Abstract
Plant dietary lipids remain one of the most intriguing and complex classes of biological molecules. Among them, medium-chain triglycerides (MCTs) have garnered recognition for their unique physico-chemical properties and potential health benefits. Despite their classification as saturated fats, they stand apart from other saturated fatty acids due to their distinctive characteristics, positioning them as a valuable component in nutrition. While traditional dietary fats primarily contain long-chain triglycerides (LCTs), MCTs consist of medium-chain fatty acids (MCFAs), naturally found in coconut and palm oils. The structural dissimilarity grants MCTs advantageous attributes, encompassing rapid digestion and absorption, providing a swift source of energy. Importantly, MCT oil derived from coconuts surpasses traditional coconut oil in efficiency and speed of energy conversion due to its higher concentration of readily metabolizable MCTs, making it a superior choice in human nutrition. This comprehensive study delves deeply into the potential of coconut-derived MCT oil, illuminating its chemical constituents, production from coconut oil, distinctive physical and chemical properties, and metabolic characteristics. Additionally, it highlights a range of potential biological activities of the oil, including its efficacy in managing gastrointestinal disorders and promising roles in anticancer, neuroprotective, and antimicrobial effects. The report also discusses the extensive applications of MCT oil across diverse industrial and technological sectors, as well as its utilization in structured lipids, oleogels, and as a carrier for capsaicin. Addressing safety concerns and providing dosage guidelines, this paper emphasizes MCT oil as an alternative to coconut oil in various applications, offering a holistic perspective on its benefits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Along with carbohydrates and proteins, lipids belong to the most principal nutrients in the human diet with a plethora of key biological functions. In addition to serving as a source of energy and building blocks of biomembranes, they also participate in numerous cellular signaling pathways and activation of transcription factors (Soto-Avellaneda and Morrison 2020; Bravo-Ruiz et al. 2021), thus significantly impacting human physiology and pathophysiology. Additionally, lipids play a crucial role in influencing the sensory characteristics of food products. In this context, they contribute to the mouthfeel and texture of various food items (Shahidi and Senanayake 2006). Moreover, owing to their excellent biocompatibility, lipids are commonly utilized as safe vehicles for food bioactive ingredients (Akhavan et al. 2018; Garcia et al. 2021; Nahum and Domb 2021) and drug delivery (Yang and Merlin 2020; Kesharwani et al. 2022). However, it is important to note that excessive consumption of lipids is linked with development of obesity which strongly contributes to several diseases, including cardiovascular diseases (atherosclerosis, hypertension), type 2 diabetes, liver diseases, cancer (breast and colon cancer) and other disorders (Shahidi and Senanayake 2006; Jin et al. 2023; Li et al. 2023).
Chemically, lipids consist of fatty, waxy, or oily compounds that exhibit solubility in nonpolar (organic) solvents while being completely insoluble in water. This structurally and functionally heterogeneous group includes: (1) fats and oils (triglycerides, TGs), (2) phospholipids, (3) waxes, and (4) steroids (Ahmed et al. 2023). Dietary lipids are predominantly present in the form of TGs, also known as neutral fats, triacylglycerols, or triacylglycerides. They consist of a glycerol (a 3-carbon sugar alcohol/polyol) backbone esterified with three fatty acids (FAs) (Woodbury 2012). In industrially developed countries, TGs serve as the primary source of dietary lipids, and their average daily consumption ranges from approximately 100 to 150 g, accounting for around 30% of an individual daily caloric intake (Zarevúcka and Wimmer 2008). Complete oxidation of FAs yields approximately 9 kcal/g (Sethi et al. 2022), which is 2.25-fold higher than the energy yield of carbohydrates or proteins (Shahidi and Senanayake 2006; Boateng et al. 2016). The physico-chemical characteristics and biological properties of FAs are strongly determined by the degree of saturation (i.e., number of double bonds in the carbon chain) and the length of the aliphatic chain. Based on these aspects, FAs can be classified as (1): saturated (i.e., SFAs), monounsaturated (i.e., MUFA), and polyunsaturated (i.e., PUFA) (Chen and Liu 2020; Jadhav and Annapure 2023), and as (2): short- (SC), medium- (MC), long- (LC) (Park et al. 2021) or very long-chained (VLC) (Shah and Limketkai 2017), respectively.
This comprehensive study offers an in-depth exploration of coconut-derived MCT oil potential, focusing on its chemical composition, production from coconut oil, unique physico-chemical properties and metabolism features, health benefits and safety. To compile this paper, an extensive literature search was conducted, drawing from internationally recognized databases like Medline/PubMed, Web of Science, Science Direct/Elsevier, and Springer. Additionally, resources like Google Books and Google Scholar were consulted. To gauge the state of coconut oil production in 2020, data from FAOSTAT (https://www.fao.org/faostat/en/#data/QCL) was analyzed. The literature cited in the report was limited to the English language sources, drawing from a total of 291 references dated between 1989 and 2023, with nearly half of them being published within the last 5 years. This approach ensures a contemporary perspective on the subject matter and reflects the latest advancements and insights in the field. The search employed a range of keywords and phrases, including “dietary lipids”, “medium-chain fatty acids”, “medium-chain triglycerides”, “coconut palm”, “coconut oil”, “physico-chemical characteristics of coconut oil”, “coconut oil processing”, “coconut-derived MCT oil”, “physico-chemical and sensory attributes of MCT oil”, “metabolism of MCT oil”, “biological activities of MCT oil”, “industrial and technological applications of MCT oil”, “MCT oil dosage”, “MCT oil safety”. The paper excluded unpublished works, case reports, or letters. Duplicate or irrelevant publications were also sifted out during the screening process. The studies and documents that met the criteria were assessed, identified, and graded by all authors independently, and any discrepancies were resolved through discussion and consensus. In cases of conflicting information or references, preference was given to more recent sources.
While some existing reviews focus either on health benefits of coconut oil (e.g., Sankararaman and Sferra 2018) or MCTs (e.g., Jadhav et al. 2022; Nimbkar et al. 2022), alongside reports evaluating differences in satiety properties between coconut oil and MCT oil (e.g., Kinsella et al. 2017; Maher et al. 2017), it is important to emphasize that they cover only specific aspects extracted from the topic of our report. In effect, this comprehensive study not only delves deeper into the dynamic and kinetic processes of MCT oil within the human body, but it importantly highlights the distinctions between coconut and MCT oil across various perspectives, empowering consumers to optimize their well-being by selecting the most suitable oil based on their preferences and health goals.
Medium-chain triglycerides and medium-chain fatty acids
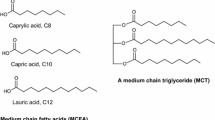
Medium-chain triglycerides (MCTs), a structurally modified type of lipids, are composed of saturated medium-chain fatty acids (MCFAs) containing 6–12 carbon (C) atoms in their chain length. The MCFAs that make up MCTs (Fig. 1) include caproic (hexanoic) acid (C6:0), caprylic (octanoic) acid (C8:0), capric (decanoic) acid (C10:0), and lauric (dodecanoic) acid (C12:0), with C8:0 and C10:0 being the most dominant, accounting for 80% and 20–50% of total MCFAs, respectively (Garcia et al. 2021; Esperón-Rojas et al. 2022; Zawistowski and Kopeć 2022). Lauric acid is a less prominent constituent (Lee et al. 2022), typically ranging from 1 to 2% (Mirzaee Ghazani and Marangoni 2020). Compared to their LC counterparts, MCFAs exhibit smaller molecular structures, lower molecular weights, viscosity, and melting points, and higher polarity (solubility) (Table 1) which facilitates their hydrolysis, absorption, and metabolism in the human body (Jala and Ganesh Kumar 2018; Esperón-Rojas et al. 2022; Watanabe and Tsujino 2022; Zawistowski and Kopeć 2022). According to Man and Manaf (2006), MCFAs have melting points of 16.7 °C and 31.3 °C for C8:0 and C10:0, respectively. They display stability when subjected to temperatures below 300 °C (Parekh et al. 2011) and demonstrate characteristics of weak electrolytes. In neutral pH environments, they undergo significant ionization, enhancing their solubility in biological fluids (Ng et al. 2021). In addition, MCTs are liquid at room temperature and have a lower energy (8.4 kcal/g) as compared to LCTs (9.2 kcal/g), providing about 8.7% less energy (Pandya and Haenlein 2009; Esperón-Rojas et al. 2022).
Structural formulas of medium-chain triglycerides and medium-chain fatty acids. a Common structure of a medium-chain triglyceride (MCT), consisting of three saturated fatty acids (represented by the "R" groups) attached to a glycerol backbone. b Structural formulas of medium chain fatty acids (MCFAs), including hexanoic (caproic) acid (6 carbons), octanoic (caprylic) acid (8 carbons), decanoic (capric) acid (10 carbons), and dodecanoic (lauric) acid (12 carbons)
Naturally, MCFAs are primarily found in vegetable oils like coconut and palm kernel oil (> 50 wt% of FAs) (Ashton et al. 2021; Duttaroy 2021; Mett and Müller 2021), as well as in the oil fraction of some Cuphea species (Zentek et al. 2011). Additionally, they are also present in dairy products (Roopashree et al. 2021), including butter (6.8%), milk (6.9%), yogurt (6.6%), and cheese (7.3%). The content of C6:0-C10:0 and C12:0 in bovine milk is estimated to be 4–12% and 2–5% of all FAs, respectively, depending on genetic background, feeding regimen, and lactation stage (Duttaroy 2021). In human breast milk, MCFAs account for 9–28% of all FAs (Schönfeld and Wojtczak 2016).
Coconut oil as a raw material for MCT oil production
Coconut palm
Coconut palm (Cocos nucifera Linn.) is a member of the palm family Arecaceae (Palmae), the subfamily Cocoideae. It is often accredited as the ‘tree of life’ because of its great versatility in usage. Indeed, this most important crop of tropical and subtropical regions provides many value-added products derived from its diverse plant parts (listed in Table 2) that sustain the local economy (DebMandal and Mandal 2011; Pham 2016; Lima and Block 2019; Rethinam 2019). Botanically, coconut is considered as a drupe rather than a nut or fruit (Sankararaman and Sferra 2018). The coconut palm is also known for its adaptability to various well-drained tropical soil types, and it demonstrates a remarkable tolerance to salinity and a wide range of pH levels, spanning from 5.0 to 8.0. However, excessively high-water tables that remain stagnant for extended periods can be detrimental to the palm growth (Lal et al. 2003; Kumar and Kunhamu 2022). In general, coconut cultivation is environmentally friendly, allowing for the coexistence of multiple plant species. It enhances soil fertility when cultivated alongside other crops and is highly adaptable to organic farming practices when suitable intercrops are grown in the spaces between coconut trees (DebMandal and Mandal 2011). This arborescent monocotyledonous perennial tree (approximately 25 m in height) with a dense canopy were first cultivated on islands in Southeast Asia and those situated between the Pacific and Indian Oceans (Lima et al. 2015). Nowadays, it is grown in more than 98 countries worldwide (Arulandoo et al. 2017). Commonly, there are two distinct types of coconut palm trees, the tall variety, and the dwarf variety. Tall coconut palm varieties exhibit a slower maturation process, typically taking 6–10 years after planting to produce their first flowers. They can have a lifespan ranging from 60 to 100 years. On the other hand, dwarf coconut palm varieties tend to reach a height of 7.5 to 9 m. They have a shorter time to first flowering, usually around 3 years after planting, and a relatively shorter lifespan, averaging around 30 years (Lal et al. 2003). Importantly, the tall coconut palm outperforms the dwarf type in terms of coconut oil production, primarily due to the unsuitability of the tough copra obtained from the dwarf coconuts for commercial purposes. Consequently, the dwarf coconut palm is predominantly planted for ornamental purposes (Amri 2011).
Coconut oil
Coconut oil is a mainstream edible oil that is extracted from the kernel or meat of matured coconuts (Lim et al. 2020). In 2020, worldwide production of coconuts (in shell) was estimated to be more than 62 mil. tonnes from approximately 11.3 mil. ha of harvested area with an average yield of more than 5 mil. hg/ha. Global production of coconut oil averaged in this year at 26.1 mil tonnes with Philippines (965 200 mil. tonnes) being the major producer country followed by Indonesia, India, Vietnam, and Mexico, contributing to 22.93%, 12.98%, 6.77%, and 5.07% of the global production, respectively (FAOSTAT 2023). As compared to other oil seed crops, coconut has higher productivity, as well as consistency in production, which is partially attributed to its less susceptibility to abnormal climatic conditions (Krishna et al. 2010; Pham 2016).
Physico-chemical and sensory characterization of coconut oil
Coconut oil possesses several peculiar characteristics that set it apart from other vegetable oils. Indeed, this oil is characterized by a distinctive and notable flavor (Pereira et al. 2023), accompanied by a color range that varies from colorless to a pale brownish-yellow hue (Man and Manaf 2006). In terms of physical properties, coconut oil is classified as a fat due to its solid state at room temperature. However, it transforms into a liquid oil when the temperature exceeds 25.6 °C. Unlike other fats that gradually soften as the temperature rises, coconut oil has a distinctive characteristic with a sharp melting point of approximately 24.4–25.6 °C. This behavior is attributed to its high concentration of lauric acid, as mentioned by Lawson (2012). Another notable feature of coconut oil is its ability to resist oxidation and polymerization reactions. This high resistance to oxidative rancidity is due to elevated levels of saturated fat and low content of unsaturated FAs in its chemical composition. As a result, coconut oil is well-suited for storage without experiencing deterioration and is a reliable and stable choice for cooking purposes (Lal et al. 2003; Sankararaman and Sferra 2018). Moreover, coconut oil is characterized by its high inductivity, the lowest turbidity (compared to other vegetable oils) and excellent electrical insulation capabilities, making it a preferred choice. Other important physical properties include its specific gravity of 0.926 at 15 °C and 0.9188 at 25 °C, a saponification value ranging from 251 to 263 (indicating its suitability for soap-making), an iodine number within the range of 8.0 to 9.6 (reflecting its level of unsaturation), a Polenske value typically falling between 15 and 18, a titre of FAs ranging from 20.4 to 25.3, and a melting point of completely hydrogenated fat estimated at 44.5 °C (Manikantan et al. 2019).
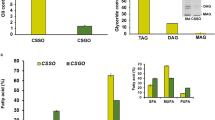
From a chemical standpoint, coconut oil is highly concentrated in SFAs (comprising approximately 92% of its composition) (Fernando et al. 2015) which differ from those found in animal fats (Boateng et al. 2016). Within the SFAs, this oil is particularly abundant in MCFAs, accounting for 60–70% of its fatty acid profile (Turpeinen and Merimaa 2011). The primary MCFA in coconut oil is C12:0, constituting 46–54% of the total (Dayrit 2015). Because of this high lauric acid content, coconut oil falls into a specific category of vegetable oils collectively known as “lauric oils”, along with palm kernel (Elaeis guineensis) oil, babassu kernel (Orbignya oleifera) oil, and cuphea (Cuphea viscosissima) oil (Pham 2016). Hewlings (2020) points to the fact that coconut oil is widely recognized as the most abundant natural source of C12:0. In addition, coconut oil contains smaller amounts of C8:0 (5–10%) and C10:0 (5–8%) (Jadhav and Annapure 2023). Most importantly, coconut oil in addition to MCFAs also contains LCFAs, including saturated ones such as myristic acid C14:0 (8%), palmitic acid C16:0 (8%), and stearic acid C18:0 (2%), and relatively low levels of unsaturated FAs like monounsaturated oleic acid C18:1 (6%), and polyunsaturated linoleic acid C18:2 (2%) (Boateng et al. 2016). The study conducted by Pereira et al. (2023) provides specific compositional data for coconut oil determined using gas chromatography analysis. The authors reported that the oil contained approximately 9.06 ± 0.05% caprylic acid, 6.15 ± 0.10% capric acid, 47.10 ± 0.15% lauric acid, 17.45 ± 0.09% myristic acid, 6.49 ± 0.06% palmitic acid, 4.13 ± 0.15% oleic acid, and 9.62 ± 0.08% minor components such as glycerol, phosphatides, sterols, and other substances present in the lipid samples. The coconut oil also contained 0.08% of free FAs. Regarding TGs (Table 3), they make up 90.0 to 98.2% of coconut oil, with the principal MCTs identified as trilaurin (3C12; 12.34–23.04%) and caprodilaurin (C10 + 2C12; 8.56–23.53%) (Appaiah et al. 2014).
Besides the dietary fat fraction, coconut oil is also endowed with diverse antioxidant compounds, such as tocopherols, tocotrienols, phytosterols, phenolics (Wagner et al. 2001; Appaiah et al. 2014), and volatile compounds (Deen et al. 2021). Total sterols in this vegetable oil have been estimated to vary between 272.3 and 535.8 mg/kg. Here, β-sitosterol (167.9–337.6 mg/kg) has been recognized as the major phytosterol followed by stigmasterol (74.5–136.5 mg/kg) and campesterol (29.9–62.8 mg/kg) (Silva et al. 2020). The primary component of tocols in coconut oil is α-tocotrienol, which can vary in concentration from 0 to 44 mg/kg. Also, minor tocols, such as α-tocopherol (0 to 17 mg/kg), β-tocopherol (0 to 11 mg/kg), and γ-tocopherols (0 to 14 mg/kg) are present. However, the refining process of crude coconut oil (ECO) reduces the amount of these tocols, subsequently diminishing the oil oxidative stability (Amri 2011). In crude coconut oil, ketones, lactones, and δ-lactones were identified as the principal volatile flavor constituents. Among these, undecan-2-one and δ-decalactone were found to be present in concentrations of 290 mg/kg and 97 mg/kg, respectively. It is worth noting that the distinct flavor and aroma of coconut oil are primarily attributed to the presence of δ-octalactone, as stated in a study by Lal et al. (2003). In addition to this, coconut oil contains trace amounts of other nutrients, including approximately 1 mg of calcium (Ca), 0.05 mg of iron (Fe), 0.02 mg of zinc (Zn), and 0.3 mg of choline. Considering a nutritional composition, 100 g of coconut oil provides 892 kcal of energy, which is equivalent to 3730 kJ. This energy content is derived primarily from the presence of 99.06 g of fat within the same 100 g sample (Ng et al. 2021). In this line, Marcus (2013) states that one tablespoon of coconut oil contains 116 cal. This amount includes 13.5 g of total fat, 11.7 g of saturated fat, 0.8 g of monounsaturated fats, and 0.2 g of polyunsaturated fats. When comparing coconut oil to other edible oils, they generally offer similar amounts of energy per 100 g due to their high-fat content. However, slight variations can exist based on the specific composition of fats in each oil. Commonly used oils such as sunflower oil, canola oil, flaxseed oil, sesame oil, and olive oil typically have an estimated energy value of 884 kcal per 100 g of oil (Bobo et al. 2021).
Coconut oil is available in two main forms: refined coconut oil (RCO; also known as copra oil) and unrefined virgin coconut oil (VCO). While the fatty acid profile is similar across these forms, there are some differences in terms of processing and additional constituents (Dayrit 2014). For instance, VCO generally contains higher levels of certain nutrients, such as vitamin E, and dietary bioactive compounds, including polyphenols, as compared to copra oil (Wallace 2019).
Processing of coconut oil
Generally, the quality and successful production of coconut oil depend on effectively implementing optimal pre- and post-harvest technological processes, as well as selecting sufficiently mature fruits, ideally aged between 11–12 months (Foale 2003; Siriphanich et al. 2011; Barlina et al. 2022). The key factor in determining the right fruit maturity is the thickness of the kernel. In effect, the quantity of oil directly correlates with kernel thickness, meaning a thicker kernel yields more copra (meat) mass and, consequently, more oil. Other indicators for assessing proper fruit maturity include the volume of liquid (coconut water) inside the fruit, which can be detected by the sloshing sound when shaking a ripe fruit, the brown husk color (as opposed to gray), and a uniformly brown soft eye without signs of moisture or a protruding embryo (Foale 2003). Equally vital is the impact of post-harvest handling on the quality of coconut products. When dealing with young nuts, it is imperative to exercise heightened care, as rough manipulation can cause extensive damage. More mature nuts are better able to withstand vibrations and potential drops on the ground. Proper storage conditions, including temperature, humidity, and atmospheric parameters, must be maintained meticulously (Siriphanich et al. 2011).
Conventionally, coconut oil can be produced using either a wet method, initialing with fresh coconuts, or dry method utilizing copra (Barlina et al. 2022). Copra is dried coconut meat with reduced moisture content from 45% to around 6%, as mentioned by Pham (2016). The author further states that coconut oil processing encompasses three main steps, such as pretreatment, extraction (pressing or solvent extraction), and post-extraction treatments. For extracting oil from copra, power-driven rotaries and expellers are used, and the extraction process is followed by separation of cake residue and mucilage using filtering or settling (Lal et al. 2003). Moreover, oil produced through the dry method requires purification due to the potential presence of compounds that may be harmful (Barlina et al. 2022). Overall, a series of steps creating the manufacturing process of the dry method can be summarized as follows: grinding, steaming, and pressing, followed by refining and bleaching. Ultimately, the oil must go through a deodorization process at high temperatures, typically around 204–205 °C (O'Brien 2012). According to Barlina et al. (2022), the dry method is usually employed on an industrial scale but necessitates purification before consumption due to variations in copra quality and potential harmful compounds. On the other hand, the wet method of coconut oil production begins with the selection of high-quality, mature coconuts. These coconuts are carefully dehusked without damaging the kernel, deshelled, and cut into halves to access the kernel. After removing the testa from the kernel, it is washed and shredded using a milling machine or manual methods (Srivastava et al. 2018). Subsequently, this shredded mass is mixed with water in a 1:2 ratio, and the result is filtered to yield coconut milk. Coconut milk then undergoes a separation process, yielding three distinct layers: cream, skim, and sediment. The cream layer is extracted and heated to approximately 100–110 °C, resulting in the final acquisition of the oil fraction. The oil is heated once more at the same temperature until clear oil is obtained. Virgin coconut oil (VCO) is obtained through subsequent cooling and pre-filtration (Barlina et al. 2022). Based on the processing technique used, three different types of coconut oil for edible purposes can be prepared. These include VCO from fresh coconuts (unrefined grade), coconut oil from dry coconuts (unrefined grade), and coconut oil utilizing solvent extraction method (refined from coconut expeller cake (Krishna et al. 2010).
