Abstract
Purpose
There is a tendency to use data generated for adults in the management of pediatric Differentiated Thyroid Carcinoma, neglecting the clinical peculiarities of this condition in childhood. This study aimed to assess and compare the clinical-epidemiological characteristics and their significance in the evolution of thyroid carcinoma diagnosed in childhood across different age groups.
Methods
Seventy-seven patients diagnosed with Differentiated Thyroid Carcinoma (DTC) up to 21 years old were selected and divided into different age groups: up to 10 years, 11 to 18 years, and 19 to 21 years old. Clinical-epidemiological data and their influence in the disease progression were analyzed and compared across age groups.
Results
Patients diagnosed below 10 years of age were associated with tumors showing extrathyroidal extension, metastasis in regional lymph nodes, higher levels of stimulated thyroglobulin in the diagnostic iodine-131 whole-body scan (WBS), and under TSH suppression in the last assessment. Additionally, pulmonary metastasis were associated in both diagnostic and post-radioiodine dose WBSs in these younger patients. Analysis of findings in the post-radioiodine therapy WBS revealed significant differences between all age groups (p = 0.0029). The time of diagnosis was identified as a factor associated with an excellent response in subgroups up to 18 years and up to 21 years. No factors associated with dynamic responses over the 1st, 3rd and 5th years of follow-up and the persistence/recurrence of the disease were identified in the subgroup up to 18 years. In the subgroup up to 21 years, having an incomplete structural response in the 3rd year of follow-up increased the chances of recurrent or persistent response by 5.5 times, and by 32.6 times if found in the 5th year of follow-up.
Conclusions
Younger patients exhibited more aggressive tumor characteristics and underwent more rigorous treatment. However, treatment response and disease status in the last assessment, whether free or recurrent/persistence, were similar when comparing the age groups of 11 to 18 and 19 to 21 years. Nonetheless, responses obtained in the 3rd and 5th years post-treatment emerged as factors associated with the persistence/recurrence of the disease in the last assessment in the age group up to 21 years but not in patients diagnosed up to 18 years, a relevant distinction considering the tumor behavior in defining the pediatric age range in thyroid cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid carcinoma accounts for 0.5% to 3% of malignant neoplasms in childhood and adolescence [1]. However, its incidence is increasing, making it the most common cancer of the endocrine system in children and young adults [2], predominantly in the form of papillary thyroid carcinoma (PTC) [2].
In childhood, PTC presents higher rates of recurrence and persistence compared to the adult population, along with increased rates of extracapsular extension (50% versus 30%), regional lymph node involvement (80% versus 50%), distant metastases (30% versus 5%), and multifocal disease (65% versus 38%) [2]. Surgical therapy, either alone or combined with radioactive iodine, yields favorable outcomes even in more advanced cases [3], reaching to five-years survival rates of 99.8% for tumors confined to the thyroid and 97.1% for PTC with regional lymph node metastasis [4].
According to “Management Guidelines for Children with Thyroid Nodules and Differentiated Thyroid Cancer” no validated postoperative staging system has been established for children with PTC. The applicability of adult staging systems in the pediatric context is limited due to observed clinical differences between the age groups. While the AJCC TNM classification system is widely used in adults, its utility in determining prognosis for children is restricted because of the low disease-specific mortality and the prevalence of stage I or II disease in young patients. Nonetheless, the TNM classification is valuable for describing disease extent and guiding evaluation and management approaches. Knowledge of lymph node status, especially the presence of advanced cervical lymph node disease, is crucial for risk stratification in pediatric PTC patients. Using the TNM system, particularly lymph node and distant metastasis staging, allows categorization of pediatric patients into risk groups, aiding in identifying those at risk of persistent disease and guiding postoperative staging decisions to screen for distant metastasis [5]. The staging is performed within 12 weeks after surgery, with the AJCC TNM classification assessing the risk of persistent disease in three categories: low (N0, Nx, or N1a), intermediate (extensive N1a and N1b), and high risk (extensive N1b, T4, or any distant metastasis) [5]. Continuous surveillance during follow-up is recommended, considering the risk of recurrence over more than 30 years, categorized as excellent response, incomplete biochemical response, incomplete structural response, or indeterminate, based on biochemical and imaging parameters [4].
