusion of meropenem in neonates with gram-negative late-onset sepsis. Pediatr Infect Dis J. 2017;36(4):358–63. https://doi.org/10.1097/inf.0000000000001445.
Sivanandan S, Soraisham AS, Swarnam K. Choice and duration of antimicrobial therapy for neonatal sepsis and meningitis. Int J Pediatr. 2011;2011:1–9. https://doi.org/10.1155/2011/712150.
Herk WV, Stocker M, Rossum AM. Recognising early onset neonatal sepsis: An essential step in appropriate antimicrobial use. J Infect. 2016;72:S77–82. https://doi.org/10.1016/j.jinf.2016.04.026.
Cotten CM. Antibiotic stewardship: reassessment of guidelines for management of neonatal sepsis. Clin Perinatol. 2015;42(1):195–206.
Voller SMB, Myers PJ. Neonatal Sepsis. Clinical Pediatric Emergency Medicine. 2016;17(2):129–33. https://doi.org/10.1016/j.cpem.2016.03.006.
Ku LC, Boggess KA, Cohen-Wolkowiez M. Bacterial meningitis in infants. Clin Perinatol. 2014;42(1):29–viii. https://doi.org/10.1016/j.clp.2014.10.004.
Manzoni P, De Luca D, Stronati M, Jacqz-Aigrain E, Ruffinazzi G, Luparia M, et al. Prevention of nosocomial infections in neonatal intensive care units. Am J Perinatol. 2013;30(2):81–8. https://doi.org/10.1055/s-0032-1333131.
Harris JB, Holmes AP. Neonatal herpes simplex viral infections and acyclovir: an update. J Pediatr Pharmacol Ther. 2017;22(2):88–93. https://doi.org/10.5863/1551-6776-22.2.88.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Infectious Disease
Rights and permissions
About this article
Cite this article
Ershad, M., Mostafa, A., Dela Cruz, M. et al. Neonatal Sepsis. Curr Emerg Hosp Med Rep 7, 83–90 (2019). https://doi.org/10.1007/s40138-019-00188-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40138-019-00188-z