The traditional method of obtaining coconut oil results in the deactivation of bioactive polyphenolic compounds and a loss of aroma and flavor (Ng et al. 2021). As a result, there is a growing focus on producing VCO from coconut milk using production processes that avoid the use of chemicals and limit the application of high temperatures and UV radiation (Villarino et al. 2007; Srivastava et al. 2018; Barlina et al. 2022). This heightened interest in VCO production has spurred the development of various extraction methods for obtaining VCO from coconut milk. These methods include the cold extraction process, hot extraction process, low pressure extraction, chilling-freezing and thawing, natural fermentation, induced fermentation, centrifugation, enzymatic extraction, supercritical fluid carbon dioxide, expeller pressing, and the wet mill method (Srivastava et al. 2018; Ng et al. 2021). In their study, Ng et al. (2021) provides a concise description and outlines the advantages of various VCO extraction methods.
Coconut-derived MCT oil
The development of MCTs is dated back to the early 1950s when researchers began exploring the nutritional properties of different types of dietary fats. Originally, MCTs were developed as by-products of coconut oil manufacturing to utilize low melting point FAs present in coconut oil (Watanabe and Tsujino 2022). These FAs, obtained from the lower boiling or top fraction of hydrolyzed coconut oil, consisted predominantly of C8:0 and C10:0. Historically, due to their tendency to cause irritation in detergent applications, these MCFAs were eliminated and deemed worthless. However, this disregard for them opened opportunities for their alternative applications and purposes (Heydinger Galante and Tenore 2006). Over time, the commercial production of MCTs became feasible as refining techniques improved, enabling the extraction and purification of MCTs on a larger scale.
Conventionally, the separation of MCTs from coconut oil can be achieved through a process called lipid fractionation (Baeza-Jiménez et al. 2017), which exploits the differences in melting points (Huey et al. 2009). This approach involves a series of sequential steps to transform coconut oil into the desired MCT product. This production process begins with the hydrolysis of coconut oil under high pressure and steam, effectively liberating the FAs from the glycerol present in the oil. The released MCFAs are then separated from the pool of other FAs through fractional distillation. This process capitalizes on the distinct melting point variations among the FAs (Lee et al. 2022). During the thermal fractionation process, FAs are subjected to elevated temperatures, reaching their respective evaporation points, all under high vacuum conditions. Subsequently, the evaporated FAs are separated in packed columns into three distinct cuts. The first cut yields MCFAs at 10–15%, the second cut predominantly comprises lauric and myristic acids, constituting the main fraction at 50–70%, while the third cut contains the remaining coconut FAs, yielding 15–20% (Mirzaee Ghazani and Marangoni 2020). Once the MCFAs are obtained, the esterification process takes center stage. The MCFAs, along with glycerol, undergo high pressure and high-temperature conditions, often surpassing 200 °C. This esterification reaction (Fig. 2) can occur with or without the presence of a catalyst, such as a metal or a slightly acidic catalyst (Lee et al. 2022). Alternatively, diverse enzymes, such as papain lipase, lipase B from Candida (C.) antarctica can also be employed (Caro et al. 2004; Kanprakobkit et al. 2023). Throughout this process, water is continuously eliminated to drive the reaction towards completion, and any remaining unreacted free FAs or partial acylglycerols are meticulously removed through various purification techniques, including alkali washing and steam refining. These purification steps play a vital role in eliminating impurities and further refining the quality of the MCTs (Lee et al. 2022). Upon the successful completion of the esterification process, any excess FAs present in the reaction mixture are eliminated through vacuum distillation, as mentioned by Heydinger Galante and Tenore (2006). Following purification, the crude ester undergoes a series of additional treatments. They include degumming, bleaching, and deodorization which collectively work to produce a bland, flavor-neutral MCT product that possesses a desirable sensory profile, devoid of any unwanted aromas or tastes (Lee et al. 2022). Indeed, degumming removes any remaining phospholipids, bleaching helps eliminate pigments and unwanted color, and deodorization eliminates volatile odor and flavor components, as well as any residual FAs (Gharby 2022).
MCT formation from glycerol and medium-chain fatty acids [Source: Heydinger Galante 2020: In: Shahidi F (ed.) Bailey’s industrial oil and fat products, 1st ed. (pp. 1–14). Wiley. https://doi.org/10.1002/047167849X.bio090]
The global market for commercial MCT products is primarily dominated by the inclusion of caprylic (65–75%) and capric (25–35%) acids. These two MCFAs play a vital role in shaping the composition of MCT formulations available to consumers (Rudkowska and Jones 2007; Baeza-Jiménez et al. 2017). Before the esterification process takes place, the combination of C8:0 and C10:0 occurs in varying proportions, giving rise to diverse types of MCT products with a spectrum of consistencies, spanning from solid to liquid. The specific C8:C10 ratio in a typical MCT product can vary between 95:5 to 5:95, with the most commonly encountered ratio being 70:30. Worth noting is that these MCT products typically contain minimal amounts (less than 6%) of other MCFAs, such as C6:0 and C12:0, further emphasizing the predominance of C8:0 and C10:0 in commercial MCT formulations (Heydinger Galante and Tenore 2006). In 1991, the first MCT-based product, known as Caprenin, was introduced. It was a low-calorie designer fat composed of C8:0, C10:0, and behenic acids (C22:0). However, the presence of C22:0, having an extended chain length compared to other FAs, limited its absorption. The combination of MCFAs and C22:0 in Caprenin resulted in a low-calorie density of 5 kcal/g. Procter & Gamble marketed Caprenin as a cocoa butter substitute, but it faced challenges in terms of heat resistance and a slight increase in serum total cholesterol levels, leading to its withdrawal from the market in September 2000 (Rudkowska and Jones 2007). The most common form of MCTs produced through the interesterification process of glycerol and C6:0, C8:0, C10:0, and C12:0 derived from coconut oil is Captrin. This widely used commercial MCT product has been found to have an energy content of 8.3 kcal/g when measured using bomb calorimetry. However, in terms of net metabolic energy, Captrin is estimated to provide around 6.8 kcal/g (Yoon 2006). Hasanah and Warnasih (2020) successfully prepared MCT from VCO. The process was initiated by isolating MCFAs from VCO through low-pressure fractionated distillation at 130–140 °C, yielding 81.74% MCFAs. Then, the synthesis of MCT was performed by an 8 h esterification reaction of the MCFAs with glycerol at 170 °C/40 kPa. This process yielded the highest glycerol conversion and MCT yield, achieving 99.17%.
Physico-chemical and sensory characterization of MCT oil
At room temperature, MCT oil is almost transparent (colorless), low-viscous “water-like” liquid oil being bland in odor and taste, which makes it a great carrier for condiments and colorants (Takeuchi et al. 2008; Hasanah and Warnasih 2020; Pereira et al. 2023). As compared to coconut oil, MCT oil has been found to be more palatable (Kinsella et al. 2017). On the other hand, its persistence in a liquid state at ambient temperature presents a notable challenge for the baking industry, manifesting as a detrimental influence on product texture (Jadhav et al. 2022). Nevertheless, this limitation can be addressed through the creation of oleogels derived from liquid MCTs, experiencing a growing trend in popularity (Puşcaş et al. 2020).
Commonly, MCT oil exhibits several distinctive characteristics, including reduced viscosity (30 mPas) (van Aken et al. 2011; Jadhav et al. 2022), heightened water solubility (Jamoussi et al. 2021), low water emulsification (Murack and Messier 2019), diminished thermal resilience (Park et al. 2022), and a lower smoke point (Matsuo et al. 2001; Takeuchi et al. 2008). This diminished smoke point (140–160 °C) of MCT oil precludes its utilization in high-temperature culinary applications, as the elevated temperatures beyond this threshold can engender the formation of hazardous compounds (e.g., acrolein and malondialdehyde) (Fullana et al. 2004; Katragadda et al. 2010). Hence, it is not suitable for frying (McCarty and DiNicolantonio 2016; Sankararaman and Sferra 2018). According to Rudkowska and Jones (2007), this characteristic is accompanied with a content of low molecular weight FAs in MCT oil, having lower smoke, flash, and fire points than other animal or vegetable fats. On the other hand, MCFAs confer to MCTs great oxidative stability, making them a preferred choice for various applications (Ceballos et al. 2016). Additionally, they are more polar as compared to LCFAs; thus, MCTs are miscible with hydrocarbons, esters, alcohols, acids, ketones, and natural oils (Pham 2016).
From a chemical perspective, MCT oil and coconut oil are distinct from each other, as mentioned earlier. While coconut oil contains significant amounts of C12:0 and lower contents of C6:0, C8:0, and C10:0, MCT oil exclusively consists of C8:0 and C10:0, omitting the presence of C12:0 and other LCFAs found in coconut oil (Clegg 2017; Pereira et al. 2023). Thus, the concept “MCT” often refers to just C8:0 and C10:0, as a content of the majority of commercially available MCT oils (Dayrit 2014; Rogawski 2016; Norgren et al. 2020).
MCTs dynamics in human body
In general, when MCTs are ingested orally, they undergo hydrolysis by lipases (triacylglycerol hydrolases; EC 3.1.1.3) in the body. The process begins in the stomach, where lingual or gastric lipases partially hydrolyze MCTs, and the complete and efficient hydrolysis of MCTs occurs in the duodenum with the assistance of pancreatic lipase (Man and Manaf 2006). The positions of MCFAs within MCT molecules are crucial determinants for their metabolic fate. These positions are designated using stereospecific numbering, referred to as sn positions. Within the context of MCTs, the glycerol backbone provides for three stereochemically distinct FA positions: the two outer positions, known as sn-1 and sn-3, and the central one, denoted as sn-2 (Zarevúcka and Wimmer 2008). In the digestive system, pancreatic lipase exhibits stereoselective hydrolysis by preferentially cleaving the ester bonds of MCTs at the sn-1 and sn-3 positions. This enzymatic action results in the formation of sn-2 monoglyceride and MCFAs as the primary products. While sn-2 monoglyceride can be absorbed intact, it is important to note that it is less thermodynamically stable compared to its sn-1 or sn-3 counterparts. As a result, there is a possibility of spontaneous isomerization or the migration of FA from the sn-2 monoglyceride to either the sn-1 or sn-3 positions. This process would ultimately lead to the production of glycerol and free MCFA as final products (Salentinig et al. 2015).
The MCFAs have unique characteristics that enable their faster and more efficient absorption and metabolism compared to LCFAs, which constitute the majority (97%) of dietary fats (Osman 2019). In general, higher water solubility (hydrophilic nature) and shorter carbon chain of MCFAs, in comparison with LCFAs, allows them to be absorbed without the need for emulsification by bile acids/salts (Bhagavan and Ha 2015). They also facilitate pancreatic lipase activity (Ferguson et al. 2016). Importantly, MCFAs can be absorbed intact by enterocytes (across the intestinal barrier) even in the presence of pancreatic enzyme deficiency, making up to 30% of absorption (Shah and Limketkai 2017; Osman 2019; Bhutia and Ganapathy 2021). These features, along with their low affinity for FA binding proteins, FA transport proteins, or FA translocase (Marten et al. 2006; Zentek et al. 2011), prevent them from being re-esterified within the intestinal mucosa and following the typical chylomicron route of LCFAs through the lymphatic system. Instead, MCFAs tend to be directly absorbed as free acids through the portal vein, partly complexed to plasma albumin, and transported to the liver for hepatic metabolism (Shah and Limketkai 2017; Duttaroy 2021; Jadhav and Annapure 2023). This allows MCFAs to be effectively converted into energy for its immediate utilization rather than being involved in cholesterol biosynthesis and fat accumulation (Duttaroy 2021). Additionally, MCFAs are less susceptible to the actions of hormone-sensitive lipase and less likely to be stored in adipose tissue compared to LCFAs (Kinsella et al. 2017; Althaher 2022). Moreover, it was found that these superior absorption characteristics make MCFAs a unique form of dietary fat that plays a fundamental role in weight management, making them highly valuable for patients with pancreatic insufficiency and those with digestive and fat absorption disorders (Watanabe and Tsujino 2022).
In the hepatocytes, MCFAs in their non-esterified form enter the mitochondrial matrix independently of the carnitine transport system (Schönfeld and Wojtczak 2016) unlike LCFAs, thus not requiring the activity of the carnitine acyltransferase-1 or carnitine palmitoyltransferase I, CPT-1. The medium-chain acyl-CoA synthetases (ACSMs), including ACSM1, ACSM2A, ACSM2B, ACSM3, and ACSM5, present in the mitochondria, catalyze the formation of fatty acyl-CoA thioesters, i.e., the activated form of MCFAs, using adenosine triphosphate (ATP) and through a thioester linkage between the carboxyl group (–OOH) of the MCFAs and the thiol (–SH) group of coenzyme A (Co-A) (Watkins 2013; Adeva-Andany et al. 2019). These crucial intermediates (fatty acyl-CoA thioesters) undergo metabolism through mitochondrial β-oxidation pathways, which involve a series of enzymes located either in the inner mitochondrial membrane or mitochondrial matrix (Fig. 3). These enzymes catalyze the fragmentation of successive two-carbon units in the form of acetyl-CoA from the carboxyl terminal of the fatty acyl-CoA chain. The degradation cycles continue until the complete reduction of the chain to compounds comprising two carbon residues (Blanco and Blanco 2017; Kumari 2018; Xia et al. 2019). In addition to mitochondrial β-oxidation, approximately 10% of MCFAs are believed to undergo oxidation in the peroxisomes in the basal state (Salway 2004). However, these subcellular organelles depend on cooperation with mitochondria, as they receive the end products of peroxisomal β-oxidation for complete degradation to CO2 and H2O (Wanders et al. 2016). Additionally, when mitochondrial β-oxidation is overwhelmed, the endoplasmic reticulum and cytoplasm can facilitate the ω-oxidation of MCFAs. This process mainly involves hydroxylation and oxidation of FAs, converting them into dicarboxylic acids. The conversion enhances their water solubility, enabling their excretion through urine (Talley and Mohiuddin 2023). Regarding the metabolic fate of MCTs and MCFAs, their plasma half-life after ingestion tends to be short, with estimates around 11 min for MCTs and 17 min for MCFAs, as reported in a study by Mingrone et al. (1995).
Schematic representation of medium-chain triglycerides (MCT) metabolism upon ingestion. A MCT enzymatic hydrolysis begins in the stomach, and it is completed in the duodenum with the release of medium-chain fatty acids (MCFAs) by gastric and pancreatic lipases, respectively, where they are directly absorbed through the portal vein. B In the liver, non-esterified MCFAs are directly transported into the hepatocyte mitochondria where medium-chain acyl-CoA synthetases catalyze the formation of fatty acyl-CoA thioesters, which undergo β-oxidation to produce acetyl-CoA that can be further metabolized through the tricarboxylic acid cycle
Most importantly, MCTs also have another unique metabolic pathway associated with its consumption. In mitochondria of liver cells, they can undergo a process called ketogenesis, apart from their role in acetyl-CoA production. This pathway leads to the generation of ketone bodies, including acetoacetate (AcAc), β-hydroxybutyrate (βHB) and acetone, without requiring a conventional ketogenic diet or extended periods of fasting (Lin et al. 2021). Here, two acetyl-CoA molecules are converted by thiolase (also known as acetyl coenzyme A acetyltransferase, ACAT) into acetoacetyl-CoA, which is then further converted into β-hydroxy β-methylglutaryl (HMG)-CoA by the enzyme HMG-CoA synthase. Subsequently, HMG-CoA lyase acts on HMG-CoA, converting it into AcAc. Acetoacetate can take two paths: it can undergo non-enzymatic decarboxylation, resulting in the production of acetone, or it can be converted to βHB via the enzyme βHB dehydrogenase, as mentioned by Dhillon and Gupta (2023). The ketone bodies serve as alternative energy substrates to glucose and are transported to tissues with high energy demands, such as heart or muscles, to provide immediate energy for their proper functioning (Modre-Osprian et al. 2009; Takeishi et al. 2021; Jadhav and Annapure 2023). Furthermore, they can cross the blood–brain barrier and serve as an alternative fuel source for the brain (Jensen et al. 2020; Omori et al. 2022; López-Ojeda and Hurley 2023). When they reach tissues, βHB can be converted back into AcAc by the enzyme βHB dehydrogenase. Similarly, AcAc can be converted back to acetyl-CoA through the enzyme β-ketoacyl-CoA transferase. Acetyl-CoA then enters the CAC, and through oxidative phosphorylation, it produces 22 ATP molecules per molecule of acetyl-CoA. However, it is important to note that acetone does not convert back into acetyl-CoA. Instead, it is either excreted through urine or expelled through exhalation (Dhillon and Gupta 2023).
There is ongoing debate regarding the classification of C12:0 as a MCFA due to its distinctive utilization within the body, as it was reviewed in the report by Clegg (2017). Unlike shorter carbon chain MCTs found in pure MCT oil (C6:0-C10:0), only a portion of C12:0 (around 20–30%) is directly transported to the liver via the portal vein to be used as energy. Consequently, approximately 23.16% of coconut oil in total consists of MCTs that are absorbed and metabolized in a similar manner to pure MCT oil. The digestion of coconut oil and MCT oil using in vitro static and dynamic digestion protocols have been compared by Pereira et al. (2023). Their results revealed that MCT oil was digested more rapidly and exhibited a higher lipolysis than coconut oil. Both oils showed similar lipolysis levels at the endpoint of the gastric phase, suggesting that gastric lipase concentration limited free FA release rather than the availability of MCFAs. During the gastric digestion, MCT oil released mainly C8:0 and C10:0, while coconut oil released C12:0. MCT oil had higher lipid bioaccessibility, indicating greater bioavailability in early digestion. However, some FAs remained esterified to glycerol, indicating incomplete lipolysis with the used lipase activities. The research conducted by Sonnay et al. (2019) investigated the differences in metabolic flux pathways and ketogenesis at the basal level of C8:0 and C10:0 using human-induced pluripotent stem cell-derived (iPSC) astrocytes. The study revealed MCFA-specific ketogenic properties, suggesting distinct β-oxidation pathways for C8:0 and C10:0. This was supported by higher extracellular concentrations and faster secretion rates of βHB and AcAc in cells metabolizing C8:0 compared to C10:0. Also, the research identified opposite directions of intracellular metabolic fluxes between the MCFA intermediates C6:0 and C8:0, further highlighting the unique characteristics of MCFA metabolism. Vandenberghe et al. (2017) investigated the ketogenic effects of different oils in an 8-h study with healthy adults. Tricaprylin (C8) was found to be the most ketogenic oil compared to coconut oil (3% C8, 5% tricaprin, C10) and other MCT oils (C8–C10; C10), or coconut oil mixed with C8-C10 or C8. The ketogenic effect of C8 was significantly higher when consumed without a meal. However, it only produced half the increase in the acetoacetate-to-βHB ratio compared to coconut oil. Also, significantly higher ketosis after intake of C8:0 compared to coconut oil (both with and without previous glucose intake) was reported in the research by Norgren et al. (2020). Additionally, their results indicate that the extent of ketosis achieved from C8:0 can vary strongly based on the timing and macronutritional composition of the overall food intake. As a result, the effectiveness of MCT supplements or coconut oil in achieving ketosis is enhanced when combined with a time-restricted feeding regimen that emphasizes carbohydrate restriction. Therefore, individuals seeking to optimize the benefits of MCT supplementation or coconut oil for achieving ketosis would benefit from implementing a time-restricted feeding approach that limits their carbohydrate intake.
Biological activities of MCT oil
Generally, coconut oil has garnered interest for its myriad potential health benefits, including anticancer, hypocholesterolemic, antidiabetic, hepatoprotective, antioxidant, anti-inflammatory, antimicrobial, skin-moisturizing, and wound-healing effects, documented across various studies (DebMandal and Mandal 2011; Kappally et al. 2015; Peedikayil et al. 2016; Sezgin et al. 2019; Widianingrum et al. 2019; Pruseth et al. 2020; Deen et al. 2021; Vásquez and Guardia 2021; Umaru et al. 2023). According to many authors (Dayrit 2014; Sheela et al. 2019), many of these advantages of coconut oil are attributed to the characteristics of MCFAs, with a particular focus on C12:0. This MCFA is also present in MCT oil; however, only in small amounts. Regarding this, it is crucial to emphasize that digestion and metabolism of C12:0 more closely resemble that of LCFAs due to its primary absorption with chylomicrons (Eyres et al. 2016; Devers and Brown 2020), raising questions about weight loss effects of coconut oil (Jayawardena et al. 2021). In contrast, MCT oil, enriched with health-beneficial C8:0 and C10:0 surpasses coconut oil in this aspect. This raises doubts about relying solely on coconut oil for similar effects. Further, coconut oil contains also various polyphenolic compounds combating oxidative stress (Sandupama et al. 2022), and its health benefits such as reduced blood pressure (Bandeira Alves et al. 2014) or neuroprotection (Lim et al. 2020) are linked to its antioxidant properties. Despite these benefits, coconut oil also contains substantial levels of other SFAs, including LCFAs. In this context, consumption of coconut oil has been associated with increased serum levels of total cholesterol, low density lipoprotein (LDL) cholesterol, and high density lipoprotein (HDL) cholesterol (Jayawardena et al. 2021), escalating concerns about its potential role as a risk factor for cardiovascular diseases (Jayawardena et al. 2020; Neelakantan et al. 2020; Schwingshackl and Schlesinger 2023; Spiazzi et al. 2023). Hence, governmental regulatory agencies in many countries have expressed skepticism regarding its consumption (Lima and Block 2019). In light of these concerns, MCT oil has emerged as a potentially more efficient substitute, largely owing to its concentrated MCFAs. Its swift conversion into energy makes it valuable for those seeking immediate and sustained energy sources. Its ability to support various health benefits and its versatility in different applications may further solidify its role as a superior choice compared to coconut oil.