The 2022 European Thyroid Association Guidelines for the management of pediatric thyroid nodules and differentiated thyroid carcinoma advocates that the assessment of postoperative staging be conducted through the surgical report, histological analysis, Tg measurement, and post-therapy I-131 scintigraphy. Additionally, it recommends the adoption of the AJCC TNM classification system to describe the extent of the disease in the pediatric population. In the subsequent follow-up, monitoring of TSH and free T4 levels is advised every 3 to 6 months during periods of growth and puberty, followed by annual assessments, with TSH suppression recommended until complete clinical remission and subsequently maintaining target TSH levels between 0.3 and 1.0 mU/L. The panel of experts also suggests semiannual monitoring of serum Tg in the first 3 years and annually thereafter, proposing the measurement of TgAb as alternative tumor markers if present. Regarding ultrasound follow-up, the Guideline recommends annual cervical ultrasounds in the initial 5 years of follow-up [6].
The relationship between patient age and Differentiated Thyroid Carcinoma (DTC) behavior is not clearly defined in the literature, and the age cutoff is arbitrary across studies [7]. Some authors suggest 18 years as the cutoff, considering that most patients have completed growth and pubertal development, impacting tumor behavior [5]. Nevertheless, studies vary in this regard, with some including individuals up to 21 years of age [5].
This study aims to assess and compare the clinical-epidemiological characteristics and their influence in the progression of thyroid carcinoma diagnosed in different age groups considered as childhood.
Methods
Study design and patients
This retrospective cross-sectional study aimed to investigate the clinical, histopathological, and progression characteristics of patients diagnosed with DTC within the age groups of up to 10 years, 11 to 18 years, and 19 to 21 years. Our sample consisted of 9 patients diagnosed before the age of 10, 39 patients diagnosed between 11 and 18 years old, and 29 patients diagnosed between 19 and 21 years old.
The age groups were defined based on the impact of physiological changes in growth and development on tumor behavior, considering individuals up to 10 years old as prepubertal, between 11 and 18 years old as pubertal, and from 19 years old onwards as transitioning to adulthood.
In our service, we recommend total thyroidectomy with or without lymphadenectomy (depending on lymph node involvement) and radioiodine therapy if there is evidence of locoregional disease not amenable to surgical resection, as well as in the presence of distant metastasis. Some patients did not undergo surgery and/or received iodine therapy at our service, however only patients who received treatment compatible with the protocols adopted were included.
A total of 1409 medical records of thyroid cancer patients under current or prior follow-up at the Thyroid Cancer Service of a tertiary hospital were evaluated for the period between 1990 and 2022. The study included patients diagnosed with thyroid carcinoma up to 21 years of age, providing clinical and pathological data suitable for initial risk stratification, with a minimum follow-up of 12 months after surgical treatment. Exclusion criteria involved incomplete medical records, definitive loss of clinical follow-up within 12 months, refusal to participate, and failure to sign the informed consent form during outpatient consultations.
Initially, 79 patients diagnosed before the age of 21 (5.6%) were selected. After applying inclusion and exclusion criteria, only 77 patients (5.46%) remained in the study, undergoing periodic clinical evaluation, assessment of serum thyroglobulin (Tg), anti-thyroglobulin antibody (TgAb), thyroid-stimulating hormone (TSH), free thyroxine (FT4), and annual cervical ultrasound.
The cutoff values for detectable thyroglobulin were analyzed according to the American Thyroid Association (ATA) guidelines (2015), with Tg during thyroid hormone suppressive therapy above 0.2 ng/ml or stimulated Tg above 1 ng/ml. The serum concentration of anti-thyroglobulin antibody was determined using electrochemiluminescence (Liaison, Diasorin), considering values detectable by the assay method as “positive,” which, in 2020, was replaced by absolute values above 10 IU/ml in our study.
The risk classification for tumor persistence after initial treatment was based on ATA criteria: low risk - disease confined to the thyroid, N0 or Nx, or with incidental metastasis in a small number of central lymph nodes; intermediate risk - extensive N1a disease or minimal N1b disease; high risk - regionally extensive disease (extensive N1b) or locally invasive (T4), with or without distant metastasis [5].
