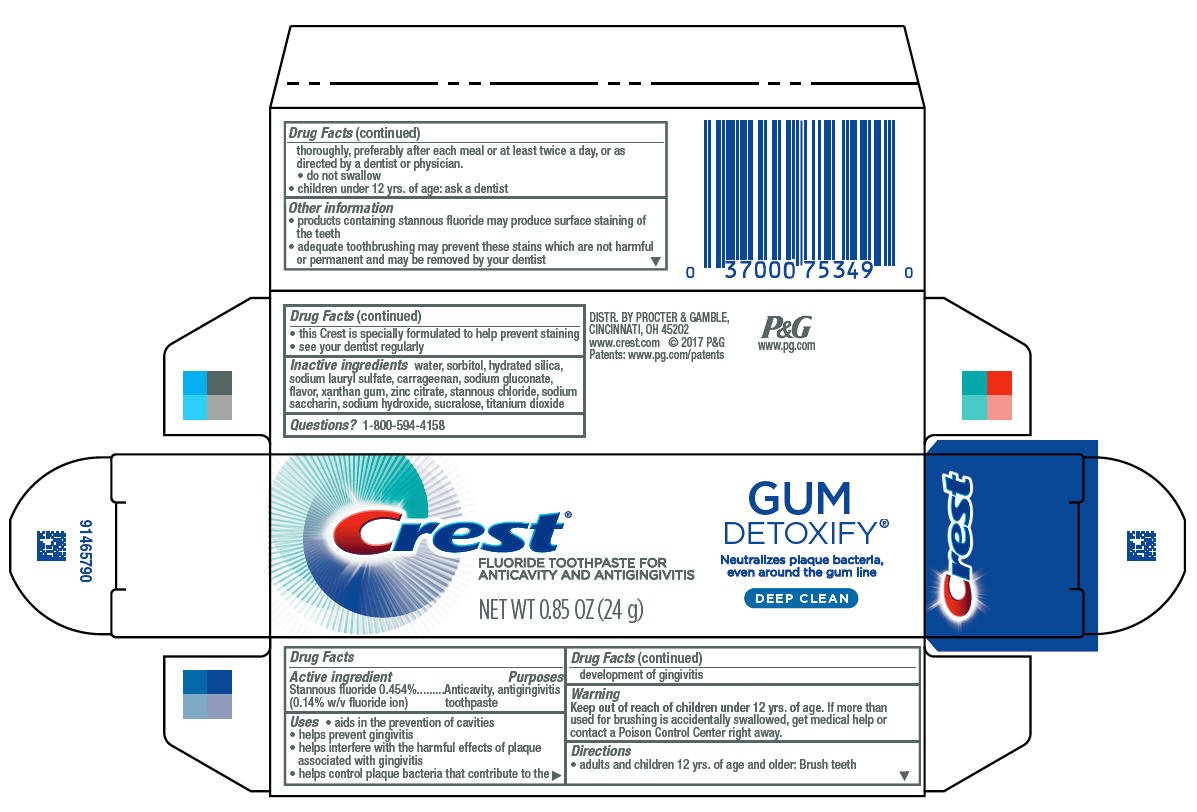
Crest Gum Detoxify
Dosage form: paste, dentifrice
Ingredients: STANNOUS FLUORIDE 1.4mg in 1g
Labeler: The Procter & Gamble Manufacturing Company
NDC code: 37000-481
Medically reviewed by Drugs.com. Last updated on Apr 4, 2024.
Gum Detoxify ®
Active ingredient
Stannous fluoride 0.454%
(0.14% w/v fluoride ion)
Anticavity, antigingivitis toothpaste
- aids in the prevention of cavities
- helps prevent gingivitis
- helps interfere with the harmful effects of plaque associated with gingivitis
- helps control plaque bacteria that contribute to the development of gingivitis
Keep out of reach of children under 12 yrs. of age. If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
- adults and children 12 yrs. of age and older: Brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or physician.
- do not swallow
- children under 12 yrs. of age: ask a dentist
- products containing stannous fluoride may produce surface staining of the teeth
- adequate toothbrushing may prevent these stains which are not harmful or permanent and may be
removed by your dentist
- this Crest is specially formulated to help prevent staining
- see your dentist regularly
water, sorbitol, hydrated silica, sodium lauryl sulfate, carrageenan, sodium gluconate, flavor, xanthan gum, zinc citrate, stannous chloride, sodium saccharin, sodium hydroxide, sucralose, titanium dioxide
1-800-594-4158
DISTR. BY PROCTER & GAMBLE,
CINCINNATI, OH 45202
| CREST
GUM DETOXIFY
stannous fluoride paste, dentifrice |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - The Procter & Gamble Manufacturing Company (004238200) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.