The following section delves into the extensive range of biological activities associated with MCT oil. Unfortunately, direct comparisons of biological activities between MCT oil and coconut oil are scarce (summarized in Table 4). Indeed, studies often focus on MCFAs or MCTs which are present in both oils but in different proportions, making it challenging to draw precise conclusions about the superior advantages of MCT oil.
Anticancer properties of MCT oil
Many preclinical (in vitro and in vivo), as well as clinical studies have indeed demonstrated the cytotoxic and anticancer effects of MCTs in diverse human cancers, encompassing liver, breast, gastric, colorectal (CRC), prostate, skin, and brain cancers (Kimoto et al. 1998; Otto et al. 2008; Dueregger et al. 2015; Crotti et al. 2016; Aminzadeh-Gohari et al. 2017; Wakana et al. 2019; Roopashree et al. 2022). However, despite this growing body of research, only a limited number of studies have specifically investigated the anticancer effects of MCT oil sourced from coconuts. Regarding this, it has been discovered that C12:0, C10:0, and C8:0 are among the major constituents of MCTs exhibiting antitumor and chemopreventive actions. These effects include their ability to modulate cellular energy metabolism, hinder tumor growth and cell proliferation, downregulate inflammatory cytokines and chemokines, regulate genes associated with cell cycle and division, induce apoptosis, or influence specific signaling pathways (Verma et al. 2019; Ramya et al. 2022).
Preclinical studies
Recently, it was suggested that targeting energy metabolism by a modified diet supplemented with 25% 8- and 10-carbon MCTs may be considered as part of a multimodal treatment regimen to improve the efficacy of neuroblastoma anticancer therapy in CD-1 Nu mouse model (Aminzadeh-Gohari et al. 2017). Furthermore, this study revealed that a ketogenic diet supplemented with 25% 8-carbon MCTs, combined with a daily dose of the chemotherapeutic drug cyclophosphamide [40 mg/kg for mice with SH-SY5Y xenografts and 13 mg/kg for mice with SK-N-BE(2) xenografts], administered for at least 36 days, was more effective than a diet supplemented with LCTs in sensitizing neuroblastomas to low-dose chemotherapy. In addition, it has been shown that MCTs have a more potent anticancer activity compared to LCTs, and they can change energy demands and glucose metabolism of benign cells towards the increased glycolytic activity and higher requirements to utilize dietary MCTs as energy source in comparison with malignant cells of the prostate. In a study conducted by Dueregger et al. (2015), MCT oil (100% mix of coconut and palm oil, C10), LCT oil (100% mix of thistle oil and line seed oil, C22), and combination of MCT/LCT (77%/23%) significantly increased the cell proliferation of benign cells (RWPE-1) within 72 h, with the most pronounced effect observed with 200 μM LCT. However, these treatments did not significantly affect the proliferation of prostate cancer cells (LNCaP, ABL, PC3). The obtained findings supported the notion that benign prostate tissue has a significantly higher ß-oxidation and glycolytic activity than cancer tissue. The anticancer effects of MCTs have also been demonstrated in other in vitro and vivo studies. For instance, dietary intervention involving 80% MCT diet (20 U/kg/day) was found to reduce both tumor proliferation and cancer cachexia in murine colon adenocarcinoma, as reported by Beck and Tisdale (1989). Furthermore, an antiproliferative action of MCTs was evidenced in a study by Otto et al. (2008). The authors showed that a ketogenic diet supplemented with omega-3 fatty acids and 21.45% MCTs (6–12 carbons) inhibited the tumor growth in a xenograft model of human gastric adenocarcinoma cells and increased the mean survival time of animals. In effect, animals on the ketogenic diet with MCTs survived for an average of 34.2 ± 8.5 days, compared to 23.3 ± 3.9 days in the control group. Suppressed tumor growth was also observed in human colon xenograft models, when the diet provided 31.1 kJ/g and was composed of 36.2% MCT, 21.8% omega-3 fat, 11% lard fat, 20% protein, and 3% carbohydrate (Hao et al. 2015). Additionally, a similar ketogenic diet, characterized by high fat, low carbohydrate, and moderate protein content, supplemented with 30% MCTs, resulted in a reduction of glioblastoma progression, inhibition of cancer cell proliferation, and increased survival in an orthotopic xenograft model. Notably, these anticancer effects were attributed to the inhibition of the mTORC1/2 pathway, as demonstrated by Martuscello et al. (2016). Regardless of the dietary type, it has also been observed that the administration of MCTs (at a dose of 60 g/week/mouse), especially C8:0, significantly inhibited chemically-induced hepatic carcinogenesis in mice when compared to the control group (Wakana et al. 2019). Additionally, the study revealed that the ketone body βHB, a main metabolite of MCTs, inhibited tumor cell proliferation in in vitro conditions and led to a decrease in the expression of inflammatory cytokines [tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), interleukin 1β (IL-1β), and interferon γ (IFN-γ,)] and chemokines [chemokine monocyte chemoattractant protein-1 (MCP-1, C–C) and motif chemokine ligand 2 (MIP-2, C-X-C)]. On the other hand, this study showed that MCTs significantly increase the expression of adipocytokines in adipose tissue after diethylnitrosamine administration, effectively inhibiting oxidative stress caused by lipid peroxidation. These findings suggest a potential therapeutic benefit in combating hepatic carcinogenesis induced by inflammatory agents through the incorporation of oral MCT supplements or MCT-rich diets. Specifically, the anticancer properties of MCFAs, including C10:0, C8:0, and C6:0, have been demonstrated in human skin, breast, and colon cancer cells (Narayanan et al. 2015). Notably, this study observed that C10:0, particularly at a concentration of 4.4 mM, exhibited the most potent inhibitory effect, mainly on colon and skin cancer cells. This was followed by C8:0 (at a dose of 7 mM) and C6:0 (6.5 mM) after 48 h of MCFA administration. All these MCFAs exerted anticancer activity by downregulating genes linked to cell division [cyclin dependent kinase 2 (CDK2), cyclin dependent kinase 4 (CDK4), CDC28 protein kinase regulatory subunit 1B (CKS1B), cyclin A2 (CCNA2), cyclin D1 (CCND1)] and upregulating the Gadd45a (growth arrest and DNA damage inducible α) gene, which plays a pivotal role in inducing apoptosis in colon cancer cells. Similarly, in skin cancer cells, the MCFAs downregulated cell division-related genes (CKS1B, CCNA2, CCND1) and upregulated NR4A1 (nuclear receptor subfamily 4 group A member 1) and P21 (cyclin dependent kinase inhibitor 1A) genes that are essential for apoptosis. In breast cancer cells, only C10:0 and C8:0, at doses of 0.6 mM and 0.7 mM, respectively, downregulated CDK4, CKS1B, CCNA2, and CCND1 genes. Nevertheless, the expression of P21, associated with apoptosis, was upregulated by all three MCFAs at doses ranging from 0.6 to 0.9 mM.
Clinical studies
The clinical application of MCTs in relation to their potent anticancer and therapeutic effects has been reported in several clinical studies. Researchers are currently investigating the efficacy of diets rich in MCTs, also known as ketone diets, in conjunction with conventional treatments like chemotherapy and radiation therapy. This is particularly relevant for advanced stage IV cancers and challenging-to-treat brain tumors such as glioblastoma multiforme, which are difficult to surgically remove (Zuccoli et al. 2010). Nebeling et al. (1995) proposed one of the first diets rich in MCTs (a diet containing 60% MCTs) as a successful therapeutic option in oncology, suggesting its potential for clinical application in pediatric patients with malignant brain tumors. Conducting comprehensive clinical research on cancer patients poses several challenges, but further studies in this direction are anticipated in the future (Chung and Park 2017; Watanabe and Tsujino 2022). Extensive research on the ketogenic diet containing MCTs has been carried out in a pilot study conducted by Schmidt et al. (2011) in patients with advanced metastatic tumors. This study demonstrated notable improvements in patients, who followed a ketogenic diet for three months (containing MCTs in a form of oil-protein mixture: 21 g of fat with MCTs, 5 g of carbohydrate, and 14 g proteins) in physical well-being and observed tumor shrinkage as a result of the ketogenic diet, without adverse side effects and changes in cholesterol or blood lipids. Moreover, a recent study showed that advanced cancer patients (stage IV), who received the ketogenic diet with MCT oil (approximately 50–80 g/day) in combination with chemotherapy and radiation therapy for three months, exhibited significantly increased total ketone bodies, reduced fasting blood sugar, and insulin levels. From one week to three months, there were significant increases in the levels of AcAc and βHB (139.9 ± 178.0 to 922.1 ± 340.1 and 573.2 ± 356.0 μmol/L, respectively) and notable reductions in levels of both fasting blood sugar (96.8 ± 11.5 to 91.2 ± 13.0 mg/dL) and insulin (8.6 ± 11.4 to 4.8 ± 4.6 μIU/mL). Additionally, the diet markedly improved the long-term prognosis of the patients and even inhibited the development of multiple liver metastases (Hagihara et al. 2020). Similar results were reported in a randomized controlled trial conducted by Khodabakhshi et al. (2020). They found that an MCT-based ketogenic diet, which contained 6% of calories from carbohydrates, 19% from protein, 20% from MCTs, and 55% from fat, when combined with standard chemotherapy for patients with metastatic breast cancer (500 ml of MCT oil administered every 2 weeks for 3 months), significantly reduced fasting blood sugar levels (84.5 ± 11.3 vs. 100.4 ± 11.8 mg/dl) in the intervention group across different time intervals (third and last visit). On the other hand, the control group exhibited no significant changes (106.4 ± 27.2 vs. 105.2 ± 15.8 mg/dl). Moreover, there was an increasing trend in serum levels of ketone bodies (0.007 ± 0.026 to 0.92 ± 0.699 mmol/l) in the intervention group during the follow-up period. This study also demonstrated that MCT-based ketogenic diet can improve the biochemical parameters, body composition, and overall survival with no substantial side effects in patients with breast cancer.
In addition, the efficacy, safety, and tolerance of lipid emulsions containing LCTs, MCTs, and PUFAs were recently investigated in patients undergoing major surgery for gastric and colorectal cancer (Ma et al. 2015). This study reported that lipid particles (containing 100 g/l of MCTs) exhibited immunomodulatory effects but had no significant influence on levels of pro-inflammatory factors, including IL-6, TNF-α, CRP, and uroporphyrinogen III decarboxylase (PCT), or clinical outcomes in patients undergoing elective surgery. Furthermore, recent research (Roopashree et al. 2022) has unveiled the potential utility of MCTs levels in human plasma as a promising screening and new prognostic biomarker for different stages and pathological subtypes of breast cancer. Specifically, the levels of C8:0 and C12:0 were found to be significantly decreased, especially in the HER2- and ER-positive breast cancer subjects (0.902% and 3.083%, respectively), in comparison with the control groups (2.256% and 3.882%, respectively). In contrast, the level of C10:0 was significantly increased in breast cancer patients (12.559%) when compared to the control group (10.709%). The findings suggest that lower levels of C8:0 and C12:0 may be indicative of a higher risk of developing breast cancer. Similar outcomes were corroborated in a study by Crotti et al. (2016) involving CRC cells. Among the MCTs examined in human plasma samples, the level of C10:0 was found to be elevated in the precancerous lesions (HGDA), early-stage CRC (I–II), and late-stage CRC (III–IV) compared to the control group. This elevation was also observed when contrasted with patients diagnosed with breast cancer or ulcerative colitis. These findings strongly indicate that C10:0 could serve as a valuable early diagnostic biomarker for colorectal cancer. Moreover, the study found notable differences in the C6:0/C10:0 ratio between individuals with HGDA and CRC patients (1.48 and 0.13, respectively). These results suggest the potential for discrimination between HGDA and CRC based on the C6:0/C10:0 ratio.
Neuroprotective properties of MCT oil
As mentioned earlier, ketone bodies serve as an alternative energy source that brain cells can utilize when glucose availability is limited, a state that can occur during prolonged fasting, exercise, or when induced deliberately through the administration of MCTs (Cunnane et al. 2016; Vandenberghe et al. 2020). The concept of nutritional ketosis has been harnessed for its neuroprotective effects and its potential in treating various neurological disorders. Generally, ketogenic diets have been employed in the management of conditions such as epilepsy and Alzheimer’s disease (AD), aiming to enhance cognitive function (D’andrea Meira et al. 2019; Thelen and Brown-Borg 2020). In AD, where there is impaired cerebral glucose and insulin metabolism, ketones offer an alternative energy source. Indeed, continuous consumption of MCT oil appears to stabilize cognition in individuals with AD, particularly those in the mild to moderate stages of the disease (Juby et al. 2022). Dietary interventions, including ketogenic diets, have been investigated as a therapeutic option for preventing and treating seizures in individuals with epilepsy for over a century. These diets can mimic the metabolic changes associated with fasting, which has been shown to possess anticonvulsant properties (Masino and Rho 2012). Over the years, dietary modifications for seizure management have been extensively studied in the field of human medicine. Among these interventions, the MCT ketogenic diet has emerged as one of the most effective therapeutic approaches, especially for children with drug-resistant epilepsy (Barañano and Hartman 2008; Liu 2008; Rho and Stafstrom 2012; Martin-McGill et al. 2020).
A study conducted by Rebello et al. (2015) revealed that consumption of 56 g/day of MCTs for 24 weeks lead to an increase in serum post-prandial βHB concentrations and improvement of memory in patients with mild cognitive impairment through supplementation with ketone precursors. Miles et al. (1991) conducted a study to explore the effects of MCT infusion on the central nervous system in dogs. For this purpose, six dogs were subjected to sequential infusions of trioctanoin at varying rates over 80-min intervals to provide different calorie levels relative to their resting energy expenditure. Results showed that plasma octanoate levels increased progressively during the infusions, peaking at 1.44 ± 0.41 mmol/l. Notably, at higher trioctanoin infusion rates, dogs experienced emesis, somnolence, and coma. Ketone body concentrations and production significantly increased, reaching 859 ± 54 μmol/l and 18.5 ± 1.7 μmol/kg/min, respectively, at the highest trioctanoin infusion rate. Plasma lactate levels also rose from 1.3 ± 0.1 to 4.3 ± 0.9 mmol/l at the highest infusion rate. EEG changes were observed, characterized by high amplitude slowing and reduced faster component amplitudes. The study concluded that MCTs had a dose-related central nervous system toxicity in dogs. Thus, caution should be exercised in clinical studies involving MCTs, with meticulous measurement of MCFA concentrations being essential. In a study conducted by Berk et al. (2022), changes in the metabolome and neurotransmitter levels relevant to epilepsy and behavioral comorbidities in dogs with idiopathic epilepsy after consuming a MCT dietary supplement (MCT-DS) were investigated. Out of the 28 dogs included in the study, over 60% experienced a reduction in seizure frequency during MCT supplementation. However, only 30% of the dogs exhibited an overall reduction in seizure frequency of ≥ 50% and were categorized as MCT responders (R). The remaining 23 dogs were classified as MCT non-responders (NR). The study revealed significant differences in four out of nine neurotransmitters between dogs consuming the control-DS and MCT-DS after 90 days of DS consumption. In all 15 dogs, there was a notable increase (p = 0.044) in γ-aminobutyric acid (GABA) concentration during the MCT phase. This increase was accompanied by a significant shift in the balance between GABA and glutamate, with a relative 50% increase on the GABAergic side (p = 0.025). Additionally, during the MCT-DS phase, significant differences were observed between R and NR. MCT-R dogs also exhibited significantly lower urinary concentrations of histamine (p = 0.006), glutamate (p = 0.046), and serotonin (p = 0.012) during the MCT-DS phase, whereas no significant differences were observed during the control-DS phase [histamine (p = 0.345); glutamate (p = 0.323); serotonin (p = 0.269)]. This pattern was consistent for all three metabolites when compared to the baseline measurements. Thavendiranathan et al. (2000) focused on male Wistar rat pups weaned at 20 days of age and assigned to either a control diet or a ketogenic diet containing MCT oil. The primary goal was to investigate the impact of these diets on seizure susceptibility and blood levels of βHB. After following a 10-day dietary regimen, the rats underwent one of four distinct seizure tests: maximal electric shock, threshold electroconvulsive shock, threshold pentylenetetrazol, or maximal pentylenetetrazol. Subsequently, the rats were humanely euthanized, and blood samples were collected to measure βHB concentration. Rats on the MCT diet displayed substantially elevated blood βHB levels, ranging from 10 to 30 times higher than those observed in the control group. These levels were not only significantly increased but also fell within or even exceeded the ranges commonly reported in clinical studies. In contrast, the control group exhibited βHB values that varied across the different experiments, ranging from 0.17 to 0.42 mM. In comparison, the ketogenic diet group showed a substantial increase in βHB levels, ranging from 3.36 to 7.14 mM. However, despite the remarkable increases in blood ketone levels, none of the seizure tests demonstrated any anticonvulsant effects. Surprisingly, the tests involving maximal seizures revealed proconvulsant actions. These findings challenge the prevailing notion that elevated ketone levels in the bloodstream are inherently associated with anticonvulsant effects. In conclusion, this study suggests that clinical levels of ketones can be present in the bloodstream without exerting any inhibitory effects on seizures.
In a case study involving a 43-year-old man with a history of nonsurgical partial epilepsy, Azzam and Azar (2013) introduced MCTs in the form of pure oil into his regular diet. The patient had previously not responded to multiple trials of antiepileptic drugs. Remarkably, the introduction of MCTs into his diet led to a significant reduction in the frequency of his seizures, transitioning from multiple daily occurrences to just one seizure every four days. This positive response was consistently observed when the patient consumed four tablespoons of MCT oil twice daily. Importantly, no adverse side effects were reported at this level of supplementation; however, at higher doses of MCTs, the patient did report significant gastrointestinal side effects, including diarrhea and flatulence. In a study conducted by Rasmussen et al. (2023), adult patients with intractable epilepsy were the focus. These participants incorporated MCT oil into their diet, following a tolerance-based regimen of twice-daily supplementation for a three-month period. This regimen included a 1–2-week titration period at the beginning, followed by a 1–2-week tapering-off phase. The data analysis yielded compelling results showing a significant estimated reduction of 42% (p < 0.0001) in the frequency of seizures among the participants.
In 2013, Chang and colleagues (2013) performed a comparative analysis, pitting the established epilepsy treatment Valproate (VPA) against a variety of MCT diet-associated fatty acids, as well as related branched compounds. Their study revealed that specific MCFAs, including those prescribed in the MCT diet and certain branched compounds positioned at the fourth carbon, exhibited significantly improved in vitro seizure control compared to VPA. Notably, VPA led to a slight yet statistically significant reduction in the frequency of epileptiform discharges, decreasing to 77.1 ± 2.0% of the baseline. On the other hand, C8:0 had no discernible impact on seizure control, registering at 98.4 ± 7.2%. Conversely, nonanoic acid and C10:0 showed marked effects, with reaching 23.2 ± 8.2% and 2.2 ± 1.4%, respectively. The introduction of a side chain to C8:0 resulted in increased suppression of epileptiform discharges when branched either at the second carbon (2-propyloctanoic acid, 11.1 ± 4.6%) or the fourth carbon (4-methyloctanoic, 50.0 ± 3.8% and 4-ethyloctanoic, 5.8 ± 3.8%). Importantly, the inhibition of epileptiform discharges was structurally specific, as compounds like 3,7-dimethyloctanoic acid (96.7 ± 2.6%) and 2-methylheptanoic acid (84.9 ± 2.5%) had limited effects. These findings support the potential therapeutic role of C10:0 and C8:0 derivatives in the direct mechanism of the diet for seizure control. Overall, this study highlights the promising prospect of MCFAs as a basis for the development of more effective and safer epilepsy treatments when compared to VPA.
Additionally, as summarized by Liu and Wang (2013), the ketogenic diet stands out as one of the most effective therapies for drug-resistant epilepsy. The MCT ketogenic diet has proven to be equally effective as the classic ketogenic diet. With careful management by healthcare professionals, MCT ketogenic diet offers hope to individuals with epilepsy for whom the classic ketogenic diet may not be a suitable option, providing them access to its benefits.