Dynamic treatment response was assessed according to ATA criteria in the first, third, and fifth years, and at the time of the last evaluation in consultation, starting from the date of the surgical procedure. An excellent response was considered for those with negative imaging, negative anti-thyroglobulin antibody, and suppressed thyroglobulin < 0.2 ng/ml or stimulated < 1 ng/ml; incomplete biochemical response for those with suppressed thyroglobulin > 1 ng/ml or stimulated Tg > 10 ng/ml, or increased TgAb levels, with negative imaging; incomplete structural response for patients with evidence of disease imaging, regardless of thyroglobulin and anti-thyroglobulin antibody levels; and indeterminate response when there were nonspecific imaging findings or iodine-131 whole-body scan with weak uptake in the thyroid bed, or when suppressed thyroglobulin levels were between 0.2 and 1 ng/ml or stimulated between 1 and 10 ng/ml or stable/declining anti-thyroglobulin antibody levels in patients without evidence of disease on imaging [5].
The study was approved by the Human Research Ethics Committee of the institution, CAAE 57425822700005404.
Demographic and histopathological tumor characteristics, follow-up, and outcome
Different age groups were assessed based on the following criteria: demographic data; diagnostic risk factors (previous exposure to cervical radiotherapy); histopathological tumor characteristics; data from the first iodine-131 whole-body scan (WBS) performed after the surgical procedure; stimulated thyroglobulin (Tg) and anti-thyroglobulin antibody (TgAb) values by thyroid-stimulating hormone (TSH); need for radioactive iodine and administered activity; WBS characteristics after the first dose of radioiodine; risk classification at diagnosis; dynamic risk stratification in the first, third, and fifth years of follow-up, and at the time of the last evaluation; late onset of metastases; treatment response at the last assessment; total follow-up time.
Clinical outcomes were evaluated based on initial cancer presentation data: disease-free survival, persistence or recurrence of the disease, and tumor-related death.
Statistical analysis
The analysis was performed using the Statistical Analysis System and R Core Team (2020). Descriptive analysis included frequency tables for categorical variables, measures of dispersion for continuous variables, and graphs when necessary. Group comparisons were performed using the Mann-Whitney/Kruskal-Wallis test (followed by Dunn’s test when necessary) for quantitative variables and Chi-square/Fisher’s Exact tests for categorical variables. Post hoc age group comparisons were conducted using logistic regression with a cumulative logit link function. Univariate logistic regression was employed to identify factors associated with the final ATA response. Multiple logistic regression, according to variable selection, was used to identify predictive factors of the final ATA response. The significance level adopted for the study was 5%.
Results
The mean total follow-up period was 119.6 months (median 108; minimum-maximum 12–396), with 161.4 months (126; 36–396) for patients diagnosed up to age 10, 117.7 (108; 12–324) months for those diagnosed between 11 and 18 years old, and 109.1 (48; 12–384) months for patients between 19 and 21 years old. It was possible to complete all proposed assessments in the study for 42 patients (54.5%), while 35 participants (45.45%) lost follow-up temporarily at some point and came back. No deaths were recorded in our sample across any of the age groups investigated.
Regarding demographic characteristics, there was a predominance of females (77.92%). The presence of a thyroid nodule was highlighted as the reason for surgery in 77.92% of the sample, with the majority having no history of prior cervical irradiation (89.61%). There was no difference between 3 age groups in terms of sex, the presence of risk factors (cervical irradiation), and the reason for surgery (data not shown).
All patients included in the study underwent total thyroidectomy. Lymphadenectomy was performed more frequently in younger patients (p = 0.0161): 49 participants (63.6%) performed lymphadenectomy, with 9 of these patients diagnosed before the age of 10 (100%), 26 between 11 and 18 years old (66.6%), and 14 between 19 and 21 years old (48.27%). Regarding radioiodine therapy, 67 patients received initial therapy (85.7%), with 8 of them diagnosed before the age of 10 (88.8%), and the only patient in this age group who did not receive initial radioiodine therapy received it later due to incomplete structural response; 34 individuals diagnosed between 11 and 18 years old received initial radioiodine therapy (87.17%), with 4 of the remaining 5 in this age group receiving radioiodine therapy later, 2 of them due to incomplete biochemical response and the other 2 due to incomplete structural response; additionally, 25 patients between 19 and 21 years old received initial radioiodine therapy (86,2%).
The nodule diameter, histological type, papillary carcinoma subtype, degree of differentiation (based on the anatomopathological report), capsular and angiolymphatic invasions were also evaluated, with no difference between age groups, as shown in Table 1.