Health benefits of MCT in gastrointestinal disorders
Gastrointestinal disorders
The remarkable ease with which MCTs are absorbed and metabolized in contrast to other dietary fats has sparked significant interest in their application for managing various gastrointestinal disorders. In this context, MCTs are mainly utilized to alleviate problems related to fat malabsorption and to provide essential calories in order to enhance overall nutritional well-being (Shah and Limketkai 2017). As per the review by Watanabe and Tsujino (2022), MCTs have been employed since the 1950s to offer energy supplementation for patients grappling with issues such as lipid malabsorption, liver dysfunction, and malnutrition. Incorporating MCTs into protein-rich diets (up to 40–70%) has been found to enhance lipid absorption in individuals with malabsorption syndrome (Murphy et al. 2002; Smart et al. 2011). Chylous leakage (CL) is an abnormal condition characterized by the seepage of chylomicron-rich fluid from the lymphatic vessels (Satala et al. 2021). Post-operative CL can occur as a surgical complication, often resulting from damage to the lymphatic system (Inoue et al. 2016). This complication can induce malnutrition and compromise the patient's immune system, potentially impacting the long-term outcomes of individuals undergoing surgical treatment for malignant diseases (Koch et al. 2011; Shyr et al. 2020). The implementation of a dietary regimen like the MCT diet in patients with CL can help alleviate stress on damaged lymphatic vessels, as MCTs are typically not absorbed through the intestinal lymphatics (Shyr et al. 2020). A study conducted by Wang et al. (2023) investigated the impact of the MCT diet and/or low-fat enteral nutrition (EN) interventions on the prognosis of patients with post-operative CL. In this analysis, a total of 63 patients with post-operative CL were included. Among them, 58 patients were successfully cured without the need for additional treatments. The authors concluded that approximately 90% of post-operative CL cases could be effectively treated using their MCT diet/EN management strategy, surpassing success rate higher than that reported in numerous studies. Based on their findings, they recommend the use of the MCT diet and EN as the primary treatment choice, favoring these options over fasting, parenteral nutrition, or octreotide. In a retrospective study involving 245 patients who underwent pancreatoduodenectomy or total pancreatectomy, 40 patients who developed CL were given an enteral formula enriched with MCTs until they could shift to a fat-free diet supplemented with oral MCTs. Notably, all these patients experienced a reduction in chyle output without needing surgical intervention or parenteral nutrition (Abu Hilal et al. 2013). For chylous fistulas that occurred post neck dissections, patients were able to achieve fistula closure after two weeks of MCTs administration (Martin et al. 1993).
In the context of chronic pancreatitis, there is a growing interest in utilizing MCTs to alleviate post-prandial pain. A small study of Shea et al. (2003), which involved 8 adult patients suffering from chronic pancreatitis and equipped with sufficient pancreatic enzymes, revealed promising results. These patients were administered with an elemental enteral formula rich in MCTs (comprising 69% of the total fat content; 9.8 g per can) at least 3 times daily for a duration of 10 weeks, with an additional requirement of maintaining a daily fat intake of less than 20 g. The outcome was noteworthy, as it resulted in minimal increases in serum plasma cholecystokinin (CCK) and a significant reduction in post-prandial abdominal pain. Specifically, mean CCK levels for the control subjects were 0.46 ± 0.29 pM at baseline, 10.75 ± 0.45 pM in response to the high-fat meal, and 7.9 ± 1.25 pM in response to the standard enteral formulation. Of note, CCK levels were 1.43 ± 0.72 pM in response to the enteral supplement containing MCT and hydrolyzed peptides. In patients with chronic pancreatitis, there was an average improvement in pain scores of 61.8% from baseline to the conclusion of the study (p = 0.01). This corresponded to a clinical improvement in 6 out of the 8 patients. The complete enteral supplement, containing MCT and hydrolyzed peptides, demonstrated the ability to minimally increase plasma CCK levels while potentially proving effective in reducing postprandial pain associated with chronic pancreatitis.
Short bowel syndrome (SBS) is a condition characterized by nutrient malabsorption and occurs following surgical resection, congenital defect, or disease of the bowel. In patients with a remaining colon, incorporating a diet containing MCTs has been proven to enhance overall fat absorption in comparison to a diet containing solely LCTs. Currently, there are limited early case reports demonstrating the potential benefits of MCTs in SBS. Jeppesen and Mortensen (1998) investigated the role of the colon in fat absorption, with a focus on MCTs and MCFAs. In total, 19 patients with SBS were included, 10 of them retaining a functional colon. In these patients, the absorption of C8:0-C10:0 FAs was significantly better as compared to those lacking a colon, even in case of severe LCFA malabsorption. However, the impact of the colon on the absorption of LCFAs (C14:0-C18:0) was minimal. The introduction of MCTs substantially enhanced fat absorption (MCT + LCT), raising it from 23 to 58% in patients with a colon and elevating overall energy absorption from 46 to 58%. Conversely, in patients without a colon, the increase in fat absorption from 37 to 46% did not improve overall energy absorption due to increased malabsorption of carbohydrates and proteins. In the case of a 78-year-old female recovering from colorectal cancer surgery and subsequent small bowel resection due to intestinal necrosis, nutritional management posed a significant challenge due to the limited length of the remaining small intestine and the absence of the ileum. Implementing MCT oil into her diet led to noticeable benefits in her recovery. Gradual improvements were seen in stool consistency and a reduction in defecation frequency. Additionally, her food intake and protein utilization improved, resulting in increased skeletal muscle mass and higher albumin levels (Kunii et al. 2022).
One of the initial studies comparing fat-modified enteral formulas, specifically those containing MCTs in contrast to standard formulas, was conducted by Viall et al. (1990). This small-scale double-blind, randomized clinical trial involved 23 non-surgically treated hospitalized patients, selected to represent the common adult population requiring enteral nutrition in general practice. The participants were randomly assigned to receive either the study formula (SF: 83.5% MCT + 16.5% LCT) or the control formula (CF: 50% MCT + 50% LCT) over a period of 65 days. Although the results showed numerical advantages for the SF, there were no significant differences observed in the number of daily bowel movements (CF: 1 ± 0.5 vs. SF: 0.6 ± 0.3) or the frequency of days with high gastric residuals (CF: 10/59 vs. SF: 2/59). Similarly, there were no substantial distinctions in the occurrence of gastrointestinal adverse effects, such as diarrhea (CF: 7/59 vs. SF: 4/59 days) and vomiting (CF: 7/59 vs. SF: 1/59 days). In a prospective double-blind study, the incidence of diarrhea episodes was compared between two groups of patients: one group receiving an MCT- and fish oil-enriched formula and the other group receiving a standard enteral formula. Unfortunately, no clinical benefit was observed in the treatment group, and the significance of these results was constrained by the small number of participants (Jakob et al. 2017). More promising results were obtained in two additional studies. In a study by Qiu et al. (2017) which involved 144 patients requiring enteral nutrition, a fat-modified enteral formula containing MCT, carnitine, and taurine was compared to a standard enteral formula. The findings indicated that the fat-modified formula improved feeding tolerance in critically ill patients. However, the individual contribution of each nutrient was not clearly established, limiting the impact of the research. In another study involving 229 patients with gastrointestinal tract cancer, Wang et al. (2010) demonstrated that an MCT- and protein-enriched enteral formula led to improved prealbumin levels and reduced hospital stay duration without causing an increase in adverse reactions compared to patients receiving isocaloric enteral nutrition.
Obesity
The global incidence of obesity is steadily increasing and is closely linked to various health issues, including type 2 diabetes, atherosclerosis, hypertension, cardiovascular diseases, and cancer. Excessive intake of nutrients plays a significant role in obesity, leading to the enlargement of white adipose tissue (WAT) and the buildup of fat in unconventional sites such as the liver, skeletal muscle, and kidneys. The surplus of visceral and ectopic fat is linked to the onset of insulin resistance (Neeland et al. 2019). As of 2020, the total number of obese people globally exceeded 600 million, which accounts for more than one-tenth of the world’s population, and this number continues to rise (Kumanyika and Dietz 2020). It is imperative to develop therapeutic approaches aimed at reducing fat accumulation and improving overall metabolic function. Thanks to their unique metabolism, MCTs offer immediate energy and can be utilized to manage obesity. MCTs have a high satiety value, preventing overconsumption of food (Kinsella et al. 2017). Numerous clinical studies have explored the potential of MCTs in preventing obesity. Since 2010, several research articles have been published, delving into the effects of MCT ingestion on body composition, weight loss, and energy expenditure (Nagao and Yanagita 2010; Rego Costa et al. 2012; Bueno et al. 2015; Mumme and Stonehouse 2015; Watanabe and Tsujino 2022). In a review conducted by Mumme and Stonehouse (2015), randomized clinical studies with a minimum ingestion duration of 3 weeks were selected and analyzed. This review specifically compared MCTs (particularly those containing C8:0 and C10:0) with LCTs concerning body composition, weight, serum lipids, and other endpoints in healthy men and women. Thirteen clinical trials, involving a total of 749 participants, were included in the analysis. The findings revealed that, in comparison to LCTs, MCTs led to a significant reduction in body weight (BW), waist circumference, hip circumference, total body fat, total subcutaneous fat, and visceral fat. In a study performed by Tsuji et al. (2001), a double-blind controlled protocol was employed to investigate the potential health benefits of MCT in comparison with LCTs in 78 healthy men and women. The participants were categorized into two groups based on their body mass index (BMI): BMI ≥ 23 kg/m2 (n = 26 for MCT, n = 30 for LCT) and BMI < 23 kg/m2 (n = 15 for MCT, n = 7 for LCT). Throughout the 12-week study period, participants in both groups were instructed to consume 9218 kJ/day and 60 g/day of total fat. Both groups experienced reductions in BW and body fat by weeks 4, 8, and 12 of the study. However, among participants with a BMI ≥ 23 kg/m2, those in the MCT group exhibited a significantly greater decrease in BW and body fat (− 3.86 ± 0.3 kg) compared to the LCT group (− 2.75 ± 0.2 kg) at the 8-week mark. Additionally, the area of subcutaneous fat decreased significantly more in the MCT group than in the LCT group at weeks 4, 8, and 12. These findings indicate that an MCT-based diet may lead to pronounced reductions in BW and fat in individuals with a BMI ≥ 23 kg/m2 when compared to an LCT-based diet. It has been observed that the consumption of MCTs is associated with increased energy expenditure, enhanced fat oxidation and improved feelings of satiety. The objective of the study by Kinsella et al. (2017) was to investigate the impact of MCT oil in comparison to coconut oil and a control substance on food intake and satiety. In the experiment, fifteen healthy participants (33 ± 12 years; BMI 23.85 ± 2.85 kg/m2) consumed a test breakfast smoothie, providing 205 kcal, with a choice of either MCT oil, coconut oil, or vegetable oil (control) following an overnight fast. The study results revealed a significant difference in energy intake during the ad libitum meal among the three oils (control 1797.7 ± 415.7 kcal; MCT 1474.2 ± 536.5 kcal; coconut oil 1710.8 ± 430.6 kcal; P = 0.018), with the most notable difference observed between the control and the MCT oils (p = 0.048). Furthermore, distinctions were observed in the visual analogue scales (VAS) for all four parameters: hunger, fullness, desire to eat, and prospective consumption (P < 0.001). These differences were noted between MCT and coconut oil for all VAS parameters (P < 0.01) and between the control and MCT for hunger and fullness (P < 0.05). The study demonstrated that MCT increased satiety more effectively than coconut oil and the control substance. In a report of Maher et al. (2021), it was concluded that MCT led to a reduction in energy intake over the following 48 h, an effect that was not observed with conjugated linoleic acid (CLA). This reduction might be mediated via delayed gastric emptying or increased βHB concentrations. In the study, 15 participants with a healthy weight (BMI: 22.7 ± 1.9 kg/m2) and 14 participants with overweight/obesity (BMI: 30.9 ± 3.9 kg/m2) were provided with breakfast meals containing either 23.06 g of vegetable oil (CON), 25.00 g of MCT oil (MCT), or 6.25 g of CLA and 16.80 g of vegetable oil (CLA). Various parameters, including appetite, peptide YY, total ghrelin, βHB, and gastric emptying, were measured. Energy intake was assessed during an ad libitum lunch and over the subsequent ~ 36 h. Neither MCT nor CLA led to a decrease in ad libitum intake; however, MCT did reduce energy intake on day 1 (P = 0.031) and over the 48-h period (P = 0.005) compared to CON. MCT also delayed gastric emptying (P ≤ 0.01) compared to CON, whereas CLA did not. Concentrations of peptide YY and total ghrelin showed no significant differences (P = 0.743 and P = 0.188, respectively), but MCT increased βHB concentrations compared to CON (P = 0.005) and CLA (P < 0.001). Importantly, βHB concentrations were higher in participants with overweight/obesity (P = 0.009). In a recent study by Jackson and Jewell (2023), the impact of different dietary components, including MCTs, long-chain PUFAs from fish oil (FO), and their combination (FO + MCTs) on the serum metabolome of dogs was thoroughly investigated. For this purpose, sixty-four dogs were randomly assigned to different diet groups: a control diet, a diet with 7% MCTs, a diet containing FO (comprising 0.18% eicosapentaenoate and 1.3% docosahexaenoate), or a diet combining FO and MCTs. The dogs adhered to these diets for 28 days following a 14-day washout period during which they consumed the control diet. Notable findings of the study included decreased levels of circulating TGs and total cholesterol with the FO (with or without MCTs) addition and reduction in N-acyl taurines with the MCTs, FO, or FO + MCTs supplementation. The study conclusion indicates that the lipidomic signatures relevant to the clinical effectiveness of FO or MCT are preserved when these oils are consumed together.
Time-restricted feeding (TRF) has emerged as a potent therapy for addressing obesity. In particular, TRF has been found to suppress diet-induced BW gain and metabolic dysfunction in mice when administered during the active phase, as demonstrated in a research of Hatori et al. (2012). The study by Abe et al. (2022) focused on 40 elderly nursing home residents, with an average age of 85.9 ± 7.7 years, in a 1.5-month randomized intervention trial. The participants were randomly allocated into two groups: one group received 6 g/day of MCTs at breakfast (breakfast group) as a test group, and the other group at dinnertime (dinner group) as a positive control group. Thirty-seven participants completed the research and were included in the analysis. The supplementation of MCTs, whether at breakfast or dinner, resulted in a significant increase (P < 0.001) in right and left arm muscle areas compared to the baseline, with an increase of 1.1 ± 0.8 cm2 and 1.1 ± 0.7 cm2, respectively, for the breakfast group, and 1.6 ± 2.5 cm2 and 0.9 ± 1.0 cm2, respectively, for the dinner one. Additionally, right knee extension time improved by 39 ± 42 s (P < 0.01) and 20 ± 32 s (P < 0.05) for the breakfast and dinner groups, respectively, while leg open and close test time increased by 1.74 ± 2.00 n/10 s (P < 0.01) for the breakfast group and 1.67 ± 2.01 n/10 s (P < 0.01) for the dinner group. The analysis showed that supplementation with 6 g of MCTs daily for 1.5 months, whether taken at breakfast or dinnertime, resulted in enhanced muscle mass and function, along with improved cognition in frail elderly adults. Abe (2022) investigated how the timing of MCT consumption affects obesity and metabolic dysfunction induced in mice by a high-fat high-sucrose diet (HFHSD). In this research, mice were subjected to an HFHSD with (M-HFHSD) or without MCT during either the active or rest phase for a duration of 9 weeks. The results revealed a significant reduction in BW, WAT weight, and adipocyte size in epididymal WAT (eWAT), as well as improved insulin sensitivity in mice fed with M-HFHSD during the active phase, but not during the rest phase. Additionally, mice consuming M-HFHSD during both active and rest phases exhibited enhanced glucose tolerance. Phosphorylated Akt, a signaling molecule, was more abundant in the gastrocnemius muscles and eWAT of mice fed M-HFHSD during the active phase compared to those fed HFHSD. The study also observed increased mRNA and protein expression of lipogenic genes in the eWAT of mice fed M-HFHSD compared to HFHSD. Furthermore, feeding mice with M-HFHSD during the active phase significantly increased the abundance of phosphorylated Ser563 and 660 of hormone-sensitive lipase, an enzyme involved in lipid metabolism, and its upstream regulator, protein kinase A, in eWAT. These findings indicated that the timing of MCT consumption modulates its effects on eWAT hypertrophy and influences glucose and lipid metabolism in mice.
A study by Nosaka et al. (2020) involved sedentary middle-aged and elderly individuals who were given 6 g of MCTs/day for 2 weeks. The results demonstrated that continuous MCT ingestion enhanced lipid metabolism, as compared to carbohydrates, during low-intensity exercise in daily activities. Similarly, Tsujino et al. (2022) conducted a study with sedentary obese individuals who were given 2 g of MCTs/day for 2 weeks. Their findings revealed that MCT ingestion increased fat oxidation during low-intensity physical activity compared to LCTs. Animal studies, as highlighted by Ooyama et al. (2008) on rats, have further shown that when MCT ingestion is combined with exercise, it leads to additional enhancements in fat metabolism and reductions in visceral fat. Given the current lifestyle changes, which often involve reduced physical activity, it is essential for individuals to not only incorporate MCTs into their diets but also integrate them with exercise and physical activity. This combination approach can contribute significantly to overall health and well-being as emphasized by Watanabe and Tsujino (2022).
Antimicrobial properties of MCT oil
While many research studies have focused on antimicrobial activity of coconut oil and MCFAs, investigations into the activity of MCT oil remain relatively rare. The antibacterial potential of coconut oil has been a matter of discussion, with certain studies failing to detect any significant activity (Hovorková et al. 2018). On the other hand, MCFAs have exhibited a spectrum of antimicrobial effects against pathogenic microorganisms (Kim and Rhee 2013; Matsue et al. 2019). Virgin coconut oil and its components were evaluated for their impact on potentially pathogenic bacteria Clostridium (C.) difficile, known to cause diarrhea. When used in its unaltered state, virgin coconut oil did not demonstrate any inhibitory effect on the bacterial growth, except when digested by lipase. Lipolyzed coconut oil displayed concentration-dependent activity with 1.2% oil inhibiting 99.9% of C. difficile. Lauric, capric, and caprylic acids individually showed significant inhibitory activity at concentrations of 1 mM, 2 mM, and 10 mM, respectively, underscoring the need for oil digestion to obtain inhibition properties (Shilling et al. 2013). Enhanced inhibitory activity of hydrolysed coconut oil was discussed in a study by Nguyen and Diep (2022), where the fraction containing MTCs exhibited the highest inhibition activity against various bacteria, including Staphylococcus (S.) aureus (with an inhibition zone of 15.67 mm), Bacillus (B.) subtilis (11.67 mm), B. cereus (10.00 mm), Escherichia (E.) coli (9.00 mm), and Salmonella (S.) typhimurium (6.00 mm). Sihombing et al. (2014) found that the antimicrobial activity of coconut oil increased significantly after 100% hydrolysis by lipase. In this case, S. aureus, S. typhmurium, and E. coli were inhibited by 71.36%, 51.82%, and 60.71%, respectively, compared to non-hydrolysed oil. Arsenault et al. (2019) tested the dietary supplementation of MTC in a small group of premature infants with Candida infection. Their findings demonstrate an 84% reduction in Candida colonies within the gastrointestinal tract after one week of treatment.
As MCTs are metabolized into MCFAs after consumption, several research studies have focused exclusively on the individual components present in MCT oil. Skrivanova et al. (2006) observed that MCFAs exhibited an inhibitory activity against E. coli, Salmonella sp., and C. perfringens. The most potent inhibitory activity was attributed to C8:0, with a MIC reaching from 1 to 3 mg/ml. Furthermore, exposure to MCFAs led to the inhibition of glucose utilization by all tested microorganisms. Lauric acid (10 mM) inhibited E. coli and Salmonella, while S. enteritidis was inhibited by 5 mM C10:0 (Martínez-Vallespín et al. 2016). In general, Salmonella strains seemed to be more sensitive to MCFAs than E. coli strain, as evidenced by a decrease in specific growth with increasing MCFA concentrations. Huang et al. (2011) evaluated the inhibitory activity of 25 μg/ml of MCFAs against various gram-positive and gram-negative bacteria found in the oral cavity. Complete inhibition was achieved against Fusobacterium (F.) nucleatum with C10:0 treatment and against Agregatibacter actinomycetemcomitans, Porphyromonas gingivalis, F. nucleatum, and S. gordonii with C12:0 treatment. Batovska et al. (2009) examined activity of MCFAs and their monoglycerides against several microorganisms, like Staphylococcus, Corynebacterium, Bacillus, and Streptococcus. They found out that monoglycerides were more active against all tested microorganisms, except Listeria monocytogenes, which exhibited pronounced inhibition with MCFAs, especially C12:0 with MIC of 31.25 μg/ml. In a study by Bergsson et al. (2001), various strains of C. albicans were tested against MCFAs. Here, C8:0 did not show any remarkable activity, while C10:0 demonstrated inhibitory action at a concentration of 10 mM. The most considerable activity was observed after C12:0 treatment, with significant inhibition at 5 mM concentration.
The mechanisms of antimicrobial actions of MCFAs have been proposed by several studies. In a summary by Desbois and Smith (2010), they discussed potential targets of free FAs for bacterial inhibition. These mechanisms include interaction with the electron transport chain, through a pathway of oxidative phosphorylation, or blocking a nutrient uptake. Inhibition of bacterial cell growth or cell death can result from the disruption of the pathogene cell wall or membrane. Destabilization of the membrane and pore formation increase cell permeability and can lead to cell lysis, which helps protect hosts against bacterial infection (Yoon et al. 2018). In the case of C12:0 treatment, it resulted in morphological changes in supported lipid bilayers and membrane lysis in S. aureus (Yoon et al. 2015). Transmission electron microscopy also showed structural changes in the cell membranes of bacteria after exposure to C12:0 (Tangwatcharin and Khopaibool 2012). Disruption of cell membrane integrity and the release of nucleic acids were observed in C. difficile following C12:0 treatment. The induction of membrane damage and the generation of reactive oxygen species resulted in cell lysis (Shilling et al. 2013; Yang et al. 2018).
Selected industrial and technological applications of MCT oil
Owing to their unique physical attributes, MCTs have proven to be valuable in a variety of applications beyond their well-documented nutritional advantages. In effect, their higher polarity renders them excellent solvents as compared to other oils (Pham 2016; Heydinger Galante 2020), and this unique characteristic is harnessed effectively in the realms of food processing and nutrient delivery (Nimbkar et al. 2022). As effective emulsifiers, MCTs also find utility in the formulation of pharmaceutical products for both human and veterinary use, and their versatility also extends into the cosmetic industry (Traul et al. 2000).
Food and beverage industries
In general, MCTs are highly regarded and widely used in the flavor industry for their superior clean organoleptic quality, not contributing any off-notes to products, and solvent capabilities (Heydinger Galante and Tenore 2006). For instance, they find utility in the formulation of salad dressings and cooking oils, as suggested by Rudkowska and Jones (2007). An intriguing application arose in the work of Ceballos et al. (2016) where they selected MCT oil with a specific composition (98%, consisting of 66% C8:0 and 32% C10:0) for the purpose of dissolving an ethylcellulose (EC) copolymer to improve the rheological and crystallization parameters of molten chocolate and cocoa butter. The authors found that the novel emulsifier blend was essentially odorless, with very faint color and proper slight taste. However, its most remarkable feature was its ability to prevent the overheating of cocoa butter, while incorporating EC, thus preserving characteristic organoleptic properties of cocoa butter. Consequently, the study concluded that a solution of EC in MCT oil serves as rheology modifier in chocolate manufacturing. The study performed by Khalil et al. (2012) has shed light on an effective method for extracting lutein esters using MCT oil, particularly valuable for preserving the integrity of these esters under food processing conditions. In their research, MCT oil demonstrated its remarkable ability to prevent lutein esters and MCT oil-based emulsions against temperature-induced (100 °C, with a recovery of 91% after 40 min of treatment) and UV light-induced degradation (at 365 nm for 72 h), respectively. Additionally, MCT oil was shown to be a proficient carrier lipid for food processing applications. Out of five different carrier lipids tested (including MCT, coconut, olive, and soybean oils, and cocoa butter), it was observed that β-caryophyllene-loaded lipid nanocarriers using MCT oil exhibited the most robust freeze–thaw stability while also undergoing the fastest lipid degradation during simulated in vitro digestion. Indeed, despite an increase in droplet sizes and polydispersity index (PDI) following freeze–thaw treatment, the lipid nanocarriers containing MCT oil maintained a high level of dispersion stability (PDI < 0.3) even after undergoing 3 freeze–thaw cycles. Interestingly, after simulated intestinal digestion, the lipid nanocarriers with MCT oil increased in size by approximately 16.5-fold as compared to their initial droplet sizes (Lee et al. 2021). As a lipid-based nanocarrier, MCT oil has also been employed in encapsulation of numerous bioactive compounds such as d-limonene (Paramita et al. 2012), β-carotene (Qian et al. 2012; Zhang et al. 2016; Fang et al. 2019), curcumin (Shah et al. 2016; Tan et al. 2021; Jiang and Charcosset 2022), lycopene (Li et al. 2018), quercetin (Pool et al. 2013), and carvacrol (Nash and Erk 2017). Moreover, in a study conducted by Amft et al. (2020), the potential of MCT oil in reducing lipid oxidation in corn extrudates was clearly demonstrated. This was achieved through an analysis of hydroperoxide concentrations and hexanal formation in different lipid fractions during storage. Indeed, their findings strongly suggest that the MCT coating effectively decreases hydroperoxide levels and hexanal formation, particularly in surface lipids, providing notable advantages in terms of stability. The mechanisms behind this coating effectiveness include crack filling, reduced volatile emissions, and diluting oxidizable lipids. Additionally, a study by Ali et al. (2022) reported a superior synergistic effect between vitamin E and MCT oil in preserving oxidative stability of β-carotene within nanoemulsions as compared to alternative lipid combinations, including tuna fish oil. The incorporation of vitamin E at concentrations of 2% and 4% in MCT-based formulations resulted in significantly higher retention of β-carotene, with rates of approximately 35.17% (2% concentration) and 33.89% (4% concentration), respectively, compared to nanoemulsions based solely on MCT. Furthermore, Jadhav et al. (2022) incorporated an MCT-based oleogel (synthesized using ultrasound) into a cookie formulation. The results revealed that these cookies maintained exceptional texture and outperformed in sensory attributes like mouthfeel, crunchiness, and hardness, making them a promising healthier alternative to saturated fats in bakery applications. The inclusion of ultrasounded MCT oil (U-MCTO) in cookie formulations yielded significant advantages. U-MCTO demonstrated sustained mechanical strength for a storage duration of 35 days, indicating its enduring stability. Furthermore, U-MCTO exhibited enhanced resistance to oxidation in comparison to alternative MCT forms, implying potential enhancements in the shelf-life extension of baked products. Additionally, U-MCTO-infused cookies presented commendable textural properties, persisting for an extended period of 30 days, underscoring its capacity to augment the quality and longevity of baked goods.
In the realm of beverages, the use of MCTs for flavor delivery also brings with it a dual benefit as a clouding agent. Frequently, the need for additional emulsifiers is eliminated, primarily because of the slight water solubility of MCTs and their specific gravity, which closely matches that of the beverage (Heydinger Galante and Tenore 2006). In a study carried out by Aditya et al. (2015), MCT oil was employed to create a water-in-oil-in-water emulsion, specifically to enhance the stability of curcumin and catechin in beverages. The incorporation of catechin into the inner aqueous phase of the double emulsion led to a stability improvement of over 20% at 23 ± 2 °C and over 40% at 4 °C following a 15-day incubation period, in contrast to free catechin. Concerning curcumin, over 80% was identified post-incubation in the emulsion-based beverage system, while it diminished to approximately 40% when curcumin was in its free form.
Nutraceuticals and pharmaceuticals
Undoubtedly, MCT oil plays a crucial role as a valuable carrier for lipid-soluble substances. It is notably applied in oral drug administration, a preferred method due to its convenience, cost-effectiveness, and its positive impact on patient compliance (Poonia et al. 2016; Mohamad 2023). Oral products hold several advantages for pharmaceutical companies, including simplified manufacturing processes, enhanced stability, ease of storage and transportation, and exemption from sterility and apyrogenicity requirements. However, certain drugs face challenges related to low oral absorption, which can limit their effectiveness when administered orally, as highlighted by Bansal et al. (2009). To overcome this limitation, MCT oil is frequently utilized as a carrier, replacing traditional carriers like ethanol or hexane, which exhibit potential toxicities. This substitution is driven by global regulations, emphasizing the use of greener alternatives in the extraction and purification of natural and food products. In this line, MCT oil, derived from vegetable sources, aligns with the principles of Green Chemistry, offering a renewable and sustainable option with low toxicity and ease of recycling (Yara-Varón et al. 2017).
Besides diverse biologically active compounds mentioned above, MCT oil has found utility as a carrier or delivery vehicle for a wide range of other nutraceuticals and pharmaceuticals such as dihydromyricetin (Wang et al. 2021), bruceine D (Dou et al. 2018), a novel calcium channel blocker—dibudipine (Keyhanfar et al. 2014), ibuprofen (Hafizah et al. 2022), cannabidiol (Feng et al. 2021), or sirolimus (rapamycin) (Sun et al. 2011). In a study conducted by Keyhanfar et al. (2014), the solubility of dibudipine in MCT oil was found to be higher (5.3 mg/ml) as compared to other tested oils including mineral oil (0.1 mg/ml), almond oil (1.5 mg/ml), thistle oil (1.7 mg/ml), or ethyl oleate (4.5 mg/ml). This superiority in solubility led to MCT oil being selected as the solvent for this drug. Also, the solubility of ibuprofen in MCT oil has been demonstrated to be superior to that in VCO or olive oil. The formula was stable at 45 °C storage for up to 14 days, at 25 °C, and at 5 °C, steady at 120 days (Hafizah et al. 2022). Indeed, previous research has consistently shown that MCTs are frequently chosen for formulations due to their high fluidity, excellent solubility properties, and emulsification capabilities (Gershanik and Benita 2000). These characteristics promote bioavailability while not possessing significant pharmacological action (Stuchlík and Zák 2001).
Cosmetics and personal care products
MCT oil is a widely used ingredient in numerous cosmetics and personal care products due to its excellent emollient properties. This compound is often incorporated into creams, lotions, moisturizers, and hair care items to provide hydration, enhance skin texture and the spreadability of these products (Heydinger Galante 2020). Generally, vegetable oils, mostly composed of either MCFAs or LCFAs, offer a dual protective effect for damaged hair. Firstly, they form a hydrophobic coating on the hair surface, and secondly, they fill gaps caused by oxidative damage as they penetrate the hair cortex (Lee and Ahn 2022). Hence, vegetable oils from various plants have been extensively studied and utilized as key ingredients in hair treatment formulations (Gode et al. 2012; Kim et al. 2021). Employing radiolabeling techniques, Gode et al. (2012) discovered that tritiated coconut oil penetrates the hair at a rate of 20.4–26.3% based on the weight of the treated hair after 6 h of treatment. As compared to LCFAs, MCFAs have been found to display a higher ability for hair penetration, providing superior protection. Among these MCFAs, C10:0 and C8:0 demonstrated the highest penetration capability. Additionally, C8:0 treatment showed a significantly higher reduction in protein leakage as compared to other hair treatment formulations. Interestingly, coconut oil provided hair protection comparable to that of MCFAs or MCT oil (Kim and Ahn 2023). In a research performed by Stortz and Marangoni (2014), thixotropic EC oleogels were developed to replace petrolatum as a structuring agent in cosmetics. This was achieved using glycerol monooleate to increase the solubility of EC in various oils, including MCT oil. The gel created with MCT oil exhibited reduced viscosity and rapid recovery viscosity, closely resembling its initial state. This anticipated trend arises from the fact that EC polymers with higher molecular weights are expected to experience more extensive swelling, consequently leading to heightened viscosity within the gel matrix. Notably, all assessed EC viscosities demonstrated nearly complete recovery, approaching 100%, in viscosity following one week of storage.
Oleogels
In the field of food and pharmaceuticals, the incorporation of hydrophobic and sensitive nutraceuticals into formulations presents challenges due to their poor water solubility and susceptibility to processing conditions. Edible oleogels and structured oils have emerged as innovative carrier systems for lipophilic active compounds, providing precise control over lipid digestion and delivery (O'Sullivan et al. 2016). Additionally, oleogels have been investigated as substitutes for unhealthy saturated and trans FAs (Davidovich-Pinhas 2016). The controlled release and stability of loaded bioactive compounds in oleogels are influenced by factors such as the type and quantity of oil, oleogelators, and additional bioactive compounds (Calligaris et al. 2020; Wang et al. 2020; Qiu et al. 2021; Shang et al. 2021). Commonly, oleogels can be prepared through direct or indirect methods, depending on the lipophilicity of oleogelators (Luo et al. 2019). Emulsion templated approaches, which involve the use of polysaccharides as oleogelators, have been reported in many studies (Jiang et al. 2018; Meng et al. 2018a, b; Espert et al. 2020). Gelatin and gellan gum, typically used as hydrogelators in tissue engineering, have been evaluated as structural agents for stabilizing MCT oleogel matrices (Wen et al. 2014; Koivisto et al. 2019; Liu et al. 2022). Their ability to carry lipophilic compounds within an oleogel matrix to enhance bioavailability and promote health benefits, particularly in the co-delivery of compounds like curcumin and resveratrol have been documented in a research of Kavimughil et al. (2022). The overall bioaccessible fraction exhibited an augmentation of up to 1.13-fold and 1.2-fold for curcumin and resveratrol, respectively, in comparison to the control (MCT oil). Furthermore, the release profile of free FAs also underscored the significant influence of gelators on lipase activity. Additionally, ex-vivo everted gut sac analysis revealed an enhanced permeation rate of approximately 1.83 times and 1.13 times for curcumin and resveratrol, respectively.
Structured lipids
MCT oil, enriched with MCFAs, tends to produce more smoke when exposed to heat and can readily form foams, making it unsuitable as a standalone replacement for traditional cooking oil, as mentioned earlier. Thus, to effectively utilize MCT oil in cooking and frying, it must be combined with other edible oils in specific proportions, as outlined by Jadhav and Annapure (2023). Accordingly, MCTs are owing to favorable physical properties commonly used in the production of structured lipids (Shahidi and Senanayake 2006; Zawistowski and Kopeć 2022). In effect, MCT derivatives created through interesterification are known as structural lipids or structural TGs. This process involves introducing LCFAs, such as C18:1, into the end product, resulting in the formation of rearranged TGs with a combination of both MCFAs and LCFAs (referred to as MLCTs) in desired ratios on the same glycerol molecule (Rudkowska and Jones 2007). These MLCTs have the potential to mitigate weight gain in high-fat foods and possess nutritional properties that can be utilized to address metabolic issues (Lee et al. 2012). Research has explored various applications for MLCTs. For instance, commercially available MLCT demonstrates a higher smoke point when blended with other plant-based oils, which can reduce production costs (Koh et al. 2009, 2011). Rice bran oil structured lipid, synthesized from rice bran oil and C8:0 with Lipozyme RM IM as biocatalyst, has proven suitable for frying and energy bars, offering unique textures and consumer appeal (Jennings et al. 2010). Also, MLCT has been found to improve butter spreadability and can be used to create healthier butter (Lee et al. 2012), particularly in margarine and shortening production (Arifin et al. 2012). MLCT contributes functional hard stock and plasticity, making it beneficial for structured lipid chocolate beverages that exhibit enhanced sweetness and reduced bubble formation (Osborn et al. 2003). In summary, the adaptability of MLCT holds promise for the development of healthier fat alternatives in a wide range of food products.
MCT oil as carrier for capsaicin
Capsaicin, a primary bioactive compound found in Capsicum fruits, is known for its intense spicy flavor and numerous health benefits, including antioxidant and anticancer properties (Han et al. 2020; Duranova et al. 2022). Due to these valuable functions, capsaicin is widely utilized in the production of nutritional products and pharmaceutical preparations (Wang et al. 2012). However, its limited solubility in water, poor stability, and pungency present challenges that restrict its extensive application (Duranova et al. 2022; Zhang et al. 2022). Essentially, MCT oil has been recognized as an effective carrier for capsaicin, as demonstrated in a study by Lu et al. (2017). Here, a nano-emulsified solution of MCT oil and capsaicin was used in a dietary study involving rats. The results showed that the nano-emulsification technique protected the gastric mucosa and alleviated gastric inflammation caused by high consumption of capsaicin. The use of nano-emulsified capsaicin yielded similar results to non-emulsified capsaicin, with the added benefit of no negative impacts on the stomach. This finding indicates that the nano-emulsification process effectively preserved the properties and effects of capsaicin while avoiding gastric irritation. The development of a food-grade nanoemulsion using MCT to increase the dissolution and bioaccessibility of capsaicin, as well as to mitigate its irritating effects, was the focus of a study by Lu et al. (2016). The authors observed a reduction in gastric mucosa irritation in rats caused by capsaicin, and the organogel-derived nanoemulsion proved to be an effective delivery system for capsaicin-based functional food products. Clegg et al. (2013) conducted research using a combination of chili and MCT oil feeding to investigate their impact on diet-induced thermogenesis (DIT) and satiety in healthy volunteers. Their findings suggested that combined chili–MCT oil feeding had a cumulative effect on DIT, as expected, since both chili and MCT oil, when fed individually, are known to increase DIT through different metabolic pathways. On the other hand, data obtained by Clegg et al. (2011) showed significant variations in energy expenditure among the various breakfast meals (P < 0.001) with chili-MCT oil exhibiting the highest values for this parameter. Specifically, the most pronounced distinctions were noted between (P = 0.011) the chili-MCT oil (47.7 ± 10.9 kcal) and chili-sunflower oil (32.5 ± 11.7 kcal), as well as between (P = 0.006) chili-MCT oil (47.7 ± 10.9 kcal) and pepper-sunflower oil (31.3 ± 11.1 kcal), and between (P = 0.005) pepper-sunflower oil (31.3 ± 11.1 kcal) and pepper-MCT oil (42.0 ± 12.2 kcal).
Most importantly, the inclusion of this subchapter in the review is motivated by our ongoing research challenge to optimize the delivery of selected chili pepper extracts for their enhanced anticancer activity. In effect, to address the issues of limited water solubility, poor stability, and pungency, and so unlocking the full potential of capsaicin for practical application in nutritional products and pharmaceutical preparations, we turned to MCT oil as a promising carrier. Effectively, nano-emulsification demonstrates a protective role in maintaining the properties and benefits of capsaicin while avoiding gastric irritation, as evidenced by previous studies. Furthermore, if the MCT oil enriched with chili pepper extract continues to exhibit irritative effects on the gastrointestinal tract of consumers, we will endeavor to eliminate these effects through a unique microencapsulation technique of the chili extract with a suitable coating material, preserving its verified anticarcinogenic effects. So, these approaches offer a promising delivery system for capsaicin-based functional food products, enhancing the bioavailability and safety of capsaicin.
Safety
As shown earlier, a wide array of studies, encompassing both animal and human trials conducted in vivo and in vitro, have explored diverse metabolic and general health aspects associated with the consumption of MCTs and MCT oil. Although most of these studies do not specifically assess the toxicological safety of MCTs, collectively, they contribute to the evidence supporting the long-standing and safe use of MCTs in diets. In this view, MCT oil is considered safe for most people when used in moderation. The US Food and Drug Administration (FDA) accepted a GRAS (Generally Recognized as Safe) affirmation petition for the use of MCTs in food products in June 1994, as mentioned by Traul et al. (2000). MCTs have been widely consumed as part of the diet for many years.
The recommended daily intake of MCT for healthy adults typically falls within the range of 30–100 g, accounting for up to 40% of their daily energy needs (Rudkowska and Jones 2007). According to Talbott and Hughes (2007), consumption of MCT oil by humans is safe up to levels of 1 g/kg BW. In accordance with this fact, McCray and Parrish (2004) suggest that doses of 60–70 g/day (4–5 tablespoons, approximately 500–600 cal) spread out throughout the day are generally tolerated. Also, Shah and Limketkai (2017) recommend a maximum daily dose of 50–100 g (equivalent to 4–7 tablespoons, 56–98 g of fat, 460–805 cal) for improved gastrointestinal tolerance. On the other hand, consuming an excessively high dose of MCT or not gradually integrating it into the diet may lead to negative effects in healthy individuals (Rudkowska and Jones 2007), causing diarrhea and gastrointestinal distress (McCray and Parrish 2004) like abdominal discomfort, cramping, gassiness, and bloating (Shah and Limketkai 2017). To prevent adverse effects, it may be advisable to briefly pause or start with a small initial dose, like half a tablespoon per day, as recommended by Rudkowska and Jones (2007) and Talbott and Hughes (2007). Combining MCT oil with various foods and beverages, including hot cereals, soups, sauces, broth, coffee, tea, fruit/vegetable juices, and smoothies, can also be an approach to avoid these side-effects (Shah and Limketkai 2017; Nimbkar et al. 2022). The general acceptance of MCT consumed in mixed meals suggests that MCTs are better tolerated with other nutrients, or that tolerance simply develops over time (Rudkowska and Jones 2007). Most importantly, MCT oil is not recommended for diabetics, unless it is a part of a medically supervised treatment, and individuals with liver diseases should avoid MCT oil because MCTs are delivered rapidly to the liver, which could put additional stress on this organ (Talbott and Hughes 2007). According to Rudkowska and Jones (2007), the maximum safe dosage of MCT in young children, pregnant or nursing women, or people with serious kidney disease has not been firmly established; however, MCT can be consumed safely in moderation at levels of up to 10% of total fat intake.
Apart from the treatment dose, various factors affecting the ketogenic response were examined, including accompanying meals, fasting duration, and caffeine consumption. Drawing from the available literature, four practical recommendations have been formulated to enhance the ketogenic impact of MCTs while minimizing undesirable side effects. First, it is advisable to commence with an initial dose of either 5 g of C8:0 or 5 g of a combination of C8:0 and C10:0. Gradually, the dose can be increased to 15–20 g of C8:0. Second, MCTs are best consumed following an overnight fast, without an accompanying meal if tolerable, or in conjunction with a low-carbohydrate meal. Third, the addition of caffeine may slightly enhance the ketogenic response. Fourth, emulsifying MCTs may have the dual benefit of increasing their ketogenic effectiveness and mitigating potential side effects (Lin et al. 2021).
Importantly, a series of toxicity tests demonstrated the non-toxic nature of MCT oil when administered to various animals at specified doses. In 90-day tests, whether given through the diet (up to 9375 mg/kg BW/day) or by intramuscular injection (up to 0.5 ml/kg/day) in rabbits, no significant signs of toxicity were observed. Reproductive performance in rats (4.28 g/kg BW/day and 12,500 mg/kg BW/day, respectively), pigs (up to 4000 mg/kg BW/day in the diet), and rabbits (1.0–4.28 g/kg BW/day) remained unaffected by intravenous or dietary administration of MCTs. Furthermore, in a two-year study in rats involving a closely related compound, tricaprylin, no evidence of carcinogenic effects was found when administered orally at levels up to 10 ml/kg (9.54 g/kg) per day (Traul et al. 2000). Also, MCT-based ketogenic diets established as safe and well-tolerated various populations, including children (Thompson et al. 2017), patients with various diseases and disorders (Azzam and Azar 2013; Rasmussen et al. 2023), and patients with advanced cancer (Schmidt et al. 2011; Khodabakhshi et al. 2020). However, recent clinical findings provided by Jain et al. (2021) have raised concerns regarding long-term ketogenic diet consumption and its impact on bone health. This study aimed to investigate whether the primary components of MCT diets, specifically octanoic (1700 mg/kg) and decanoic acids (1100 mg/kg) might contribute to bone loss in Swiss albino mice, like what is observed with the traditional ketogenic diet. The results revealed significant and adverse changes in trabecular bone microarchitecture and bone markers in the octanoic acid-treated groups. This suggests that C8:0 may have notable adverse effects on bone health. Hence, further research and validation are necessary to provide clinically relevant data, which may lead to adjustments in the composition of the MCT ketogenic diet to enhance bone health.