The Table 2 shows the comparison between age groups according to postoperative tumor stratification. In the younger age group, associations were observed with extrathyroidal extension, involvement of regional lymph nodes, higher levels of stimulated thyroglobulin (Tg), pulmonary metastases detected in the diagnostic whole-body scan (WBS), pulmonary metastases, and lymph nodes affected in the post-radioiodine dose WBS, along with higher administered iodine activity.
Post hoc comparison between age groups revealed that the distribution of findings in the first diagnostic WBS was different between patients up to 10 years old at diagnosis and those aged 19 to 21 years, with no differences between the other age groups (p = 0.0047). Additionally, the analysis of the distribution of findings in the WBS post-radioiodine dose revealed that all age groups differed from each other (p = 0.0029).
During follow-up, 10 patients underwent late lymphadenectomy (12.9%), one of them diagnosed before the age of 10 (10%), 6 between 11 and 18 years old (15.38%), and 3 between 19 and 21 years old (10.4%). The mean number of radioiodine doses administered to patients diagnosed up to 10 years old was 2, between 11 and 18 years old it was 1.46 and between 19 and 21 years old it was 1.1.
Regarding the characteristics analyzed during follow-up, the last suppressed thyroglobulin (Tg) was higher in younger patients (p = 0.0338). Table 3 compares age groups regarding the characteristics analyzed during follow-up.
Regarding the risk classification at diagnosis and dynamic treatment responses evaluated during the 1st, 3rd, and 5th years after treatment, as well as the final classification, there were no differences between the groups, as shown in Table 4.
Factors associated with the persistence or recurrence of Differentiated Thyroid Carcinoma (DTC) according to age group
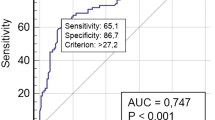
Univariate logistic regression analysis was performed regarding the persistence or recurrence of the disease in two age groups: up to 18 years and up to 21 years. It was no possible to conduct the analysis separately for the age groups up to 10 years and from 11 to 18 years due to the number of patients. The presence of positive TgAb in the context of suppressed TSH at the last evaluation was identified as a factor associated with recurrent/persistent response in both groups. Additionally, the time elapsed since confirmation of diagnosis by the anatomopathological report (time elapsed since diagnosis) was identified as a factor associated with an excellent response in both groups. In the subgroup of patients up to 18 years, no factors associated with dynamic responses over the 1st, 3rd, and 5th years of follow-up and the persistence/recurrence of the disease were identified. However, in the analysis for patients up to 21 years of age was observed that structurally incomplete response in the 3rd year of follow-up increased the chance of recurrent or persistent response at the end of the follow-up by 5.5 times, and by 32.6 times if this finding was verified in the fifth year of follow-up. On the other hand, the following factors were not associated with the persistence/recurrence of the disease: number of tumor foci, diameter of the largest nodule, capsular invasion, angiolymphatic invasion, extrathyroidal extension, tumor extension, lymph node metastasis, distant metastasis, TNM staging, ATA risk classification at diagnosis, radioactive iodine dose, dynamic response in 1st year of follow-up, late metastasis, late lymphadenectomy, and total administered radioiodine therapy activity (Table 5).
Discussion
In this study, we evaluated the characteristics of patients diagnosed with differentiated thyroid carcinoma up to 21 years of age, divided into different age groups, possible cutoff points to ensure more homogeneous analyses regarding the child’s growth and development [5, 7]. It is worth noting that data on puberty, important for this patient development categorization, were challenging to assess due to incomplete records, preventing a reliable evaluation.
A higher prevalence of extrathyroidal extension was observed in younger children, up to 10 years of age, similar to findings by Alessandri et al. [8], Lazar et al. [9], and Silva-Vieira et al. [10], although this varied in other studies [11]. We found a higher prevalence of regional lymph node metastasis at diagnosis in younger children, consistent with O’Gorman et al. [8] and Lazar et al. [9].
Differences were noted in pulmonary and nodal involvement in the initial imaging assessment at the diagnostic iodine-131 whole-body scan between age groups up to 10 years compared to 19 to 21 years and across all age groups in findings after the first radioiodine dose. This agrees with other studies that demonstrated a more aggressive tumor presentation in prepubertal children [9,10,11].
In contrast to these findings, the initial tumor (TNM) staging did not differ among different age groups; however, tumors diagnosed before 10 years of age were clearly more aggressive. A potential bias in this finding is related to the long-term follow-up of patients, which, while important for evaluating tumor evolution and prognosis, naturally underwent the influence of changes in treatment and follow-up protocols, as well as improvements in imaging sensitivity and specificity.