Conclusions
Coconut-derived MCT oil and coconut oil are both popular dietary fats that offer various potential benefits. The debate over the superiority of MCT oil compared to traditional coconut oil is a nuanced and multifaceted one. While both oils share a common origin in coconuts, their distinctions in terms of biological activities and potential health benefits are subject to ongoing research and discussion. The higher concentration of MCFAs in MCT oil has led some to believe that it may offer more targeted health benefits, particularly in areas such as weight management and cognitive health. It appears to be a potential alternative that emphasizes the concentrated benefits of MCFAs while minimizing the concerns associated with the SFAs found in coconut oil. Essentially, the choice between the two oils depends on individual health goals and dietary preferences, with still ongoing research shedding light on their distinct advantages and applications. Most importantly, moderation is a fundamental key, as both oils are calorie-dense and should be consumed in accordance with overall dietary requirements.
Availability of data and materials
The authors declare that all data supporting the findings of this study are available within the article.
Code availability
Not applicable.
References
Abe T (2022) Timing of medium-chain triglyceride consumption modulates effects in mice with obesity induced by a high-fat high-sucrose diet. Nutrients 14:5096. https://doi.org/10.3390/nu14235096
Abe S, Ezaki O, Suzuki (2022) Effects of timing of medium-chain triglycerides (8:0 and 10:0) supplementation during the day on muscle mass, function and cognition in frail elderly adults. J Frailty Aging 11:100–108. https://doi.org/10.14283/jfa.2021.33
Abu Hilal M, Layfield DM, Di Fabio F, Arregui-Fresneda I, Panagiotopoulou IG, Armstrong TH, Pearce NW, Johnson CD (2013) Postoperative chyle leak after major pancreatic resections in patients who receive enteral feed: risk factors and management options. World J Surg 37:2918–2926. https://doi.org/10.1007/s00268-013-2171-x
Adeva-Andany MM, Carneiro-Freire N, Seco-Filgueira M, Fernández-Fernández C, Mouriño-Bayolo D (2019) Mitochondrial β-oxidation of saturated fatty acids in humans. Mitochondrion 46:73–90. https://doi.org/10.1016/j.mito.2018.02.009
Aditya NP, Aditya S, Yang H-J, Kim HW, Park SO, Lee J, Ko S (2015) Curcumin and catechin co-loaded water-in-oil-in-water emulsion and its beverage application. J Funct Foods 15:35–43. https://doi.org/10.1016/j.jff.2015.03.013
Ahmed S, Shah P, Ahmed O (2023) Biochemistry, lipids. StatPearls. StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK525952/
Akhavan S, Assadpour E, Katouzian I, Jafari SM (2018) Lipid nano scale cargos for the protection and delivery of food bioactive ingredients and nutraceuticals. Trends Food Sci Technol 74:132–146. https://doi.org/10.1016/j.tifs.2018.02.001
Ali A, Rehman A, Jafari SM, Ranjha MMAN, Shehzad Q, Shahbaz HM, Khan S, Usman M, Kowalczewski PŁ, Jarzębski M, Xia W (2022) Effect of co-encapsulated natural antioxidants with modified starch on the oxidative stability of β-carotene loaded within nanoemulsions. Appl Sci 12:1070. https://doi.org/10.3390/app12031070
Althaher AR (2022) An overview of hormone-sensitive lipase (HSL). Sci World J 2022:1964684. https://doi.org/10.1155/2022/1964684
Amft J, Bauer JL, Rostek J, Spielvogel S, Döring F, Schwarz K (2020) MCT oil coating improves the oxidative stability of surface lipids in corn extrudates. Eur J Lipid Sci Technol 122:1900350. https://doi.org/10.1002/ejlt.201900350
Aminzadeh-Gohari S, Feichtinger RG, Vidali S, Locker F, Rutherford T, O’Donnel M, Stöger-Kleiber A, Mayr JA, Sperl W, Kofler B (2017) A ketogenic diet supplemented with medium-chain triglycerides enhances the anti-tumor and anti-angiogenic efficacy of chemotherapy on neuroblastoma xenografts in a CD1-nu mouse model. Oncotarget 8:64728–64744. https://doi.org/10.18632/oncotarget.20041
Amri IN (2011) The lauric (coconut and palm kernel) oils. In: Gunstone FD (ed) Vegetable oils in food technology. Wiley-Blackwell, West Sussex, pp 169–197
Appaiah P, Sunil L, Prasanth Kumar PK, Gopala Krishna AG (2014) composition of coconut testa, coconut kernel and its oil. J Am Oil Chem Soc 91:917–924. https://doi.org/10.1007/s11746-014-2447-9
Arifin N, Soo-Peng K, Long K, Chin-Ping T, Yusoff MSA, Oi-Ming L (2012) Modeling and optimization of lipozyme rm im-catalyzed esterification of medium- and long-chain triacyglycerols (MLCT) using response surface methodology. Food Bioprocess Technol 5:216–225. https://doi.org/10.1007/s11947-010-0325-5
Arsenault AB, Gunsalus KTW, Laforce-Nesbitt SS, Przystac L, DeAngelis EJ, Hurley ME, Vorel ES, Tucker R, Matthan NR, Lichtenstein AH, Kumamoto CA, Bliss JM (2019) Dietary supplementation with medium-chain triglycerides reduces Candida gastrointestinal colonization in preterm infants. Pediatr Infect Dis J 38:164–168. https://doi.org/10.1097/INF.0000000000002042
Arulandoo X, Sritharan K, Subramaniam M (2017) The coconut palm. In: Thomas VB, Murray BG, Murphy DJ (eds) Encyclopedia of applied plant sciences, 2nd edn. Academic Press, London, pp 426–430. https://doi.org/10.1016/B978-0-12-394807-6.00237-9
Ashton JS, Roberts JW, Wakefield CJ, Page RM, MacLaren DPM, Marwood S, Malone JJ (2021) The effects of medium chain triglyceride (MCT) supplementation using a C8:C10 ratio of 30:70 on cognitive performance in healthy young adults. Physiol Behav 229:113252. https://doi.org/10.1016/j.physbeh.2020.113252
Azzam R, Azar NJ (2013) Marked seizure reduction after MCT supplementation. Case Rep Neurol Med 2013:e809151. https://doi.org/10.1155/2013/809151
Baeza-Jiménez R, López-Martínez LX, García-Varela R, García HS (2017) Lipids in fruits and vegetables: chemistry and biological activities. In: Yahia EM (ed) Fruit and vegetable phytochemicals: chemistry and human health, vol 2. Wiley, London, pp 423–450
Bandeira Alves NF, Alves N, Porpino S, Monteiro M, Queiroz T, Montenegro K, Braga V (2014) Coconut oil supplementation reduces blood pressure and oxidative stress in spontaneously hypertensive rats. BMC Proc 8:P68. https://doi.org/10.1186/1753-6561-8-S4-P68
Bansal T, Akhtar N, Jaggi M, Khar RK, Talegaonkar S (2009) Novel formulation approaches for optimising delivery of anticancer drugs based on P-glycoprotein modulation. Drug Discov Today 14:1067–1074. https://doi.org/10.1016/j.drudis.2009.07.010
Barañano KW, Hartman AL (2008) The ketogenic diet: uses in epilepsy and other neurologic illnesses. Curr Treat Options Neurol 10:410–419. https://doi.org/10.1007/s11940-008-0043-8
Barlina R, Dewandari KT, Mulyawanti I, Herawan T (2022) Chemistry and composition of coconut oil and its biological activities. In: Mariod AA (ed) Multiple biological activities of unconventional seed oils. Academic Press, London, pp 383–395. https://doi.org/10.1016/B978-0-12-824135-6.00025-8
Batovska DI, Todorova IT, Tsvetkova IV, Najdenski HM (2009) Antibacterial study of the medium chain fatty acids and their 1-monoglycerides: individual effects and synergistic relationships. Pol J Microbiol 58:43–47
Beck SA, Tisdale MJ (1989) Effect of insulin on weight loss and tumour growth in a cachexia model. Br J Cancer 59:677–681
Bergsson G, Arnfinnsson J, Steingrímsson Ó, Thormar H (2001) In vitro killing of Candida albicans by fatty acids and monoglycerides. Antimicrob Agents Chemother 45:3209–3212. https://doi.org/10.1128/AAC.45.11.3209-3212.2001
Berk BA, Ottka C, Hong Law T, Packer RMA, Wessmann A, Bathen-Nöthen A, Jokinen TS, Knebel A, Tipold A, Lohi H, Volk HA (2022) Metabolic fingerprinting of dogs with idiopathic epilepsy receiving a ketogenic medium-chain triglyceride (MCT) oil. Fron Vet Sci 9:935430. https://doi.org/10.3389/fvets.2022.935430
Bhagavan NV, Ha C-E (2015) Gastrointestinal digestion and absorption. In: Bhagavan NV, Ha C-E (eds) Essentials of medical biochemistry, 2nd edn. Academic Press, London, pp 137–164. https://doi.org/10.1016/B978-0-12-416687-5.00011-7
Bhutia YD, Ganapathy V (2021) In: Feldman M, Friedman LS, Brandt LJ (eds) Sleisenger and Fordtran’s gastrointestinal and liver disease, 11th ed. Elsevier, pp 1636–1656.e4
Blanco A, Blanco G (2017) Medical biochemistry. Academic Press, London
Boateng L, Ansong R, Owusu WB, Steiner-Asiedu M (2016) Coconut oil and palm oil’s role in nutrition, health and national development: a review. Ghana Med J 50:189–196
Bobo G, Nicolau-Lapeña I, Aguiló-Aguayo I (2021) Conventional oils and oilseeds: composition and nutritional importance. In: Lafarga T, Bobo G, Aguiló-Aguayo I (eds) Oil and oilseed processing: opportunities and challenges, 1st edn. Wiley, London, pp 23–40
Bravo-Ruiz I, Medina MÁ, Martínez-Poveda B (2021) From food to genes: transcriptional regulation of metabolism by lipids and carbohydrates. Nutrients 13:1513. https://doi.org/10.3390/nu13051513
Bueno NB, de Melo IV, Florêncio TT, Sawaya AL (2015) Dietary medium-chain triacylglycerols versus long-chain triacylglycerols for body composition in adults: systematic review and meta-analysis of randomized controlled trials. J Am Coll Nutr 34:175–183. https://doi.org/10.1080/07315724.2013.879844
Calligaris S, Alongi M, Lucci P, Anese M (2020) Effect of different oleogelators on lipolysis and curcuminoid bioaccessibility upon in vitro digestion of sunflower oil oleogels. Food Chem 314:126146. https://doi.org/10.1016/j.foodchem.2019.126146
Caro Y, Turon F, Villeneuve P, Pina M, Graille J (2004) Enzymatic synthesis of medium-chain triacylglycerols by alcoholysis and interesterification of copra oil using a crude papain lipase preparation. Eur J Lipid Sci Technol 106:503–512. https://doi.org/10.1002/ejlt.200400955
Ceballos MR, Bierbrauer KL, Faudone SN, Cuffini SL, Beltramo DM, Bianco ID (2016) Influence of ethylcellulose—medium chain triglycerides blend on the flow behavior and β-V polymorph retention of dark chocolate. Food Struct 10:1–9. https://doi.org/10.1016/j.foostr.2016.10.004
Chang P, Terbach N, Plant N, Chen PE, Walker MC, Williams RSB (2013) Seizure control by ketogenic diet-associated medium chain fatty acids. Neuropharmacology 69:105–114. https://doi.org/10.1016/j.neuropharm.2012.11.004
Chen J, Liu H (2020) Nutritional indices for assessing fatty acids: a mini-review. Int J Mol Sci 21:5695. https://doi.org/10.3390/ijms21165695
Chung H-Y, Park YK (2017) Rationale, feasibility and acceptability of ketogenic diet for cancer treatment. J Cancer Prev 22:127–134. https://doi.org/10.15430/JCP.2017.22.3.127
Clegg ME (2017) They say coconut oil can aid weight loss, but can it really? Eur J Clin Nutr 71:1139–1143. https://doi.org/10.1038/ejcn.2017.86
Clegg ME, Golsorkhi M, Henry CJ (2011) The effect of combined medium chain triglyceride and chilli feeding on energy expenditure. Proc Nutr Soc 70(OCE6):E361. https://doi.org/10.1017/S0029665111004460
Clegg ME, Golsorkhi M, Henry CJ (2013) Combined medium-chain triglyceride and chilli feeding increases diet-induced thermogenesis in normal-weight humans. Eur J Nutr 52:1579–1585. https://doi.org/10.1007/s00394-012-0463-9
Crotti S, Agnoletto E, Cancemi G, Di Marco V, Traldi P, Pucciarelli S, Nitti D, Agostini M (2016) Altered plasma levels of decanoic acid in colorectal cancer as a new diagnostic biomarker. Anal Bioanal Chem 408:6321–6328. https://doi.org/10.1007/s00216-016-9743-1
Cunnane SC, Courchesne-Loyer A, St-Pierre V, Vandenberghe C, Pierotti T, Fortier M, Croteau E, Castellano C-A (2016) Can ketones compensate for deteriorating brain glucose uptake during aging? Implications for the risk and treatment of Alzheimer’s disease. Ann N Y Acad Sci 1367:12–20. https://doi.org/10.1111/nyas.12999
D’Andrea Meira I, Romão TT, Pires do Prado HJ, Krüger LT, Pires MEP, da Conceição PO (2019) Ketogenic diet and epilepsy: what we know so far. Front Neurosci 13:5. https://doi.org/10.3389/fnins.2019.00005
Davidovich-Pinhas M (2016) Oleogels: a promising tool for delivery of hydrophobic bioactive molecules. Ther Deliv 7:1–3. https://doi.org/10.4155/tde.15.83
Dayrit FM (2014) Lauric acid is a medium-chain fatty acid, coconut oil is a medium-chain triglyceride. Philipp J Sci 143:157–166
Dayrit FM (2015) The properties of lauric acid and their significance in coconut oil. J Am Oil Chem Soc 92:1–15. https://doi.org/10.1007/s11746-014-2562-7
DebMandal M, Mandal S (2011) Coconut (Cocos nucifera L.: Arecaceae): in health promotion and disease prevention. Asian Pac J Trop Med 4:241–247. https://doi.org/10.1016/S1995-7645(11)60078-3
Deen A, Visvanathan R, Wickramarachchi D, Marikkar N, Nammi S, Jayawardana BC, Liyanage R (2021) Chemical composition and health benefits of coconut oil: an overview. J Sci Food Agric 101:2182–2193. https://doi.org/10.1002/jsfa.10870
Desbois AP, Smith VJ (2010) Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl Microbiol Biotechnol 85:1629–1642. https://doi.org/10.1007/s00253-009-2355-3
Devers PM, Brown WM (2020) Fatty acid profiling. In: Pizzorno JE, Murray MT (eds) Textbook of natural medicine, 5th edn. Churchill Livingstone, London, pp 127-133.e2
Dhillon KK, Gupta S (2023) Biochemistry, Ketogenesis. StatPearls. StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK493179/
Dou Y-X, Zhou J-T, Wang T-T, Huang Y-F, Chen VP, Xie Y-L, Lin Z-X, Gao J-S, Su Z-R, Zeng H-F (2018) Self-nanoemulsfiying drug delivery system of bruceine D: a new approach for anti-ulcerative colitis. Int J Nanomed 13:5887–5907. https://doi.org/10.2147/IJN.S174146
Dueregger A, Schöpf B, Eder T, Höfer J, Gnaiger E, Aufinger A, Kenner L, Perktold B, Ramoner R, Klocker H, Eder IE (2015) Differential utilization of dietary fatty acids in benign and malignant cells of the prostate. PLoS ONE 10:e0135704. https://doi.org/10.1371/journal.pone.0135704
Duranova H, Valkova V, Gabriny L (2022) Chili peppers (Capsicum spp.): the spice not only for cuisine purposes—an update on current knowledge. Phytochem Rev 21:1379–1413. https://doi.org/10.1007/s11101-021-09789-7
Duttaroy AK (2021) Bioactive lipids in metabolic syndromes and hemostatic factors and fibrinolysis. In: Duttaroy AK (ed) Evidence-based nutrition and clinical evidence of bioactive foods in human health and disease. Academic Press, London, pp 63–78. https://doi.org/10.1016/B978-0-12-822405-2.00007-4
Espert M, Salvador A, Sanz T (2020) Cellulose ether oleogels obtained by emulsion-templated approach without additional thickeners. Food Hydrocoll 109:106085. https://doi.org/10.1016/j.foodhyd.2020.106085
Esperón-Rojas AA, Mendoza-Sánchez LG, García HS (2022) In: Kuddus M, Aguilar CN (eds) Value-addition in food products and processing through enzyme technology. Academic Press, pp 221–233
Eyres L, Eyres MF, Chisholm A, Brown RC (2016) Coconut oil consumption and cardiovascular risk factors in humans. Nutr Rev 74:267–280. https://doi.org/10.1093/nutrit/nuw002
Fang S, Zhao X, Liu Y, Liang X, Yang Y (2019) Fabricating multilayer emulsions by using OSA starch and chitosan suitable for spray drying: application in the encapsulation of β-carotene. Food Hydrocoll 93:102–110. https://doi.org/10.1016/j.foodhyd.2019.02.024
FAOSTAT (2023) Food and Agriculture Organization of the United Nations (FAO). FAOSTAT Statistics database. https://www.fao.org/faostat/en/#data/QCL. Accessed 15 May 2023
Feng W, Qin C, Cipolla E, Lee JB, Zgair A, Chu Y, Ortori CA, Stocks MJ, Constantinescu CS, Barrett DA, Fischer PM, Gershkovich P (2021) Inclusion of medium-chain triglyceride in lipid-based formulation of cannabidiol facilitates micellar solubilization in vitro, but in vivo performance remains superior with pure sesame oil vehicle. Pharmaceutics 13:1349. https://doi.org/10.3390/pharmaceutics13091349
Ferguson LR, Laing B, Ellett S, Marlow G (2016) Medium chain triglyceride oil: an intended placebo with unexpected adverse effects. Ann Clin Lab Res 4:3. https://doi.org/10.21767/2386-5180.1000105
Fernando WM, Martins IJ, Goozee KG, Brennan CS, Jayasena V, Martins RN (2015) The role of dietary coconut for the prevention and treatment of Alzheimer’s disease: potential mechanisms of action. Br J Nutr 114:1–14. https://doi.org/10.1017/S0007114515001452
Foale M (2003) Coconut Odyssey: the bounteous possibilities of the tree of life. Australian Centre for International Agricultural Research, ACIAR Monograph No. 101, 132 p
Fullana A, Carbonell-Barrachina ÁA, Sidhu S (2004) Volatile aldehyde emissions from heated cooking oils. J Sci Food Agric 84:2015–2021. https://doi.org/10.1002/jsfa.1904
Garcia CV, Shin GH, Kim JT (2021) Formulation of lipid nanocarriers for the food bioactive ingredients. In: Galanakis CM (ed) Food structure and functionality. Academic Press, London, pp 133–156. https://doi.org/10.1016/B978-0-12-821453-4.00007-7
Gershanik T, Benita S (2000) Self-dispersing lipid formulations for improving oral absorption of lipophilic drugs. Eur J Pharm Biopharm 50:179–188. https://doi.org/10.1016/S0939-6411(00)00089-8
Gharby S (2022) Refining vegetable oils: chemical and physical refining. Sci World J 2022:6627013. https://doi.org/10.1155/2022/6627013
Gode V, Bhalla N, Shirhatti V, Mhaskar S, Kamath Y (2012) Quantitative measurement of the penetration of coconut oil into human hair using radiolabeled coconut oil. J Cosmet Sci 63:27–31
Hafizah AU, Sugita P, Khotib M, Cahyaningsih U, Sadiah S (2022) Characteristics and stability of ibuprofen microemulsion per injection using a combination of medium-chain triglyceride oil, extra virgin olive oil, and virgin coconut oil as the oil phase. J Southwest Jiaotong Univ 57:625–639. https://doi.org/10.35741/issn.0258-2724.57.1.56
Hagihara K, Kajimoto K, Osaga S, Nagai N, Shimosegawa E, Nakata H, Saito H, Nakano M, Takeuchi M, Kanki H, Kagitani-Shimono K, Kijima T (2020) Promising effect of a new ketogenic diet regimen in patients with advanced cancer. Nutrients 12:1473. https://doi.org/10.3390/nu12051473
Han J, Zhang S, Liu X, Xiao C (2020) Fabrication of capsaicin emulsions: improving the stability of the system and relieving the irritation to the gastrointestinal tract of rats. J Sci Food Agric 100:129–138. https://doi.org/10.1002/jsfa.10002
Hao G-W, Chen Y-S, He D-M, Wang H-Y, Wu G-H, Zhang B (2015) Growth of human colon cancer cells in nude mice is delayed by ketogenic diet with or without omega-3 fatty acids and medium-chain triglycerides. Asian Pac J Cancer Prev 16:2061–2068. https://doi.org/10.7314/APJCP.2015.16.5.2061
Hasanah U, Warnasih S (2020) Synthesis and characterization of medium-chain triglyceride (MCT) from virgin coconut oil (VCO). AIP Conf Proc 2243:020007. https://doi.org/10.1063/5.0001449
Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JAJ, Ellisman MH, Panda S (2012) Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab 15:848–860. https://doi.org/10.1016/j.cmet.2012.04.019
Hewlings S (2020) Coconuts and health: different chain lengths of saturated fats require different consideration. J Cardiovasc Dev Dis 7:59. https://doi.org/10.3390/jcdd7040059
Heydinger Galante J (2020) In: Shahidi F (ed) Bailey’s industrial oil and fat products, 1st ed. Wiley, pp 1–14. https://doi.org/10.1002/047167849X.bio090
Heydinger Galante J, Tenore RR (2006) In: Akoh CC (ed) Handbook of functional lipids. Taylor & Francis, Boca Raton, pp 177–184
Hovorková P, Laloučková K, Skřivanová E (2018) Determination of in vitro antibacterial activity of plant oils containing medium-chain fatty acids against Gram-positive pathogenic and gut commensal bacteria. Czech J Anim Sci 63:119–125. https://doi.org/10.17221/70/2017-CJAS
Huang CB, Altimova Y, Myers TM, Ebersole JL (2011) Short- and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Arch Oral Biol 56:650–654. https://doi.org/10.1016/j.archoralbio.2011.01.011
Huey SM, Hock CC, Lin SW (2009) Characterization of low saturation palm oil products after continuous enzymatic interesterification and dry fractionation. J Food Sci 74:E177–E183. https://doi.org/10.1111/j.1750-3841.2009.01122.x
Inoue M, Nakatsuka S, Yashiro H, Tamura M, Suyama Y, Tsukada J, Ito N, Oguro S, Jinzaki M (2016) Lymphatic intervention for various types of lymphorrhea: access and treatment. Radiographics 36:2199–2211. https://doi.org/10.1148/rg.2016160053
Jackson MI, Jewell DE (2023) Feeding of fish oil and medium-chain triglycerides to canines impacts circulating structural and energetic lipids, endocannabinoids, and non-lipid metabolite profiles. Front Vet Sci 10:1168703. https://doi.org/10.3389/fvets.2023.1168703
Jadhav HB, Annapure US (2023) Triglycerides of medium-chain fatty acids: a concise review. J Food Sci Technol 60:2143–2152. https://doi.org/10.1007/s13197-022-05499-w
Jadhav HB, Pratap AP, Gogate PR, Annapure US (2022) Ultrasound-assisted synthesis of highly stable MCT based oleogel and evaluation of its baking performance. Appl Food Res 2:100156. https://doi.org/10.1016/j.afres.2022.100156
Jain S, Rai R, Singh D, Vohora D (2021) Octanoic acid a major component of widely consumed medium-chain triglyceride ketogenic diet is detrimental to bone. Sci Rep 11:7003. https://doi.org/10.1038/s41598-021-86468-9
Jakob SM, Bütikofer L, Berger D, Coslovsky M, Takala J (2017) A randomized controlled pilot study to evaluate the effect of an enteral formulation designed to improve gastrointestinal tolerance in the critically ill patient-the SPIRIT trial. Crit Care 21:140. https://doi.org/10.1186/s13054-017-1730-1
Jala RCR, Ganesh Kumar C (2018) Designer and functional food lipids in dietary regimes: current trends and future prospects. In: Holban AM, Grumezescu AM (eds) Alternative and replacement foods. Academic Press, London, pp 283–316. https://doi.org/10.1016/B978-0-12-811446-9.00010-1
Jamoussi Y, Zaiter T, Desrumaux C, Acar N, Pellequer Y, Béduneau A (2021) Investigation of the spontaneous nanoemulsification process with medium and long-chain triglycerides. Colloids Surf B Biointerfaces 197:1–7. https://doi.org/10.1016/j.colsurfb.2020.111432
Jayawardena R, Swarnamali H, Lanerolle P, Ranasinghe P (2020) Effect of coconut oil on cardio-metabolic risk: a systematic review and meta-analysis of interventional studies. Diabetes Metab Syndr 14:2007–2020. https://doi.org/10.1016/j.dsx.2020.09.033
Jayawardena R, Swarnamali H, Ranasinghe P, Misra A (2021) Health effects of coconut oil: summary of evidence from systematic reviews and meta-analysis of interventional studies. Diabetes Metab Syndr 15:549–555. https://doi.org/10.1016/j.dsx.2021.02.032
Jennings BH, Shewfelt RL, Akoh CC (2010) Food applications of a rice bran oil structured lipid in fried sweet potato chips and an energy bar. J Food Qual 33:679–692. https://doi.org/10.1111/j.1745-4557.2010.00355.x
Jensen NJ, Wodschow HZ, Nilsson M, Rungby J (2020) Effects of ketone bodies on brain metabolism and function in neurodegenerative diseases. Int J Mol Sci 21:8767. https://doi.org/10.3390/ijms21228767
Jeppesen PB, Mortensen PB (1998) The influence of a preserved colon on the absorption of medium chain fat in patients with small bowel resection. Gut 43:478–483. https://doi.org/10.1136/gut.43.4.478
Jiang T, Charcosset C (2022) Encapsulation of curcumin within oil-in-water emulsions prepared by premix membrane emulsification: impact of droplet size and carrier oil on the chemical stability of curcumin. Food Res Int 157:111475. https://doi.org/10.1016/j.foodres.2022.111475
Jiang Y, Liu L, Wang B, Sui X, Zhong Y, Zhang L, Mao Z, Xu H (2018) Cellulose-rich oleogels prepared with an emulsion-templated approach. Food Hydrocoll 77:460–464. https://doi.org/10.1016/j.foodhyd.2017.10.023
Jin X, Qiu T, Li L, Yu R, Chen X, Li C, Proud CG, Jiang T (2023) Pathophysiology of obesity and its associated diseases. Acta Pharm Sin B 13:2403–2424. https://doi.org/10.1016/j.apsb.2023.01.012
Juby AG, Blackburn TE, Mager DR (2022) Use of medium chain triglyceride (MCT) oil in subjects with Alzheimer’s disease: a randomized, double-blind, placebo-controlled, crossover study, with an open-label extension. Alzheimer’s Dement TRCI 8:e12259. https://doi.org/10.1002/trc2.12259
Kanprakobkit W, Kielarova SW, Wichai U, Bunyapraphatsara N, Kielar F (2023) Incrementing MCT character of coconut oil using enzyme catalyzed interesterification. J Oleo Sci 72:87–97. https://doi.org/10.5650/jos.ess22269
Kappally S, Shirwaikar A, Shirwaikar A (2015) Coconut oil: a review of potential applications. Hygeia J D Med 7:34–41. https://doi.org/10.15254/H.J.D.Med.7.2015.149
Katragadda HR, Fullana A, Sidhu S, Carbonell-Barrachina ÁA (2010) Emissions of volatile aldehydes from heated cooking oils. Food Chem 120:59–65. https://doi.org/10.1016/j.foodchem.2009.09.070
Kavimughil M, Leena MM, Moses JA, Anandharamakrishnan C (2022) 3D printed MCT oleogel as a co-delivery carrier for curcumin and resveratrol. Biomaterials 287:121616. https://doi.org/10.1016/j.biomaterials.2022.121616
Kesharwani R, Jaiswal P, Patel DK, Yadav PK (2022) Lipid-based drug delivery system (LBDDS): an emerging paradigm to enhance oral bioavailability of poorly soluble drugs. Biomed Mater Devices. https://doi.org/10.1007/s44174-022-00041-0
Keyhanfar F, Khani S, Bohlooli S (2014) Evaluation of lipid-based drug delivery system (phytosolve) on oral bioavailability of dibudipine. Iran J Pharm Res 13:1149–1156
Khalil M, Raila J, Ali M, Islam KMS, Schenk R, Krause J-P, Schweigert FJ, Rawel H (2012) Stability and bioavailability of lutein ester supplements from Tagetes flower prepared under food processing conditions. J Funct Foods 4:602–610. https://doi.org/10.1016/j.jff.2012.03.006
Khodabakhshi A, Akbari ME, Mirzaei HR, Mehrad-Majd H, Kalamian M, Davoodi SH (2020) Feasibility, safety, and beneficial effects of mct-based ketogenic diet for breast cancer treatment: a randomized controlled trial study. Nutr Cancer 72:627–634. https://doi.org/10.1080/01635581.2019.1650942
Kim S, Ahn C (2023) Determination of penetration and protection of fatty acids in bleached hair according to the fatty acid chain length and the application to understanding the protective effects of MCT oil and coconut oil. Fash Text 10:10. https://doi.org/10.1186/s40691-023-00332-0
Kim SA, Rhee MS (2013) Marked synergistic bactericidal effects and mode of action of medium-chain fatty acids in combination with organic acids against Escherichia coli O157:H7. Appl Environ Microbiol 79:6552–6560. https://doi.org/10.1128/AEM.02164-13
Kim H-R, Sung Y-W, Choi W-J (2021) Effects of hair treatment with shea butter on bleached hair. J Converg Inf Technol 11:212–219. https://doi.org/10.22156/CS4SMB.2021.11.03.212
Kimoto Y, Tanji Y, Taguchi T, Sugimoto T, Watanabe T, Tsukamoto F, Kim S, Yoneda K, Takamura Y, Izukura M, Shiba E, Takai S (1998) Antitumor effect of medium-chain triglyceride and its influence on the self-defense system of the body. Cancer Detect Prev 22:219–224. https://doi.org/10.1046/j.1525-1500.1998.0oa32.x
Kinsella R, Maher T, Clegg ME (2017) Coconut oil has less satiating properties than medium chain triglyceride oil. Physiol Behav 179:422–426. https://doi.org/10.1016/j.physbeh.2017.07.007
Koh SP, Arifin N, Lai OM, Yusoff MSA, Long K, Tan CP (2009) Oxidative stability of palm- and soybean-based medium- and long-chain triacylglycerol (MLCT) oil blends. J Sci Food Agric 89:455–462. https://doi.org/10.1002/jsfa.3473
Koh SP, Arifin N, Tan CP, Yusoff MSA, Long K, Lai OM (2011) Deep frying performance of enzymatically synthesized palm-based medium- and long-chain triacylglycerols (MLCT) oil blends. Food Bioprocess Technol 4:124–135. https://doi.org/10.1007/s11947-008-0138-y
Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, Fan ST, Yokoyama Y, Crawford M, Makuuchi M, Christophi C, Banting S, Brooke-Smith M, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey J-N, Greig P, Rees M, Nimura Y, Figueras J, DeMatteo RP, Büchler MW, Weitz J (2011) Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 149:680–688. https://doi.org/10.1016/j.surg.2010.12.002
Koivisto JT, Gering C, Karvinen J, Maria Cherian R, Belay B, Hyttinen J, Aalto-Setälä K, Kellomäki M, Parraga J (2019) mechanically biomimetic gelatin–gellan gum hydrogels for 3d culture of beating human cardiomyocytes. ACS Appl Mater Interfaces 11:20589–20602. https://doi.org/10.1021/acsami.8b22343
Krishna AG, Gaurav R, Singh BA, Kumar PP, Preeti C (2010) Coconut oil: chemistry, production and its applications: a review. Indian Coconut J 53:15–27
Kumanyika S, Dietz WH (2020) Solving population-wide obesity—progress and future prospects. N Engl J Med 383:2197–2200. https://doi.org/10.1056/NEJMp2029646
Kumar BM, Kunhamu TK (2022) Nature-based solutions in agriculture: a review of the coconut (Cocos nucifera L.)-based farming systems in Kerala, “the Land of Coconut Trees.” Nat Based Solut 2:100012. https://doi.org/10.1016/j.nbsj.2022.100012
Kumari A (2018) In: Kumari A (ed) Sweet biochemistry. Academic Press, pp 17–19. https://doi.org/10.1016/B978-0-12-814453-4.00004-2
Kunii E, Kurokawa T, Shike F, Ono S, Inanuma Ch, Suzuki Y, Kimura J, Kobayashi N, Komatsu A, Sawano T, Ozaki A, Kanzaki N, Ejiri T (2022) Patient with short bowel syndrome discharged home with full oral intake by utilizing medium-chain fatty acids: a case report. Am J Gastroenterol 117:2180–2181. https://doi.org/10.14309/01.ajg.0000870484.75923.fa
Lal JJ, Kumar CS, Indira M (2003) In: Caballero (ed) Encyclopedia of food sciences and nutrition, 2nd ed. Academic Press, pp 1464–1475. https://doi.org/10.1016/B0-12-227055-X/00263-7
Lawson HW (2012) Standards for fats & oils. Springer, New York
Lee SH, Ahn C (2022) Effect of rinse-off hair conditioner containing argan oil or camellia oil on the recovery of hair damaged by bleaching. Fash Text 9:17. https://doi.org/10.1186/s40691-021-00282-5
Lee Y-Y, Tang T-K, Lai O-M (2012) Health benefits, enzymatic production, and application of medium- and long-chain triacylglycerol (MLCT) in food industries: a review. J Food Sci 77:R137–R144. https://doi.org/10.1111/j.1750-3841.2012.02793.x
Lee MH, Lee IY, Chun YG, Kim B-K (2021) Formulation and characterization of β-caryophellene-loaded lipid nanocarriers with different carrier lipids for food processing applications. LWT 149:111805. https://doi.org/10.1016/j.lwt.2021.111805
Lee Y-Y, Tang T-K, Chan E-S, Phuah E-T, Lai O-M, Tan C-P, Wang Y, Ab Karim NA, Mat Dian NH, Tan JS (2022) Medium chain triglyceride and medium-and long chain triglyceride: metabolism, production, health impacts and its applications—a review. Crit Rev Food Sci Nutr 62:4169–4185. https://doi.org/10.1080/10408398.2021.1873729
Li D, Li L, Xiao N, Li M, Xie X (2018) Physical properties of oil-in-water nanoemulsions stabilized by OSA-modified starch for the encapsulation of lycopene. Colloids Surf A Physicochem Eng Asp 552:59–66. https://doi.org/10.1016/j.colsurfa.2018.04.055
Li X, Liu Q, Pan Y, Chen S, Zhao Y, Hu Y (2023) New insights into the role of dietary triglyceride absorption in obesity and metabolic diseases. Front Pharmacol 14:1097835. https://doi.org/10.3389/fphar.2023.1097835
Lim SM, Rahim NS, Ramasamy K (2020) In: Martin CR, Preedy VR (eds) Oxidative stress and dietary antioxidants in neurological diseases. Academic Press, pp 231–247. https://doi.org/10.1016/B978-0-12-817780-8.00016-5
Lima EBC, Sousa CNS, Meneses LN, Ximenes NC, Santos MA, Vasconcelos GS, Lima NBC, Patrocínio MCA, Macedo D, Vasconcelos SMM (2015) Cocos nucifera (L.) (Arecaceae): a phytochemical and pharmacological review. Braz J Me Biol Res 48:953–964. https://doi.org/10.1590/1414-431X20154773
Lima RDS, Block JM (2019) Coconut oil: what do we really know about it so far? Food Qual Saf 3:61–72. https://doi.org/10.1093/fqsafe/fyz004
Lin T-Y, Liu H-W, Hung T-M (2021) The ketogenic effect of medium-chain triacylglycerides. Front Nutr 8:747284. https://doi.org/10.3389/fnut.2021.747284
Liu YC (2008) Medium-chain triglyceride (MCT) ketogenic therapy. Epilepsia 49:33–36. https://doi.org/10.1111/j.1528-1167.2008.01830.x
Liu YC, Wang H-S (2013) Medium-chain triglyceride ketogenic diet, an effective treatment for drug-resistant epilepsy and a comparison with other ketogenic diets. Biomed J 36:9–15. https://doi.org/10.4103/2319-4170.107154
Liu L, Zhang D, Song X, Guo M, Wang Z, Geng F, Zhou X, Nie S (2022) Compound hydrogels derived from gelatin and gellan gum regulates the release of anthocyanins in simulated digestion. Food Hydrocoll 127:107487. https://doi.org/10.1016/j.foodhyd.2022.107487
López-Ojeda W, Hurley RA (2023) Ketone bodies and brain metabolism: new insights and perspectives for neurological diseases. J Neuropsychiatry Clin Neurosci 35:104–109. https://doi.org/10.1176/appi.neuropsych.20230017
Lu M, Cao Y, Ho C-T, Huang Q (2016) Development of organogel-derived capsaicin nanoemulsion with improved bioaccessibility and reduced gastric mucosa irritation. J Agric and Food Chem 64:4735–4741. https://doi.org/10.1021/acs.jafc.6b01095
Lu M, Cao Y, Ho C-T, Huang Q (2017) The enhanced anti-obesity effect and reduced gastric mucosa irritation of capsaicin-loaded nanoemulsions. Food Funct 8:1803–1809. https://doi.org/10.1039/C7FO00173H
Luo S-Z, Hu X-F, Jia Y-J, Pan L-H, Zheng Z, Zhao Y-Y, Mu D-D, Zhong X-Y, Jiang S-T (2019) Camellia oil-based oleogels structuring with tea polyphenol-palmitate particles and citrus pectin by emulsion-templated method: preparation, characterization and potential application. Food Hydrocoll 95:76–87. https://doi.org/10.1016/j.foodhyd.2019.04.016
Ma C-J, Wu J-M, Tsai H-L, Huang C-W, Lu C-Y, Sun L-C, Shih Y-L, Chen C-W, Chuang J-F, Wu M-H, Wang M-Y, Lin M-T, Wang J-Y (2015) Prospective double-blind randomized study on the efficacy and safety of an n-3 fatty acid enriched intravenous fat emulsion in postsurgical gastric and colorectal cancer patients. Nutr J 14:9. https://doi.org/10.1186/1475-2891-14-9
Maher T, Kinsella R, Clegg ME (2017) The effect of coconut oil and MCT on satiety and food intake. Proc Nutr Soc 76(OCE1):E2. https://doi.org/10.1017/S0029665117000027
Maher T, Deleuse M, Thondre S, Shafat A, Clegg ME (2021) A comparison of the satiating properties of medium-chain triglycerides and conjugated linoleic acid in participants with healthy weight and overweight or obesity. Eur J Nutr 60:203–215. https://doi.org/10.1007/s00394-020-02235-y
Man YBC, Manaf MA (2006) In: Shahidi F (ed) Nutraceutical and specialty lipids and their co-products. CRC Press, pp 27–56
Manikantan MR, Pandiselvam R, Beegum S, Mathew AC (2019) In: Nampoothiri KUK, Krishnakumar V, Thampan PK, Nair MA (eds) The coconut palm (Cocos nucifera L.)—research and development perspectives. Springer, pp 635–722
Marcus JB (2013) In: Marcus JB (ed) Culinary nutrition. Academic Press, pp 231–277. https://doi.org/10.1016/B978-0-12-391882-6.00006-6
Marten B, Pfeuffer M, Schrezenmeir J (2006) Medium-chain triglycerides. Int Dairy J 16:1374–1382. https://doi.org/10.1016/j.idairyj.2006.06.015
Martin IC, Marinho LH, Brown AE, McRobbie D (1993) Medium chain triglycerides in the management of chylous fistulae following neck dissection. Br J Oral Maxillofac Surg 31:236–238. https://doi.org/10.1016/0266-4356(93)90146-n
Martin-McGill KJ, Bresnahan R, Levy RG, Cooper PN (2020) Ketogenic diets for drug-resistant epilepsy. Cochrane Database Syst Rev 6:CD001903. https://doi.org/10.1002/14651858.CD001903.pub5
Martínez-Vallespín B, Vahjen W, Zentek J (2016) Effects of medium-chain fatty acids on the structure and immune response of IPEC-J2 cells. Cytotechnology 68:1925–1936. https://doi.org/10.1007/s10616-016-0003-1
Martuscello RT, Vedam-Mai V, McCarthy DJ, Schmoll ME, Jundi MA, Louviere CD, Griffith BG, Skinner CL, Suslov O, Deleyrolle LP, Reynolds BA (2016) A supplemented high-fat low-carbohydrate diet for the treatment of glioblastoma. Clin Cancer Res 22:2482–2495. https://doi.org/10.1158/1078-0432.CCR-15-0916
Masino SA, Rho JM (2012) In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV (eds) Jasper’s basic mechanisms of the epilepsies, 4th ed. National Center for Biotechnology Information (US). http://www.ncbi.nlm.nih.gov/books/NBK98219/
Matsue M, Mori Y, Nagase S, Sugiyama Y, Hirano R, Ogai K, Ogura K, Kurihara S, Okamoto S (2019) Measuring the antimicrobial activity of lauric acid against various bacteria in human gut microbiota using a new method. Cell Transplant 28:1528–1541. https://doi.org/10.1177/0963689719881366
Matsuo T, Matsuo M, Kasai M, Takeuchi H (2001) Effects of a liquid diet supplement containing structured medium- and long-chain triacylglycerols on bodyfat accumulation in healthy young subjects. Asia Pac J Clin Nutr 10:46–50. https://doi.org/10.1046/j.1440-6047.2001.00196.x
McCarty MF, DiNicolantonio JJ (2016) Lauric acid-rich medium-chain triglycerides can substitute for other oils in cooking applications and may have limited pathogenicity. Open Heart 3:e000467. https://doi.org/10.1136/openhrt-2016-000467
McCray S, Parrish CR (2004) When chyle leaks: nutrition management options. Pract Gastroenterol 28:60–77
Meng Z, Qi K, Guo Y, Wang Y, Liu Y (2018a) Effects of thickening agents on the formation and properties of edible oleogels based on hydroxypropyl methyl cellulose. Food Chem 246:137–149. https://doi.org/10.1016/j.foodchem.2017.10.154
Meng Z, Qi K, Guo Y, Wang Y, Liu Y (2018b) Macro-micro structure characterization and molecular properties of emulsion-templated polysaccharide oleogels. Food Hydrocoll 77:17–29. https://doi.org/10.1016/j.foodhyd.2017.09.006