Furthermore, patients were stratified over a long follow-up based on the dynamic response classification to treatment at 1st, 3rd, and 5th years after initial treatment [5]. In this regard, predicting the outcome and prognosis based on the early treatment phases was not possible, as the evolution characteristics did not allow differentiation between patients who would progress free of disease or with persistent/recurrent disease when diagnosed up to 18 years of age. Only when extending the analysis to patients up to 21 years of age did the treatment response from the 3rd year of follow-up emerge as a predictive factor for disease progression in the 5th year and at the end of follow-up.
In agreement with these findings, we observed that the time of diagnosis influenced the treatment response in the last assessment, with a longer time since diagnosis emerging as a predictive factor for an excellent response. However, it’s worth noting that we reviewed medical records of thyroid cancer patients currently or previously monitored at our institution from 1990 to 2022. It’s known that there has been an increase in the incidence of advanced tumors in recent years, changes in the therapeutic strategy over the years and eventual undetected recurrences in patients lost to follow-up. Despite the average follow-up time of 9.9 years in our sample, it was not feasible to implement a single treatment protocol for the entire group. Additionally, not all patients received initial treatment at our institution, which could be potential confounding factors for this analysis.
Interestingly, despite younger patients having higher rates of extrathyroidal extension and greater extension of the disease to regional lymph nodes, the treatment response and disease-free or recurrence/persistence status did not differ among the three age groups evaluated. In patients up to 10 years of age, higher stimulated thyroglobulin values were observed at the 1st diagnostic iodine-131 whole-body scan and suppressed thyroglobulin at the last assessment, even after more aggressive treatment, such as a higher frequency of cervical surgical lymph nodes approach and greater total administered iodine activity. This reaffirms that age up to 10 years is associated with more aggressive disease characteristics, despite long survival, similar to patients up to 21 years. It is noteworthy that no deaths occurred in our total sample, suggesting a good prognosis with a high disease-specific survival rate [4].
Female sex was more prevalent (75%), and the primary reason for surgery in most cases was the identification of a thyroid nodule (77.92%), in line with the literature [1, 2, 4], as well as the prevalence of the papillary histological type (94.81%) [12]. Additionally, a minority had a history of previous cervical irradiation (10.39%), accordingly to other authors [12], and we found a higher frequency of multifocal carcinoma (51.95%) than in other studies [1, 2]. None of these characteristics or even those related to the initial tumor stratification were associated with disease persistence or recurrence. Thus, the clinical, histopathological, and initial treatment assessments did not allow distinguishing which patients would have a better or worse outcome during long-term follow-up.
This study has some limitations, such as its retrospective, single-center nature, based on clinical record data collection. Also, some participants temporarily interrupted their follow-up at some point, compromising the complete assessment as initially planned, however we included in the study only patients who received treatment compatible with the protocols adopted in the service. In contrast, it is a structured and reference service for thyroid cancer treatment, which may enhance the credibility of the obtained data. Furthermore, due to the long-term follow-up time, there were changes in some values of biochemical assessment, particularly anti-thyroglobulin antibody, yet in our service, we prioritized evaluating the trend of antibody concentrations over the follow-up.
It is essential to highlight that the relationship between patient age and DTC behavior is not clearly defined in the literature, and the cutoff point used in many studies is arbitrary. In our sample, we included patients diagnosed up to 21 years of age, since data on puberty were incomplete in the medical records. There is a discussion on whether the adoption of a universal age would allow for a more accurate comparison of results, increasing reliability [7], or if the division between age groups should follow the onset of puberty [5].
In conclusion, we were able to show that CDT in the pediatric age group exhibited some characteristics of more aggressive behavior at diagnosis, especially in patients under 10 years of age. However, age did not influence the overall outcome and prognosis of patients when considering individuals up to 21 years old. Treatment responses obtained in the 3rd and 5th years of follow-up were predictive of persistence/recurrence of the disease in the last assessment for patients diagnosed up to 21 years of age, which was not evident in patients up to 18 years old. This difference is relevant when considering tumor behavior in defining the pediatric age range in differentiated thyroid cancer. However, additional studies are needed to confirm these findings.
Data availability
No datasets were generated or analysed during the current study.
References
K. Guo et al. Clinical and molecular characterizations of papillary thyroid cancer in children and young adults: a multicenter retrospective study. Thyroid 31, 1693–1706 (2021). https://doi.org/10.1089/thy.2021.000
G.L. Banik et al. Prevalence and risk factors for multifocality in pediatric thyroid cancer. JAMA Otolaryngol. Head Neck Surgery 147, 1100–1106 (2021). https://doi.org/10.1001/jamaoto.2021.3077
A.R. Hogan, Y. Zhuge, E.A. Perez et al. Pediatric thyroid carcinoma: incidence and outcomes in 1753 patients. J. Surg Res. 156, 167–172 (2019). https://doi.org/10.1016/j.jss.2009.03.098
L. E. Braverman, D.S. Cooper, S. C. Werner, S. H. Ingbar. Werner & ingbar’s the thyroid: a fundamental and clinical text. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins Health (2021).
G.L. Francis, S.G. Waguespack, A.J. Bauer et al. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid 25, 716–759 (2015). https://doi.org/10.1089/thy.2014.0460
C.A. Lebbink, T. P. Links, A. Czarniecka, R.P. Dias, R. Elisei, L. Izatt, H. Krude, K. Lorenz., M. Luster, K. Newbold, A. Piccardo, M. Sobrinho-Simões, T. Takano, A.S.P. van Trotsenburg, F. A. Verburg, H.M. & van Santen. European Thyroid Association Guidelines for the management of pediatric thyroid nodules and differentiated thyroid carcinoma. Eur. Thyroid J., 11 (2022). https://doi.org/10.1530/ETJ-22-0146
K. Sugino, M. Nagahama, W. Kitagawa et al. Cutoff age between pediatric and adult thyroid differentiated cancer: is 18 years old appropriate? Thyroid 32, 145–152 (2021). https://doi.org/10.1089/thy.2021.0255
A.J. Alessandri, K.J. Goddard, G.K. Blair, C.J. Fryer, K.R. Schultz, Age is the major determinant of recurrence in pediatric differentiated thyroid carcinoma. Med. Pediatr. Oncol. 35, 41–46 (2000).
L. Lazar, Y. Lebenthal, A. Steinmetz, M. Yackobovitch-Gavan, M. Phillip, Differentiated thyroid carcinoma in pediatric patients: comparison of presentation and course between pre-pubertal children and adolescents. J. Pediatr. 154, 708–714 (2009). https://doi.org/10.1016/j.jpeds.2008.11.059
M. Silva-Vieira, R. Santos, V. Leite, E. Limbert, Review of clinical and pathological features of 93 cases of well-differentiated thyroid carcinoma in pediatric age at the Lisbon Centre of the Portuguese Institute of Oncology between 1964 and 2006. Int. J. Pediatr. Otorhinolaryngol. 79, 1324–1329 (2015). https://doi.org/10.1016/j.ijporl.2015.06.002
C.S. O’Gorman, J. Hamilton, M. Rachmiel, A. Gupta, B.Y. Ngan, D. Daneman, Thyroid cancer in childhood: a retrospective review of childhood course. Thyroid 20, 375–380 (2010). https://doi.org/10.1089/thy.2009.0386
G. Keefe, K. Culbreath, C.E. Cherella, J.R. Smith, B. Zendejas, R.C. Shamberger, D.M. Richman, M.L. Hollowell, B.P. Modi, A.J. Wassner, Autoimmune thyroiditis and risk of malignancy in children with thyroid nodules. Thyroid 32, 1109–1117 (2022). https://doi.org/10.1089/thy.2022.0241
Acknowledgements
We acknowledge all patients who voluntarily participated in our study. We thank Cleide Aparecida Moreira Silva, Marcelo Tavares and the institution’s research committee for carrying out the statistical analysis.
Author information
Authors and Affiliations
Contributions
J.C.G. and D.E.Z.W.: Conceptualization, Methodology and Manuscript writing and review L.V.M.A. and M.C.R.P.: Manuscript review and editing D.E.Z.W.: Supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Garcia, J.C., de Assumpção, L.V.M., Parisi, M.C.R. et al. Impact of age on tumor characteristics and treatment outcomes in pediatric Differentiated Thyroid Carcinoma. Endocrine (2024). https://doi.org/10.1007/s12020-024-03870-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12020-024-03870-9