Mett J, Müller U (2021) The medium-chain fatty acid decanoic acid reduces oxidative stress levels in neuroblastoma cells. Sci Rep 11:6135. https://doi.org/10.1038/s41598-021-85523-9
Miles JM, Cattalini M, Sharbrough FW, Wold LE, Wharen RE Jr, Gerich JE, Haymond MW (1991) Metabolic and neurologic effects of an intravenous medium-chain triglyceride emulsion. J Parenter Enteral Nutr 15:37–41. https://doi.org/10.1177/014860719101500137
Mingrone G, De Gaetano A, Greco AV, Capristo E, Castagneto M, Gasbarrini G (1995) Medium-chain triglycerides for parenteral nutrition: kinetic profile in humans. Nutrition 11:418–422
Mirzaee Ghazani S, Marangoni A (2020) Fractionated coconut oil and MCT oil production: facts and fiction. Int News Fats Oils Relat Mater 31:29–32. https://doi.org/10.21748/inform.10.2020.29
Modre-Osprian R, Osprian I, Tilg B, Schreier G, Weinberger KM, Graber A (2009) Dynamic simulations on the mitochondrial fatty acid beta-oxidation network. BMC Syst Biol 3:2. https://doi.org/10.1186/1752-0509-3-2
Mohamad N-V (2023) Strategies to enhance the solubility and bioavailability of tocotrienols using self-emulsifying drug delivery system. Pharmaceuticals 16:1403. https://doi.org/10.3390/ph16101403
Mumme K, Stonehouse W (2015) Effects of medium-chain triglycerides on weight loss and body composition: a meta-analysis of randomized controlled trials. J Acad Nutr Diet 115:P249-263. https://doi.org/10.1016/j.jand.2014.10.022
Murack M, Messier C (2019) The impact of lactic acid and medium chain triglyceride on blood glucose, lactate and diurnal motor activity: a re-examination of a treatment of major depression using lactic acid. Physiol Behav 208:1–13. https://doi.org/10.1016/j.physbeh.2019.112569
Murphy JL, Badaloo AV, Chambers B, Forrester TE, Wootton SA, Jackson AA (2002) Maldigestion and malabsorption of dietary lipid during severe childhood malnutrition. Arch Dis Child 87:522–525. https://doi.org/10.1136/adc.87.6.522
Nagao K, Yanagita T (2010) Medium-chain fatty acids: functional lipids for the prevention and treatment of the metabolic syndrome. Pharmacol Res 61:208–212. https://doi.org/10.1016/j.phrs.2009.11.007
Nahum V, Domb AJ (2021) Recent developments in solid lipid microparticles for food ingredients delivery. Foods 10:400. https://doi.org/10.3390/foods10020400
Narayanan A, Ananda Baskaran S, Amalaradjou MAR, Venkitanarayanan K (2015) Anticarcinogenic properties of medium chain fatty acids on human colorectal, skin and breast cancer cells in vitro. Int J Mol Sci 16:5014–5027. https://doi.org/10.3390/ijms16035014
Nash JJ, Erk KA (2017) Stability and interfacial viscoelasticity of oil-water nanoemulsions stabilized by soy lecithin and Tween 20 for the encapsulation of bioactive carvacrol. Colloids Surf A Physicochem Eng Asp 517:1–11. https://doi.org/10.1016/j.colsurfa.2016.12.056
Nebeling LC, Miraldi F, Shurin SB, Lerner E (1995) Effects of a ketogenic diet on tumor metabolism and nutritional status in pediatric oncology patients: two case reports. J Am Coll Nutr 14:202–208. https://doi.org/10.1080/07315724.1995.10718495
Neelakantan N, Seah JYH, van Dam RM (2020) The effect of coconut oil consumption on cardiovascular risk factors: a systematic review and meta-analysis of clinical trials. Circulation 141:803–814. https://doi.org/10.1161/CIRCULATIONAHA.119.043052
Neeland IJ, Ross R, Després J-P, Matsuzawa Y, Yamashita S, Shai I, Seidell J, Magni P, Santos RD, Arsenault B, Cuevas A, Hu FB, Griffin B, Zambon A, Barter P, Fruchart J-C, Eckel RH (2019) Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol 7:715–725. https://doi.org/10.1016/S2213-8587(19)30084-1
Ng YJ, Tham PE, Khoo KS, Cheng CK, Chew KW, Show PL (2021) A comprehensive review on the techniques for coconut oil extraction and its application. Bioprocess Biosyst Eng 44:1807–1818. https://doi.org/10.1007/s00449-021-02577-9
Nguyen V, Diep T (2022) Efficacy of enriched medium-chain fatty acids in virgin coconut oil in their antibacterial activity against food pathogens. Philipp J Sci 151:813–821. https://doi.org/10.56899/151.03.02
Nimbkar S, Leena MM, Moses JA, Anandharamakrishnan C (2022) Medium chain triglycerides (MCT): state-of-the-art on chemistry, synthesis, health benefits and applications in food industry. Compr Rev Food Sci Food Saf 21:843–867. https://doi.org/10.1111/1541-4337.12926
Norgren J, Sindi S, Sandebring-Matton A, Kåreholt I, Daniilidou M, Akenine U, Nordin K, Rosenborg S, Ngandu T, Kivipelto M (2020) Ketosis after intake of coconut oil and caprylic acid—with and without glucose: a cross-over study in healthy older adults. Front Nutr 7:40. https://doi.org/10.3389/fnut.2020.00040
Nosaka N, Tsujino S, Honda K, Suemitsu H, Kato K (2020) Enhancement of fat oxidation during submaximal exercise in sedentary persons: variations by medium-chain fatty acid composition and age group. Lipids 55:173–183. https://doi.org/10.1002/lipd.12222
O’Brien RD (2012) Fats and oils: formulating and processing for applications, 3rd edn. CRC Press, Boca Raton. https://doi.org/10.1201/9781420061673
Omori NE, Woo GH, Mansor LS (2022) Exogenous ketones and lactate as a potential therapeutic intervention for brain injury and neurodegenerative conditions. Front Hum Neurosci 16:846183. https://doi.org/10.3389/fnhum.2022.846183
Ooyama K, Wu J, Nosaka N, Aoyama T, Kasai M (2008) Combined intervention of medium-chain triacylglycerol diet and exercise reduces body fat mass and enhances energy expenditure in rats. J Nutr Sci Vitaminol 54:136–141. https://doi.org/10.3177/jnsv.54.136
Osborn HT, Shewfelt RL, Akoh CC (2003) Sensory evaluation of a nutritional beverage containing canola oil/caprylic acid structured lipid. J Am Oil Chem Soc 80:357–360. https://doi.org/10.1007/s11746-003-0703-3
Osman A (2019) In: Ramadan MF (ed) Fruit oils: chemistry and functionality. Springer, pp 209–221. https://doi.org/10.1007/978-3-030-12473-1_9
O’Sullivan CM, Barbut S, Marangoni AG (2016) Edible oleogels for the oral delivery of lipid soluble molecules: composition and structural design considerations. Trends Food Sci Technol 57:59–73. https://doi.org/10.1016/j.tifs.2016.08.018
Otto C, Kaemmerer U, Illert B, Muehling B, Pfetzer N, Wittig R, Voelker HU, Thiede A, Coy JF (2008) Growth of human gastric cancer cells in nude mice is delayed by a ketogenic diet supplemented with omega-3 fatty acids and medium-chain triglycerides. BMC Cancer 8:122. https://doi.org/10.1186/1471-2407-8-122
Pandya AJ, Haenlein GFW (2009) In: Park YW (ed) Bioactive components in milk and dairy products. Wiley, pp 105–158
Paramita V, Furuta T, Yoshii H (2012) High-oil-load encapsulation of medium-chain triglycerides and d-limonene mixture in modified starch by spray drying. J Food Sci 77:E38–E44. https://doi.org/10.1111/j.1750-3841.2011.02534.x
Parekh VJ, Rathod VK, Pandit AB (2011) Substrate hydrolysis. In: Moo-Young M (ed) Comprehensive biotechnology, 2nd edn. Academic Press, London, pp 103–118. https://doi.org/10.1016/B978-0-08-088504-9.00094-5
Park J, Choi J, Kim D-D, Lee S, Lee B, Lee Y, Kim S, Kwon S, Noh M, Lee M-O, Le Q-V, Oh Y-K (2021) Bioactive lipids and their derivatives in biomedical applications. Biomol Ther 29:465–482. https://doi.org/10.4062/biomolther.2021.107
Park SY, Kim M-J, Lee JH (2022) Physicochemical properties and volatile formation mechanism of medium-chain triacylglycerols during heating. J Food Sci 87:2616–2625. https://doi.org/10.1111/1750-3841.16162
Peedikayil FC, Remy V, John S, Chandru TP, Sreenivasan P, Bijapur GA (2016) Comparison of antibacterial efficacy of coconut oil and chlorhexidine on Streptococcus mutans: an in vivo study. J Int Soc Prev Community Dent 6:447–452. https://doi.org/10.4103/2231-0762.192934
Pereira E, Fernandes J-M, Gonçalves R, Pinheiro AC, Salomé Duarte M, Madalena Alves M, Meirelles AJA, Maximo GJ, Vicente AA (2023) Evaluating the in vitro digestion of lipids rich in medium-chain fatty acids (MCFAs) using dynamic and static protocols. Food Chem 406:135080. https://doi.org/10.1016/j.foodchem.2022.135080
Pham LJ (2016) Coconut (Cocos nucifera). In: McKeon TA, Hayes DG, Hildebrand DF, Weselake RJ (eds) Industrial oil crops. Elsevier, Amsterdam, pp 231–242. https://doi.org/10.1016/B978-1-893997-98-1.00009-9
Pool H, Mendoza S, Xiao H, Julian McClements D (2013) Encapsulation and release of hydrophobic bioactive components in nanoemulsion-based delivery systems: impact of physical form on quercetin bioaccessibility. Food Funct 4:162–174. https://doi.org/10.1039/C2FO30042G
Poonia N, Kharb R, Lather V, Pandita D (2016) Nanostructured lipid carriers: versatile oral delivery vehicle. Future Sci OA 2:FSO135. https://doi.org/10.4155/fsoa-2016-0030
Pruseth B, Banerjee S, Ghosh A (2020) Integration of in silico and in vitro approach to reveal the anticancer efficacy of Virgin Coconut Oil. CORD 36:1–9. https://doi.org/10.37833/cord.v36i.415
Puşcaş A, Mureşan V, Socaciu C, Muste S (2020) Oleogels in food: a review of current and potential applications. Foods 9:1–27. https://doi.org/10.3390/foods9010070
Qian C, Decker EA, Xiao H, McClements DJ (2012) Nanoemulsion delivery systems: influence of carrier oil on β-carotene bioaccessibility. Food Chem 135:1440–1447. https://doi.org/10.1016/j.foodchem.2012.06.047
Qiu C, Chen C, Zhang W, Kou Q, Wu S, Zhou L, Liu J, Ma G, Chen J, Chen M, Luo H, Zhang X, Lai J, Yu Z, Yu X, Liao W, Guan X, Ouyang B (2017) Fat-modified enteral formula improves feeding tolerance in critically ill patients: a multicenter, single-blind, randomized controlled trial. JPEN J Parenter Enter Nutr 41:785–795. https://doi.org/10.1177/0148607115601858
Qiu H, Qiu Z, Chen Z, Liu L, Wang J, Jiang H, Zhang H, Liu G (2021) Antioxidant properties of blueberry extract in different oleogel systems. LWT 137:110364. https://doi.org/10.1016/j.lwt.2020.110364
Ramya V, Shyam KP, Kowsalya E, Balavigneswaran CK, Kadalmani B (2022) Dual roles of coconut oil and its major component lauric acid on redox nexus: focus on cytoprotection and cancer cell death. Front Neurosci 16:833630. https://doi.org/10.3389/fnins.2022.833630
Rasmussen E, Patel V, Tideman S, Frech R, Frigerio R, Narayanan J (2023) Efficacy of supplemental MCT oil on seizure reduction of adult drug-resistant epilepsy: a single-center open-label pilot study. Nutr Neurosci 26:535–539. https://doi.org/10.1080/1028415X.2022.2065816
Rebello CJ, Keller JN, Liu AG, Johnson WD, Greenway FL (2015) Pilot feasibility and safety study examining the effect of medium chain triglyceride supplementation in subjects with mild cognitive impairment: a randomized controlled trial. BBA Clin 3:123–125. https://doi.org/10.1016/j.bbacli.2015.01.001
Rego Costa AC, Rosado EL, Soares-Mota M (2012) Influence of the dietary intake of medium chain triglycerides on body composition, energy expenditure and satiety: a systematic review. Nutr Hosp 27:103–108. https://doi.org/10.1590/S0212-16112012000100011
Rethinam P (2019) In: Nampoothiri KUK, Krishnakumar V, Thampan PK, Nair MA (eds) The coconut palm (Cocos nucifera L.)—research and development perspectives. Springer, pp 21–56
Rho JM, Stafstrom CE (2012) The ketogenic diet: what has science taught us? Epilepsy Res 100:210–217. https://doi.org/10.1016/j.eplepsyres.2011.05.021
Rogawski MA (2016) A fatty acid in the MCT ketogenic diet for epilepsy treatment blocks AMPA receptors. Brain 139:306–309. https://doi.org/10.1093/brain/awv369
Roopashree PG, Shetty SS, Suchetha Kumari N (2021) Effect of medium chain fatty acid in human health and disease. J Funct Foods 87:104724. https://doi.org/10.1016/j.jff.2021.104724
Roopashree PG, Shetty SS, Shetty VV, Nalilu SK (2022) Medium-chain fatty acids and breast cancer risk by receptor and pathological subtypes. Nutrients 14:5351. https://doi.org/10.3390/nu14245351
Rudkowska I, Jones PJH (2007) In: Henry CJK (ed) Novel food ingredients for weight control. Elsevier, pp 305–325. https://doi.org/10.1533/9781845693114.3.305
Salentinig S, Yepuri NR, Hawley A, Boyd BJ, Gilbert E, Darwish TA (2015) Selective deuteration for molecular insights into the digestion of medium chain triglycerides. Chem Phys Lipids 190:43–50. https://doi.org/10.1016/j.chemphyslip.2015.06.007
Salway JG (2004) Metabolism at a glance, 3rd edn. Wiley, Malden
Sandupama P, Munasinghe D, Jayasinghe M (2022) Coconut oil as a therapeutic treatment for alzheimer’s disease: a review. J Future Foods 2:41–52. https://doi.org/10.1016/j.jfutfo.2022.03.016
Sankararaman S, Sferra TJ (2018) Are we going nuts on coconut oil? Curr Nutr Rep 7:107–115. https://doi.org/10.1007/s13668-018-0230-5
Satala CB, Bara TJ, Jung I, Tudorache V, Gurzu S (2021) Chylous ascites, unusual association with ductal pancreatic adenocarcinoma with plasmacytoid morphology: a case report and literature review. Surg J 07:e158–e162. https://doi.org/10.1055/s-0041-1728651
Sethi S, Joshi A, Arora B, Chauhan OP (2022) Chemical composition of foods. In: Chauhan OP (ed) Advances in food chemistry: food components, processing and preservation. Springer, Berlin, pp 1–37. https://doi.org/10.1007/978-981-19-4796-4_1
Sezgin Y, Memis Ozgul B, Alptekin NO (2019) Efficacy of oil pulling therapy with coconut oil on four-day supragingival plaque growth: a randomized crossover clinical trial. Complement Ther Med 47:102193. https://doi.org/10.1016/j.ctim.2019.102193
Shah ND, Limketkai BN (2017) The use of medium-chain triglycerides in gastrointestinal disorders. Pract Gastroenterol 160:20–28
Shah BR, Zhang C, Li Y, Li B (2016) Bioaccessibility and antioxidant activity of curcumin after encapsulated by nano and Pickering emulsion based on chitosan-tripolyphosphate nanoparticles. Food Res Int 89:399–407. https://doi.org/10.1016/j.foodres.2016.08.022
Shahidi F, Senanayake SPJN (2006) In: Shahidi F (ed) Nutraceutical and specialty lipids and their co-products. CRC Press, pp 1–26
Shang J, Zhong F, Zhu S, Huang D, Li Y (2021) Formation, structural characteristics and physicochemical properties of beeswax oleogels prepared with tea polyphenol loaded gelators. Food Funct 12:1662–1671. https://doi.org/10.1039/D0FO02772C
Shea JC, Bishop MD, Parker EM, Gelrud A, Freedman SD (2003) An enteral therapy containing medium-chain triglycerides and hydrolyzed peptides reduces postprandial pain associated with chronic pancreatitis. Pancreatology 3:36–40. https://doi.org/10.1159/000069144
Sheela D, Narayanankutty A, Nazeem P, Raghavamenon A, Muthangaparambil S (2019) Lauric acid induce cell death in colon cancer cells mediated by the epidermal growth factor receptor downregulation: an in silico and in vitro study. Hum Exp Toxicol 38:753–761. https://doi.org/10.1177/0960327119839185
Shilling M, Matt L, Rubin E, Visitacion MP, Haller NA, Grey SF, Woolverton CJ (2013) Antimicrobial effects of virgin coconut oil and its medium-chain fatty acids on Clostridium difficile. J Med Food 16:1079–1085. https://doi.org/10.1089/jmf.2012.0303
Shyr B-U, Shyr B-S, Chen S-C, Shyr Y-M, Wang S-E (2020) Chyle leakage after robotic and open pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci 27:273–279. https://doi.org/10.1002/jhbp.716
Schmidt M, Pfetzer N, Schwab M, Strauss I, Kämmerer U (2011) Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: a pilot trial. Nutr Metab 8:54. https://doi.org/10.1186/1743-7075-8-54
Schönfeld P, Wojtczak L (2016) Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. J Lipid Res 57:943–954. https://doi.org/10.1194/jlr.R067629
Schwingshackl L, Schlesinger S (2023) Coconut oil and cardiovascular disease risk. Curr Atheroscler Rep 25:231–236. https://doi.org/10.1007/s11883-023-01098-y
Sihombing N, Silalahi J, Suryanto D (2014) Antibacterial activity of aqueous garlic (Allium sativum) extracts and virgin coconut oil and their combination against Bacillus cereus ATCC 14579 and Escherichia coli ATCC 8939. Int J PharmTech Res 6:2774–2782
Silva SAD, Sampaio GR, Torres EAFDS (2020) Phytosterols content in vegetable oils of Brazil: coconut, safflower, linseed and evening primrose. Braz Arch Biol Technol 63:e20190216. https://doi.org/10.1590/1678-4324-2020190216
Siriphanich J, Saradhuldhat P, Romphophak T, Krisanapook K, Pathaveerat S, Tongchitpakdee S (2011) In: Yahia EM (ed) Postharvest biology and technology of tropical and subtropical fruits. Woodhead Publishing, pp 8–35e. https://doi.org/10.1533/9780857092885.8
Skrivanova E, Marounek M, Benda V, Brezina P (2006) Susceptibility of Escherichia coli, Salmonella sp. and Clostridium perfringens to organic acids and monolaurin. Vet Med 51:81–88. https://doi.org/10.17221/5524-VETMED
Smart KM, Alex G, Hardikar W (2011) Feeding the child with liver disease: a review and practical clinical guide. J Gastroenterol Hepatol 26:810–815. https://doi.org/10.1111/j.1440-1746.2011.06687.x
Sonnay S, Chakrabarti A, Thevenet J, Wiederkehr A, Christinat N, Masoodi M (2019) Differential metabolism of medium-chain fatty acids in differentiated human-induced pluripotent stem cell-derived astrocytes. Front Physiol 10:657. https://doi.org/10.3389/fphys.2019.00657
Soto-Avellaneda A, Morrison BE (2020) Signaling and other functions of lipids in autophagy: a review. Lipids Health Dis 19:214. https://doi.org/10.1186/s12944-020-01389-2
Spiazzi BF, Duarte AC, Zingano CP, Teixeira PP, Amazarray CR, Merello EN, Wayerbacher LF, Farenzena LP, Correia PE, Bertoluci MC, Gerchman F, Colpani V (2023) Coconut oil: an overview of cardiometabolic effects and the public health burden of misinformation. Arch Endocrinol Metab 67:e000641. https://doi.org/10.20945/2359-3997000000641
Srivastava Y, Semwal AD, Sharma GK (2018) In: Grumezescu AM, Holban AM (eds) Therapeutic, probiotic, and unconventional foods. Academic Press, pp 291–301. https://doi.org/10.1016/B978-0-12-814625-5.00015-7
Stortz TA, Marangoni AG (2014) The replacement for petrolatum: thixotropic ethylcellulose oleogels in triglyceride oils. Green Chem 16:3064–3070. https://doi.org/10.1039/C4GC00052H
Stuchlík M, Zák S (2001) Lipid-based vehicle for oral drug delivery. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 145:17–26
Sun M, Zhai X, Xue K, Hu L, Yang X, Li G, Si L (2011) Intestinal absorption and intestinal lymphatic transport of sirolimus from self-microemulsifying drug delivery systems assessed using the single-pass intestinal perfusion (SPIP) technique and a chylomicron flow blocking approach: linear correlation with oral bioavailabilities in rats. Eur J Pharm Sci 43:132–140. https://doi.org/10.1016/j.ejps.2011.04.011
Takeishi J, Tatewaki Y, Nakase T, Takano Y, Tomita N, Yamamoto S, Mutoh T, Taki Y (2021) Alzheimer’s disease and type 2 diabetes mellitus: the use of MCT oil and a ketogenic diet. Int J Mol Sci 22:12310. https://doi.org/10.3390/ijms222212310